Abstract
Purpose
To evaluate the acceptability, retention, and efficacy of face-to-face intervention, incorporating education and Motivational Interviewing (MI) to support persons with relapsing-remitting multiple sclerosis (PwRRMS) and increase self-reported medication adherence.
Patients and Methods
PwRRMS (N = 60) prescribed Disease Modifying Treatment (DMT), who were identified as non-adherent and consented to participate in an intervention, received verbal education and counseling from their treating physician, a tailored MI counseling and a booster session via telephone with a health psychologist, and a concluding MI counseling six months later. Each PwRRMS filled a battery of patient-reported outcomes (PROs) at baseline, six and 12 months later. The design was a quasi-experimental pre-test post-test across a year.
Results
Of the sixty identified persons who consented to enroll, 52 completed the intervention and 46 completed the follow-up. At six months following the baseline, adherence scores increased (median = 12.0) and were significantly different than at baseline (median=10.0, p = 0.030). Still, at 12 months follow-up there was no significant difference from baseline in reported adherence (median = 11.0, p = 0.106).
Conclusion
This study demonstrated reasonable retention and initial efficacy of a combined psycho-education and MI protocol for PwRRMS to enhance medication adherence to DMT. To maintain the change, a more sustained intervention is required.
Plain Language Summary
The study focused on persons with relapsing-remitting multiple sclerosis (PwRRMS) who do not adhere to their prescribed medication.
Following the identification of non-adherent persons, PwRRMS were offered an intervention to increase their adherence. The study examined how many of those identified consented to enroll in the intervention, how many remained in the intervention, and whether the intervention was efficacious in terms of self-reported adherence.
The intervention included verbal education and counseling from the treating physician, immediately followed by tailored counseling by a psychologist. There was a booster session via telephone with the psychologist, and a concluding counseling meeting six months later. Participants were followed for a year after the initial counseling.
Two-thirds of PWMS identified as non-adherent consented to enroll (n = 60), 52 completed the intervention and 46 completed the follow-up. At six months following counseling, self-reported adherence scores significantly increased, but at 12 months follow-up there was no significant difference from baseline in reported adherence. To maintain the change, a more sustained intervention is required.
Introduction
Disease-modifying therapies (DMTs), currently a mainstay of treatment strategies for persons with relapsing-remitting multiple sclerosis (PwRRMS), have been shown to limit the number of relapses, prevent new lesion formation, as well as reduce the accumulation of disability.Citation1 Adherence to DMT has also been associated with improved quality of life, a decrease in neuropsychological issues and fewer days of work loss.Citation2,Citation3 Alongside, adherence to DMT also reduces hospitalizations, emergency visits, and outpatient visits, also manifested in reduced annual medical care costs at the societal level.Citation4,Citation5 Adherence is especially important in chronic conditions, and more so when disease onset is at an early age, requiring many years of treatment.
