Abstract
Osteoporosis is an age-related systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility. Bone remodeling involves two types of cells: osteoblasts and osteoclasts. Receptor activator of nuclear factor-κB ligand (RANKL) is a key regulator of the formation and function of bone-resorbing osteoclasts, and its cell surface receptor, receptor activator of nuclear factor-κB (RANK), is expressed by both osteoclast precursors and mature osteoclasts. Denosumab is a fully human monoclonal anti-RANKL antibody that inhibits the binding of RANKL to RANK, thereby decreasing osteoclastogenesis and bone-resorbing activity of mature osteoclasts. Although there are many medications available for the treatment of osteoporosis, inhibition of RANKL by denosumab has been shown to significantly affect bone metabolism. Denosumab appears to be a promising, highly effective, and safe parenteral therapy with good adherence for osteoporosis. Moreover, denosumab may be cost-effective therapy compared with existing alternatives. Therefore, in this review, we focus on studies of denosumab and the risks and benefits identified for this type of treatment for osteoporosis.
Keywords:
Introduction
Remodeling of bone, which begins in the early fetal stages, is a process that is maintained in the adult skeleton. It mediates the repair of microdamage while also regulating the mechanical strength and structure of bone. The bone remodeling cycle involves a series of highly regulated steps that depend on interactions between two cell lineages: the mesenchymal bone-forming osteoblastic lineage and the hematopoietic bone-resorbing osteoclastic lineage.Citation1 The latter are differentiated from monocyte– macrophage lineage precursor cells in response to cytokines and chemokines produced by cells lining the bone surface, and these cells initiate bone remodeling.Citation2,Citation3 Subsequent interactions between osteoclast precursors and osteoblastic cells leads to the differentiation, migration, and fusion of large multinucleated osteoclasts.Citation4 These mature osteoclasts then attach to a mineralized bone surface and initiate resorption by secreting hydrogen ions and lysosomal enzymes. In particular, cathepsin K is secreted, and this enzyme is able to degrade the bone matrix, including collagen, at low pH. Osteoclastic bone resorption produces irregular scalloped cavities on the trabecular bone surface, called Howship’s lacunae, or cylindrical haversian canals in cortical bone. Following this resorptive phase, the bone surface is repopulated by osteoblasts, which deposit bone matrix and eventually undergo mineralization to form a new bone surface. Generally, the same amount of bone that is removed is replaced. However, when an imbalance between these two processes leads to an increase in bone resorption, the result is focal articular bone loss and generalized osteoporosis.
Various diseases, drugs, and metabolic abnormalities adversely affect bone health and contribute to the development of osteoporosis. Activation of osteoclastic bone resorption is a common factor in the pathogenesis of bone loss and fractures,Citation5 while estrogen deficiency during menopause or androgen deficiency in males can also lead to an unbalanced increase in bone resorption versus bone formation. As a result, bone loss can occur rather rapidly, accompanied by the destruction of bone microarchitecture.Citation6 In older adults who commonly experience vitamin D deficiency,Citation7 calcium absorption is impaired and secondary hyperparathyroidism can develop. Consequently, bone loss occurs and the risk of fracture increases.Citation8 Painful vertebral fractures are the most common complication of osteoporosis and account for ~50% of reported fractures. In addition, height loss, kyphosis, back pain, and impaired physical and psychological function can occur following such fractures. The presence of a spine fracture is also the strongest risk factor for experiencing another fracture of either hip or spine,Citation9 with the former representing the most challenging type of fracture for patient recovery. Considering that the cost of care for patients with fractures is expensive, the incidence of fractures increases progressively with advancing age, and the global population is growing older; it has been estimated that the number of fractures worldwide will double or triple by the year 2050.Citation10
For patients at risk for osteoporosis, or those having already experienced a fracture, prevention of new or additional fractures is key. Antiresorptive (anticatabolic) drugs that are currently available include estrogen, raloxifene, and bisphosphonates. These have been shown to effectively prevent bone loss in postmenopausal women without osteoporosis.Citation11–Citation13 For postmenopausal women and men with osteoporosis, treatment with either an antiresorptive drug or teriparatide, an anabolic agent, has been shown to preserve or improve bone mass and substantially reduce the risk of fracture.Citation14 Unfortunately, however, these treatments can only be safely administered for a limited period of time. For example, anabolic agents, such as teriparatide, can only be administered for a maximum of 2 years. Moreover, for bisphosphonates, prolonged administration increases the potential for rare, yet serious, adverse events such as osteonecrosis of the jaw (ONJ), atypical fractures, and esophageal cancer.Citation15 The treatment efficacy of these drugs in clinical practice has also been limited by real or perceived intolerance, as well as poor adherence to therapy.Citation16,Citation17 Phase I trials of anti-sclerostin antibody, which up-regulates the interaction between Wnt ligand and LRP5/6 coreceptor on osteoblasts, showed increase in bone formation in healthy men and postmenopausal women, and Phase II trials are underway.Citation18,Citation19 Inhibition of sclerostin is an interesting prospect for the next generation of osteoporosis drugs. In this review, we will focus on a fully human monoclonal anti-receptor activator of nuclear factor-κB ligand (RANKL) antibody, denosumab (), and its potential as a long-term treatment for osteoporosis with appropriate administration.