Despite the above benefits, there are specific challenges to consistent DMT use. While the need for DMT may not seem clear in times of disease inactivity or mild course, as disease progression is unnoticeable to PwRRMS,Citation6 the costs of medication taking is prevalent: PwRRMS report on many adverse side effectsCitation6,Citation7 which are a main component of satisfaction with medication.Citation8
The therapeutic and economic benefits, however, are contingent upon adherence to DMT dose and its administration schedule. Though adherence may be relatively high among PwMS, as compared to other illness,Citation9 a recent meta-analysis on oral DMT adherence found that before one year of treatment, 20% of PwRRMS were not taking it as prescribed,Citation10 known as non-adherence,Citation11 and one in four PwRRMS discontinued treatment, known as non-persistence.Citation12 The rates of adherence to injectable DMT range 25–50% within 1–5 years.Citation4 Reasons for non-adherence vary,Citation13 spanning from social-economic, health care system, condition-related, therapy-related to patient-related. Among PwMS, most of the studies on adherence focused on social-economic or therapy-related factor (eg, administration modality), and studies on reasons uncovered both intentional and non-intentional ones. In our longitudinal observational study of PwRRMS we found that the most consistent reason across times was intentional, specifically beliefs about medications.Citation14
A meta-analysis on 771 intervention trials to increase medication adherence reported a moderate effect size,Citation15,Citation16 if any; the meta-analysis’ authors recommended using face-to-face interventions and focus on behavioral strategies and less on knowledge and beliefs.Citation15 Medication adherence interventions among PwMS are few;Citation17–22 the reported interventions are tailored, using either digital tools or motivational interviewing (MI), the latter delivered either via telephone or face-to-face. The studies on adherence among PwMS identified the following determinants of non-adherence: Medication beliefs, adverse events, treatment satisfaction, affective states, and memory. However, most of the studies focused on persistence or re-initiation of DMT,Citation20,Citation23,Citation24 and not on adherence; the studies on adherenceCitation19,Citation21 did not find the interventions efficacious or were very small.Citation22 Specifically, an intervention using MI showed no significant differences between the visitsCitation19, possibly due to a ceiling effect, and neither did an intervention using smartphone reminders;Citation21 another intervention employing randomized trial included only 19 PwMS in both arms.Citation22 The present study is, therefore, an additional attempt to examine acceptability – willingness of PwRRMS to enroll in an adherence-promoting intervention, retention in the intervention, and initial efficacy of a medication adherence intervention focusing on identified non-adherent individuals. The intervention was designed to be tailored to the specific reasons of non-adherence, aiming at empowering the PwRRMS. MI, previously shown to increase patients’ adherence to treatment,Citation22,Citation25 was utilized. The main research question was whether the developed intervention see full details in (26) increased adherence, and if so, whether changes also took place in related variables: medication habit and beliefs about medications.
Materials and Methods
Participants
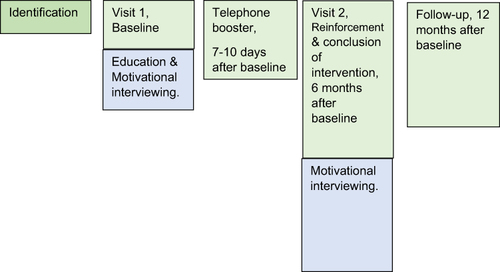
This study is part of a larger observational study on medication adherenceCitation14 where PwRRMS (n = 230) were assessed every six months for two years. Participants in the current study were screened for eligibility and enrolled if eligible and in agreement. Eligibility criteria included being prescribed oral/injectable DMT (Fingolimod, Dimethyl Fumarate, Interferon beta-1a and Glatiramer Acetate, Teriflunomide) for >6 months, and identification as non-adherent by self-reports or medication claims records (being in low or medium-low categories of ProMas (see below) or <80% medication claims per regimen). Exclusion criteria were as follows: (1) discontinuation of the medication on which non-adherence was identified, (2) being non-Hebrew speaking, as the intervention was only available in Hebrew. The decision to participate in the intervention was determined jointly by the PwRRMS and the attending physician. Ninety-one PwRRSM were identified as eligible, 60 agreed to enroll (Visit 1), 52 completed the intervention (Visit 2) and 46 also completed the 12-months follow-up. The study was carried out at Carmel Medical Center’s specialized MS clinic in Haifa, Israel, serving about 3000 PwMS who reside mostly in northern Israel.
Design
The pilot study utilized quasi-experimental pretest posttest design and a follow-up assessment; this within-subject design was employed assuming a moderate degree of recruitment consent and retention, which could limit the power detecting a difference in efficacy. Anticipating that we may end up with 40 participants at follow-up, a power of 0.79 was estimated to be sufficient to identify a difference of 0.45 standard deviation (effect size) at a 0.05 significance level.
Procedure
The intervention consisted of two main components: psycho-educational, carried out by the treating neurologist, and behavioral, utilizing Motivational Interviewing (MI) and carried out by a health psychologist.
The education component took place on Visit 1. It included: a brief explanation on MS, a review of the participant’s medical status, a review of scientific evidence supporting the benefits of the specific medication taken by the participant (delayed progression of disability, reduced proliferation of lesions, sustained cognitive ability), a discussion of potential side effects with a focus on those experienced by the participant and strategies for reducing them. The behavioral component followed immediately, lasting for a median of 30 minutes. Questionnaires were administered prior to the behavioral part. The MI component on Visit 1 started with a discussion on experiences of coping with the challenges of MS, as well as goals and hopes for life with MS. Pros and cons for DMT use/non-use/partial use were then identified, and cases of recent missed doses and medication adherence were reviewed. Then the psychologist and participant reviewed potential strategies for creating sustained medication adherence (eg, participants identified personal high-risk situations of a missed dose; a behavioral analysis of a typical missed dose; discussing ways of preventing circumstances leading to a missed dose).
A pre-scheduled telephone conversation between the health psychologist and the participant followed 7–10 days later. Missed doses that occurred (if any) were analyzed, the importance of adherence and participant’s self-efficacy were reinforced, and the main behavioral change techniques (BCTs) agreed upon in the previous session (Visit 1) were repeated, eg, goals setting, implementation intentions, planning coping, self-monitoring, and problem-solving. It lasted for a median of 11 minutes.
Visit 2 included, again, medical and behavioral parts. A routine appointment was conducted in the medical part. In the behavioral session, personalized MI was employed again, reviewing what happened in the past six months, reviewing barriers, successful and unsuccessful BCTs, reinforcing self-efficacy, discussing mid-range future (how one sees oneself in five years’ time), and concluding the intervention. This behavioral component lasted for a median of 20 minutes. At 12 months after baseline, a follow-up included a routine medical appointment and survey administration. describes the intervention. Full details on the intervention development can be found in a previous report.Citation26
Assessments
The primary endpoint of the study was a change in a patient reported outcomes (PRO) score of ProMAS,Citation27 assessed at baseline (Visit 1), six and 12 months after baseline. The ProMAS is an 18-item questionnaire assessing adherence behaviors, and respondents were asked about the last month (eg, “I have never changed my medicine use myself”, “When I am away from home, I occasionally do not take my medicines”). Following reversal of pre-determined items, responses to the 18 items were averaged, with higher scores representing better adherence rates. Adherence categories are low (sum score 0–4), medium-low (sum score 5–9), medium-high (sum score 10–14) and high (sum score 15–18). Internal reliabilities of the ProMAS were α= 0.83, 0.82 and 0.83 at Visit 1, Visit 2, and follow-up, respectively.
PwRRMS were also assessed with the Expanded Disability Status Scale (EDSS)Citation28 and a battery of PRO, described in detail elsewhere,Citation14 including medication habits, beliefs about medication, illness perceptions, affective states, and quality of life.Citation29 All PROs were administered electronically during clinic visits. In addition, the health psychologist administering the MI component recorded her perception of the importance the participant attached to medication adherence and perceived efficacy (importance and efficacy “rulers” within MI), each ranging on a scale from 1 to 10.
Demographic details (age, sex, education, social-economic status, and self-rated health) were retrieved from the observational study.Citation14
Ethics
All applicable patient privacy requirements and the ethical principles outlined in the Declaration of Helsinki 2008 were followed. Written informed consent was obtained, confirming that PwMS was free to leave the study at any time. The study was reviewed and approved by the Carmel Medical Center Institutional Review Board (IRB), Israel and was registered (#NCT02488343; registration (ClinicalTrials.gov) IDCMC-14-0061-CTIL).