Figure 1 The mechanism of action of denosumab on bone metabolism.
Abbreviations: c-Fms, colony stimulating factor-1 receptor; M-CSF, macrophage colony-stimulating factor; RANK, receptor activator of nuclear factor-κB; RANKL, receptor activator of nuclear factor-κB ligand.
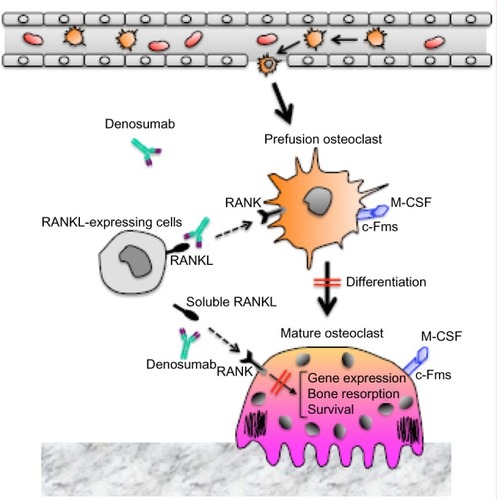
Identification of the osteoclast differentiation factor, RANKL
Bone-resorbing osteoclasts originate from hematopoietic cells, which are hypothesized to be members of the colony forming unit-megakaryocyte-derived monocyte–macrophage family. Takahashi et al and Udagawa et al developed a mouse coculture system of hematopoietic cells and primary osteoblasts to investigate osteoclast formation in vitro.Citation20–Citation22 In this coculture system, several systemic and local factors were found to induce the formation of tartrate resistant acid phosphatase-positive multinucleated cells,Citation23 and these cells exhibited a number of osteoclast characteristics. In addition, cell-to-cell contact between osteoblastic cells and osteoclast progenitors was shown to be essential for the induction of osteoclastogenesis. Based on these findings, Suda et al proposed that osteoblastic cells induce the membrane-associated osteoclast differentiation factor in response to various osteotropic factors.Citation23 In 1997, it was first reported that RANKL and its receptor, receptor activator of nuclear factor-κB (RANK), regulate interactions between dendritic cells and T-cells.Citation24 Furthermore, when osteoclast differentiation factor was cloned from a complimentary DNA library of mouse stromal ST2 cells treated with bone-resorbing factors,Citation25 it was found to be identical to RANKL, to tumor necrosis factor (TNF)-related activation-induced cytokine, and to the osteoprotegerin (OPG) ligand. These results were independently validated by other research groups.Citation26–Citation28 In these studies, RANKL was also shown to induce osteoclast differentiation from mouse hematopoietic cells and human peripheral blood mononuclear cells in the presence of macrophage colony-stimulating factor.Citation25,Citation28 To date, RANK is the only signaling receptor that has been identified for RANKL for the induction of osteoclastogenesis and the activation of mature osteoclasts.Citation4 OPG, which lacks transmembrane and cytoplasmic domains and is released in a soluble form by a variety of cells including osteoblasts, also serves as a decoy receptor for RANKL. As such, OPG competes with RANK to inhibit the differentiation and activity of osteoclasts.Citation4
A crucial role for RANKL in bone metabolism
RANKL is a membrane-anchored molecule that is released from the cell surface following proteolytic cleavage by matrix metalloproteinases such as matrix metalloproteinases 14.Citation29,Citation30 Both the soluble and membrane-bound forms of RANKL function as agonistic ligands for RANK, with the membrane-bound form functioning more efficiently.Citation29,Citation31,Citation32 Using a knockout mouse model of OPG, a natural inhibitor of RANKL, a link between RANKL and the development of osteoporosis was demonstrated.Citation33 Furthermore, overexpression of OPG in mice results in lower numbers of osteoclasts and greater bone mass.Citation34 Correspondingly, for patients experiencing estrogen deficiency, hyperparathyroidism, or other disorders that stimulate bone resorption, perturbations in the ratio of OPG to RANKL have been detected.Citation35–Citation37 More recently, Nakashima et al reported that purified osteocytes express higher levels of RANKL and undergo enhanced osteoclastogenesis in vitro, while osteocyte-specific RANKL knockout mice exhibit a severe osteopetrotic phenotype.Citation38 Taken together, these results indicate that osteocytes represent a major source of RANKL for bone remodeling in vivo. Mutations in RANKL, RANK, and OPG genes have also been identified in patients with bone disorders such as autosomal recessive osteopetrosis, familial expansile osteolysis, and juvenile Paget’s disease, respectively.Citation39 Moreover, when RANKL expression is upregulated in response to factors such as vitamin D3, prostaglandin E2, parathyroid hormone, interleukin (IL)-1, IL-6, IL-11, IL-17, and TNF-α, pathological osteoclastogenesis has been observed.Citation34,Citation40,Citation41 Therefore, regulation of the RANKL/RANK/OPG axis represents a potential therapeutic target for the treatment of osteoporosis, rheumatoid arthritis, and cancer bone metastasis.