Statistical Analysis
First, descriptive analyses for demographic and clinical characteristics were reported for all participants. For categorical variables, counts and percentages are provided, whereas means and standard deviations (SDs) are presented for continuous variables. In order to examine acceptability (ie enrollment) and retention (completion of intervention), differences in demographic, clinical and PRO variables were examined between those who consented to enroll and those who declined, as well as between completers of the intervention and those who dropped out. Categorical variables were analyzed using a chi-square test, and continuous variables were analyzed using either t-test or Mann–Whitney U-test or Kruskal–Wallis non-parametric tests (depending on the normality of distribution, tested using the Kolmogorov–Smirnov test). Second, efficacy of the intervention was evaluated by comparing self-reported adherence at Visit 1 vs Visit 2, and at Visit 1 vs follow-up, using Wilcoxon Signed-Rank non-parametric test; a parametric test could not be used due to a non-normal distribution of the adherence score. Additionally, changes across time in other PRO which could mediate the change in adherence (eg, habits, medication beliefs) were also examined. Statistical significance was set for p < 0.05. All statistical analyses were performed using SPSS version 26.0.
Results
Description of Participants
The study consisted of 60 PwRRMS who met inclusion criteria and consented to participate. Their characteristics at baseline are depicted in . PwRRMS were predominantly women, assessed their health as below average (Mean = 1.95 on a 5-point scale) and their physical disability (as measured by EDSS) was relatively low-to-moderate (Mean = 2.73, SD = 2.01, Median = 2.50, IQR = 1.00–4.00). Respondents have had MS for a mean duration of 8.84 years.
Table 1 Baseline Demographic and Clinical Characteristics (n = 60)
Acceptability and Retention - Recruitment and Retention in the Intervention
Acceptability was examined by the consent to enroll in the intervention study. PwRRMS were followed in a prospective observational study.Citation14,Citation30 Out of 229 participants who completed a third observational assessment (18 months into the observational study), 91 were identified as low in adherence (see details inCitation30) and approached by the treating neurologist for enrollment in an intervention. Out of the 91 identified, 62 consented to enroll and 60 of them completed the first session (2/3). The consenting group (n = 60) was compared to the non-consenting group (n = 31) on demographic and clinical characteristics: sex, age, education, social-economic status (SES), disability status, and MS duration. No significant differences were found on any of the variables (p’s > 0.05). It should be noted that several PwRRMS questioned their being identified as non-adherent.
Retention. We examined whether there were differences in the above demographic and clinical characteristics by the number of sessions a participant attended (Visit 1, phone, Visit 2, follow-up: four vs three or less sessions). No significant differences were found (p’s > 0.05).
We also examined whether the PRO at Visit 1 (medication habits, medication beliefs, illness perceptions, affective states, and quality of life) were different by retention in the intervention. We found no significant differences in the medication habits and medication beliefs (P’s > 0.05), and we found significant differences in affective states, quality of life (specifically, health and psychological) and illness perception: those who participated in all assessment points reported on significantly more depression and anxiety, lower health and psychological quality of life, and had worse illness perceptions (Z=−2.28, p= 0.022; Z =−2.66, p= 0.005; Z =−2.25, p= 0.023; Z =−2.95, p= 0.001; Z =−2.84, p= 0.003, respectively, for depression, anxiety, health and psychological quality of life, and illness perceptions).
Primary Outcomes: Self-Reported Medication Adherence
Paired comparison was carried out on self-reported medication adherence (ProMas) at Visit 1 and Visit 2 (6 months later) using Wilcoxon Signed-Rank non-parametric test (the distribution was not normal and could not afford a parametric test). Data at all time points were available for 39 PwMS. The difference between ProMas at Visit 2 to ProMas at Visit 1 was significant, Z = 2.17, p = 0.030, so that the adherence was significantly higher at Visit 2 with an effect size d = 0.26. displays descriptive statistics of medication adherence at Visit 1, Visit 2 and follow-up. Another paired comparison using Wilcoxon Signed-Rank non-parametric test was conducted between self-reported adherence at Visit 1 and follow-up (12 months later). The difference was non-significant, Z = 1.62, p = 0.106 with an effect size d = 0.21.
Table 2 Adherence ProMas Score by Time (Visit 1, Visit 2 and Follow-Up)
Auxiliary Outcomes: PRO Measures of Medication’s Habit and Beliefs and Rating of Importance and Efficacy
Paired comparison on PRO of medication habits and beliefs about medication at different times were also conducted. The PROs were postulated as potential mediators of the change in adherence. No significant differences were found between these PRO at Visit 1 and Visit 2 (P’s > 0.05).
Paired comparisons were also carried out on the importance attached to medication adherence and adherence efficacy. These were recorded at Visit 1, phone session, and Visit 2. There was a significant difference in importance between Visit 1 (Mean = 7.80, SD = 2.26) to importance recorded at the phone conversation (Mean = 8.90, SD = 1.67), Z = 2.93, p = 0.003, so that recorded importance was significantly higher at the phone conversation. However, the difference between Visit 1 and Visit 2 (Mean = 8.38, SD = 2.12) was non-significant, Z = 1.17, p = 0.241. Importance recorded at phone conversation (Mean = 8.93, SD = 1.66) was significantly higher than the importance at Visit 2, Z = 2.15, p = 0.032. No significant difference in adherence efficacy was found between the sessions (P’s > 0.05).
Discussion
The findings demonstrated the feasibility of a collaborative multi-professional team (a neurologist and a psychologist) and the challenges faced in promoting long-term medication adherence. Three-fourth of PwRRMS (46/60) who enrolled completed the intervention and a follow-up. Self-reported medication adherence significantly increased six months following baseline but at 12 months receded. Those who participated in more sessions were more depressed, anxious, and had negative perceptions regarding their health and their illness. No significant differences across time were observed in medication habits and persistence.
The study faced similar issues of retention of participants as previous medication adherence interventions among PwMS.Citation18,Citation19 The intervention’s results are more promising than the null effects in previous medication adherence intervention studiesCitation19,Citation21 and the effect size is congruent with the results of the meta-analysis on medication adherence interventionsCitation15 and other psycho-social interventions among PwMS,Citation31 exhibiting mostly a small effect size 0.20–0.30.
The study has several important implications. First, the study demonstrated the retention and initial efficacy of a personalized intervention: self-reported adherence increased. The clinical implications of increased adherence are clear: fewer relapses, fewer new lesions, and less accumulated disability. Second, the importance of follow-up was demonstrated, uncovering a disappointing “triangle effect”, namely an increase in adherence followed by a fade-out of the intervention and a return to baseline’s level of adherence. Third, the increased retention in the intervention of PwRRMS who were more anxious, more depressed and had lower quality of life in the psychological and health domains, possibly exhibited the need for psycho-social support among the participants: those who were worse-off psychologically apparently derived benefits from the additional resources offered, even though the intervention did not address psychological well-being but rather focused on medication adherence. A qualitative component, not employed in the study, could have uncovered their motivation. Fourth, the psycho-educational materials concerning MS and DMT and the MI format focused on challenges and coping could be implemented among PwMS not only in the context of non-adherence but also as the need for psycho-social support is evident.Citation32
The study is hampered by several limitations. First, the study’s sample size was small. This allowed for a personalized intervention (eg, using the MRI of the PwMS as a demonstration), yet upscaling and integrating the intervention into routine clinical care would be challenging and may require structuring the intervention, hence making it less personalized. For example, the educational materials can be produced as a booklet or a video (rather than a conversation with a neurologist), but thus be less tailored to the individual. Still, engagement could be attained by using questionnaires, games and other activities. The small N also did not allow subgroup analyses for example, by age, gender, or disability status. Second, Health Maintenance Organization’s regulations did not allow for online communication outside the clinic’s premises (eg, weekly/daily reporting of an adherence diary, using a commercial app along with the