Immunological function of RANKL
Prior to their identification in bone cells, RANKL and RANK were found to effect T-cell activation and dendritic cell survival.Citation24,Citation27,Citation42 Moreover, during early development, RANKL signaling regulates the microenvironment of the thymus, thereby facilitating the deletion of self-reactive T-cells to provide self-tolerance and prevent autoimmunity.Citation43 These results imply that inhibition of RANKL by denosumab may alter immune function, or increase susceptibility to infections. In studies of mice deficient in RANKL or RANK, an absence of lymph nodes and significantly smaller Payer’s patches were observed.Citation28,Citation43 These findings demonstrate the critical role that RANK activation has in the early stages of lymphoid tissue inducer cell development in peripheral lymphoid organs. A pathological model of inflammatory bowel disease has also demonstrated a role for RANKL in the stimulation of dendritic cells,Citation44,Citation45 suggesting that RANKL may mediate the activation of dendritic cells under certain autoimmune conditions. On the other hand, inhibition of RANKL by OPG has not been found to alter cellular or humoral immunity, nor does it render mice susceptible to bacterial challenge.Citation46 Thus, although dendritic cells and T lymphocytes express RANK and RANKL, it would appear that they play a minor or redundant role in the mammalian immune response. However, these results do not guarantee the safety of denosumab treatments.
In vitro studies of denosumab
Direct binding assays have demonstrated that denosumab is able to bind human RANKL, yet does not bind murine RANKL, human TNF-related apoptosis-inducing ligand, and other human TNF family members.Citation47 Denosumab also does not suppress bone resorption in normal mice or rats, although it prevented a resorptive response in mice challenged with human RANKL (huRANKL). huRANKL knock-in mice have been generated, and these mice exclusively express chimeric (human/murine) RANKLCitation47 and are responsive to denosumab. In studies of young huRANKL mice treated with denosumab, trabecular osteoclast surfaces were reduced by 95% and bone density and volume increased.Citation47 In contrast, adult huRANKL mice treated with denosumab exhibited reduced bone resorption, increased cortical and cancellous bone mass, and improved trabecular microarchitecture.Citation47 The same group also reported that subcutaneous administration of denosumab (25 or 50 mg/kg/month) for up to 16 months prevented the loss of cancellous bone and preserved indices of bone strength for adult ovariectomized cynomolgus monkeys.Citation48
Clinical development of denosumab as a prophylactic and/or therapeutic agent for osteoporosis
To evaluate whether inhibition of RANKL has clinical utility, 52 healthy postmenopausal women were given single doses of an osteoprotegerin-immunoglobulin Fc segment complex (Fc:OPG) (0.1, 0.3, 1.0, or 3.0 mg/kg) in a Phase I randomized placebo-controlled study.Citation49 Urinary levels of the cross-linked N-telopeptide of type I collagen (NTX), a specific marker of bone resorption, and bone-specific alkaline phosphatase (BSAP), an index of bone formation, were subsequently monitored for 84 days. Within 12 hours of receiving Fc:OPG, a dose-dependent decrease in NTX/creatinine ratios was observed. Furthermore, this ratio decreased by 70% to 80% within 5 days for the highest doses of Fc:OPG. After several weeks, levels of NTX/creatinine returned to baseline. A significant decrease in levels of BSAP were also observed for the 1.0 mg/kg and 3.0 mg/kg doses. For the latter group, inhibition of BSAP occurred more slowly, with levels 30% below baseline observed after 60 days. There were also no serious adverse events reported in this study. In one patient, a transient neutralizing antibody to OPG was detected, although this did not have any obvious clinical effect. These data provide evidence that inhibition of RANKL by its natural inhibitor, OPG, can result in clinically measurable effects. However, the development of OPG as a therapy for osteoporosis was not further pursued due to its potential immunogenicity, and because immunologic resistance to OPG could have negative effects on the skeleton.Citation50