psychologist). Such communication constraints have eased since the COVID-19 pandemic, which ushered in many secured digital health services. Future re-design of the intervention could include ecological momentary assessment (EMA) that will allow capturing times of need and a prompt, just-in-time response to a decrease in medication adherence (see response to micro-temporal events in smoking.Citation33 The online continuous communication would also address the time constraints of PwRRMS. These EMA could be integrated into patient support programs. A third limitation is that about a third of the PwMS identified as non-adherent declined to participate. Qualitative work is needed in order to understand their reasons and design a suitable product for their needs. A fourth limitation is that the design of this feasibility study did not allow for examining the mechanism of change, eg, which component of the intervention worked best (eg, education, MI), or what mediated the change. Still, the study did examine whether habits and medication beliefs changed across the different times; as there were no significant differences across the times, they were not tested as potential mediators of the change in adherence. The fifth limitation is the quasi-experimental pretest-posttest design of the study, lacking a control group, and the reliance on self-reported data. Alongside, the method of recruiting non-adherent PwMS, focused only on identified non-adherent people, rather than utilizing random sampling or stratification by adherence levels, thus possibly introducing a selection bias. Lastly, the current intervention addressed mostly patient (eg, beliefs, motivation) and therapy-related (eg, side effects, scheduling) factors associated with adherence and did not address such factors as healthcare system and condition-related factors.Citation13
The study also had several strengths. First, it tailored the intervention to the specific participant, employing in-person sessions with two health professionals and demonstrating a commitment to extensive patient-provider communication. Second, the researchers utilized an intervention mapping approach. This meant that there was a stage of needs assessment, comprising a longitudinal observational study among PwRRMS served by the clinic, behavioral determinants were identified and targeted, objectives were set, PwRRMS were consulted, and theory and evidence-based intervention methods (befitting the identified determinants) were selected. Third, the relatively long follow-up uncovered a fade-off, indicating that a six months’ interval is too long, and telephone or online support is necessary in order to support a change.
Conclusions
A multi-professional psycho-education and MI intervention, anchored in patient-centered approach,Citation34 is a feasible way forward in promoting DMT adherence among PwRRMS. Outcome (self-reported adherence) at six months indicates that the intervention is feasible and efficacious in enhancing medication adherence, yet outcome at a year’s follow-up underscored its fade-out. The latter, in conjunction with variability within personCitation35 and the dynamic nature of medication taking, indicate that the present intervention worked for a short term only, and further study is needed on how to maintain the intervention’s effect (eg, adding contact points with the PwMS), while balancing resources and addressing the aforementioned study’s limitations. Considering the time constraints of many of the prospective clients, a blended approach - in-person encounters augmented with non-synchronous IT-mediated resources - is recommended.
Ethical Approval
The study was reviewed and approved by the Carmel Medical Center Institutional Review Board (IRB), Israel and was registered (#NCT02488343; registration (ClinicalTrials.gov) IDCMC-14-0061-CTIL). Written informed consent was obtained.
Consent for Publication
Respondents were notified of the research aims of the study and expressed their consent in the above written form.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Dr Miller has served on the scientific advisory board, and received personal compensation for consulting and/or speaking activities and/or honoraria and/or received grant support for research from: Avanir Pharmaceuticals; Bayer-Schering Pharma; Biogen Idec; Mapi Pharma; Medison Pharma Ltd.; MerckSerono; Novartis, Sanofi; Teva Pharmaceutical Industries Ltd. The authors report no other conflicts of interest in this work.
Acknowledgments
The authors thank all the clinic staff.
Data Sharing Statement
The datasets analyzed during the current study are available upon request from the corresponding author.
Additional information
Funding
References
- Reich D, Lucchinetti C, Calabresi PA, Longo DL. Multiple Sclerosis. N Engl J Med. 2018;378:169–180. doi:10.1056/NEJMra1401483
- Yermakov S, Davis M, Calnan M, et al. Impact of increasing adherence to disease-modifying therapies on healthcare resource utilization and direct medical and indirect work loss costs for patients with multiple sclerosis. J Med Econ. 2015;18(9):711–720. doi:10.3111/13696998.2015.1044276
- Hao J, Pitcavage J, Jones JB, Hoegerl C, Graham J. Measuring adherence and outcomes in the treatment of patients with multiple sclerosis. J Am Osteopath Assoc. 2017;117(12):737. doi:10.7556/jaoa.2017.145
- Burks J, Marshall TS, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. Clin Outcomes Res. 2017;9:251–260. doi:10.7224/1537-2073.2022-018
- Lizàn L, Comellas M, Paz S, Poveda JL, Meletiche D, Polanco C. Treatment adherence and other patient-reported outcomes as cost determinants in multiple sclerosis: a review of the literature. Patient Prefer Adherence. 2014;8:1653–1664. doi:10.1586/ern.11.161
- Schoor R, Bruce A, Bruce J, et al. Reasons for nonadherence and response to treatment in an adherence intervention trial for relapsing–remitting multiple sclerosis patients. J Clin Psychol. 2018;75(3):380–391. doi:10.2147/PPA.S67253
- Bruce J, Lynch SG. Multiple sclerosis: MS treatment adherence-how to keep patients on medication? Nat Rev Neurol. 2011;7(8):421–422. doi:10.1038/nrneurol.2011.106
- Haase R, Kullmann JS, Ziemssen T. Therapy satisfaction and adherence in patients with relapsing-remitting multiple sclerosis: the THEPA-MS survey. Ther Adv Neurol Disord. 2016;9(4):250–263. doi:10.1177/1756285616634247
- Evans C, Marrie RA, Yao S, et al. Medication adherence in multiple sclerosis as a potential model for other chronic diseases: a population-based cohort study. BMJ Open. 2021;11(2):1–13. doi:10.1136/bmjopen-2020-043930
- Nicholas JA, Edwards NC, Edwards RA, Dellarole A, Grosso M, Phillips AL. Real-world adherence to, and persistence with, once- And twice-daily oral disease-modifying drugs in patients with multiple sclerosis: a systematic review and meta-analysis. BMC Neurol. 2020;20(1):1–15. doi:10.1186/s12883-020-01830-0
- World Health Organization (WHO). Adherence to Long-Term Therapies. Evidence for Action; 2003.
- Marangi A, Farina G, Vicenzi V, et al. Changing therapeutic strategies and persistence to disease-modifying treatments in a population of multiple sclerosis patients from Veneto region, Italy. Mult Scler Relat Disord. 2020;41:102004. doi:10.1016/j.msard.2020.102004
- World Health Organisation. Adherence to Long-Term Therapies: Evidence for Action.; 2003. doi:10.1016/j.neuron.2012.03.011
- Neter E, Glass-marmor L, Wolkowitz A, Lavi I, Miller A. Beliefs about medication as predictors of medication adherence in a prospective cohort study among persons with multiple sclerosis. BMC Neurol. 2021;21:1–9. doi:10.1186/s12883-020-02014-6
- Conn VS, Ruppar TM. Medication adherence outcomes of 771 intervention trials: systematic review and meta-analysis. Prev Med. 2017;99:269–276. doi:10.1016/j.ypmed.2017.03.008
- Conn VS, Enriquez M, Ruppar TM, Chan KC. Meta-analyses of theory use in medication adherence intervention research. Am J Heal Behav. 2016;40(2):155–171. doi:10.5993/AJHB.40.2.1
- Bruce J, Bruce A, Lynch S, et al. A pilot study to improve adherence among MS patients who discontinue treatment against medical advice. J Behav Med. 2016;39(2):276–287. doi:10.1007/s10865-015-9694-6
- Berger BA, Liang H, Hudmon KS. Evaluation of software-based telephone counseling to enhance medication persistency among patients with multiple sclerosis. J Am Pharm Assoc. 2005;45(4):466–472. doi:10.1331/1544345054475469
- Schreiber K, Kant M, Pfleger C, et al. High treatment adherence, satisfaction, motivation, and health-related quality of life with fingolimod in patients with relapsing-remitting multiple sclerosis – results from a 24-month, multicenter, open-label Danish study. Patient Prefer Adherence. 2018;12:1139–1150. doi:10.2147/PPA.S166278
- Roche J, McCarry Y, Mellors K. Enhanced patient support services improve patient persistence with multiple sclerosis treatment. Patient Prefer Adherence. 2014;8:805–811. doi:10.2147/PPA.S59496
- Golan D, Sagiv S, Glass-Marmor L, Miller A. Mobile phone-based e-diary for assessment and enhancement of medications adherence among patients with multiple sclerosis. Mult Scler J Exp Transl Clin. 2020;6(3):205521732093930. doi:10.1177/2055217320939309
- Turner AP, Sloan AP, Kivlahan DR, Haselkorn JK. Telephone counseling and home telehealth monitoring to improve medication adherence: results of a pilot trial among individuals with multiple sclerosis. Rehabil Psychol. 2014;59(2):136–146. doi:10.1037/a0036322
- Moeckli J, Stewart KR, Ono S, et al. Mixed-Methods study of uptake of the extension for community health outcomes (ECHO) Telemedicine Model for Rural Veterans With HIV. J Rural Health. 2016. doi:10.1111/jrh.12200
- Berger-Achituv S, Shohat T, Romano-Zelekha O, et al. Widespread use of soy-based formula without clinical indications. J Pediatr Gastroenterol Nutr. 2005;41(5):660–666. doi:10.1097/01.mpg.0000181855.77488.bf
- Palacio A, Garay D, Langer B, Taylor J, Wood BA, Tamariz L. Motivational interviewing improves medication adherence: a systematic review and meta-analysis. J Gen Intern Med. 2016;31(8):929–940. doi:10.1007/s11606-016-3685-3
- Neter E, Miller A. Using an intervention mapping approach to improve adherence to Disease-Modifying Treatment in Multiple Sclerosis. Int J Mult Scler Care. 2023;25:206–213. doi:10.7224/1537-2073.2022-018
- Kleppe M, Lacroix J, Ham J, Midden C. The development of the ProMAS: A probabilistic medication adherence scale. Patient Prefer Adherence. 2015;9:355–367. doi:10.2147/Ppa.S76749
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444–1452. doi:10.1212/WNL.33.11.1444
- Whoqol Group T, WHOQOL Group. the World Health Organization Quality of Life Assessment (Whoqol): development and General Psychometric Properties. Soc Sci Med. 1998;46(12):1569–1585. doi:10.1016/S0277-9536(98)00009-4
- Neter E, Wolkowitz A, Glass-Marmor L, et al. Multiple modality approach to assess adherence to medications across time in Multiple Sclerosis. Mult Scler Relat Disord. 2020;40:101951. doi:10.1016/j.msard.2020.101951
- Sesel AL, Sharpe L, Naismith SL. Efficacy of psychosocial interventions for people with Multiple Sclerosis: a meta-analysis of specific treatment effects. Psychother Psych. 2018;87(2):105–111. doi:10.1159/000486806
- Kidd T, Carey N, Mold F, et al. A systematic review of the effectiveness of self-management interventions in people with multiple sclerosis at improving depression, anxiety and quality of life. PLoS One. 2017;12(10):1–16. doi:10.1371/journal.pone.0185931
- Huh J, Cerrada CJ, Dzubur E, Dunton GF, Spruijt-Metz D, Leventhal AM. Effect of a mobile just-in-time implementation intention intervention on momentary smoking lapses in smoking cessation attempts among Asian American young adults. Transl Behav Med. 2021;11(1):216–225. doi:10.1093/tbm/ibz183
- Lejbkowicz I, Caspi O, Miller A. Participatory medicine and patient empowerment towards personalized healthcare in multiple sclerosis. Expert Rev Neurother. 2012;12(3):343–352. doi:10.5993/AJHB.40.2.1
- Inauen J, Bierbauer W, Lüscher J, et al. Assessing adherence to multiple medications and in daily life among patients with multimorbidity. Psychol Health. 2017:1–16. doi:10.1080/08870446.2016.1275632