Since denosumab specifically binds RANKL,Citation47 it is less likely to affect the immune system or other regulatory systems. Moreover, denosumab does not have the potential for autoimmunization against a vital regulatory protein and is characterized by a longer half-life, which permits less frequent dosing.Citation51 Each of these attributes makes denosumab a more attractive therapeutic agent than forms of OPG. To evaluate the safety, pharmacokinetics (PK), and possible bone resorption effects of denosumab, a Phase I study was conducted. Subcutaneous administration of various concentrations of denosumab (0.01 mg/kg to 3.0 mg/kg) were administered to 49 healthy postmenopausal women.Citation51 The PK of denosumab were found to be nonlinear with dose. A prolonged absorption phase also occurred, with maximum serum concentrations reached between 5 days and 21 days after the women received the initial dose. Conversely, the disappearance of denosumab from the serum occurred in two phases: a slow phase and a fast phase. The initial slow phase was associated with half-lives of approximately 20 days for the lower doses of denosumab, and approximately 32 days for the higher doses. When circulating levels of denosumab were ∼1,000 ng/mL, clearance occurred more rapidly. Urinary NTX levels were also found to decrease within 12 hours of dosing. Overall, the magnitude of the initial response was similar among the doses, although the duration of the effect was dose-dependent. These results are consistent with the pharmacokinetic data. By the end of the 9-month follow-up period, NTX levels had returned to baseline for all of the doses. Alternatively, serum levels of BSAP remained stable for the first two weeks following dosing, then decreased in a dose-dependent manner. Taken together, these results suggest that the effect of denosumab on bone formation is indirect.
Optimizing the dose of denosumab for osteoporosis
To evaluate the safety, tolerability, PK, and pharmacodynamics (PD) of denosumab, a randomized double-blind dose-escalation study was conducted. For a group of healthy postmenopausal Japanese women, denosumab was administered subcutaneously at doses of 0.03, 0.1, 0.3, 1.0, or 3.0 mg/kg, and was compared with a placebo.Citation52 Suppression of bone turnover markers (BTM) was rapidly detected (within 2 days of dosing) and the duration of suppression was dose-dependent. Moreover, there was no marked differences in the PK and PD profiles between JapaneseCitation52 and non-Japanese subjects,Citation51 and denosumab was well tolerated. In another study, the efficacy and safety of three doses of denosumab (14, 60, and 100 mg) were compared with a placebo over 12 months for a group of postmenopausal Japanese women with osteoporosis. The results associated with the 60 mg dose of denosumab were consistent with the results of a similar Phase II study of osteoporosis in a Caucasian population that was conducted in the United States.Citation53–Citation56
Reduced fracture risk with denosumab
A total of 7,868 women between the ages of 60 and 90 years who had a bone mineral density (BMD) T-score <−2.5 and >−4.0 at the lumbar spine or total hip received either 60 mg denosumab or a placebo subcutaneously every 6 months for 36 months.Citation57 In this study, it was observed that denosumab reduced the risk of new radiographic vertebral fractures by 68% (P<0.001), with the risk of hip fractures and nonvertebral fractures decreasing by 40% and 20%, respectively. Moreover, this effect did not significantly differ for any of the nine subgroups analyzed according to patient age, body mass index, femoral neck BMD T-score, prevalent vertebral fracture, prior nonvertebral fracture, estimated creatinine clearance, geographic region, ethnicity, and prior use of osteoporosis medications.Citation58
Long-term denosumab treatment
To evaluate denosumab efficacy and safety for up to 10 years of treatment, participants who completed the FREEDOM (Fracture REduction Evaluation of Denosumab in Osteoporosis every 6 Months) trialCitation57 were eligible to receive an additional 2 years of denosumab treatment (the long-term group). For comparison, patients from the FREEDOM placebo group could receive 2 years of denosumab treatment (the cross-over group). A total of 4,550 women elected to participate in the extended trial, with 2,343 women in the long-term group and 2,207 women in the cross-over group.Citation59 In the former group, BMD for the lumbar spine and the total hip further increased, resulting in 5-year gains of 13.7% and 7.0%, respectively. BMD for the lumbar spine and the total hip also increased for the latter group, with values of 7.7% and 4.0%, respectively, over the 2-year denosumab treatment period. Regarding adverse events, the number did not increase for the long-term group. However, for the cross-over group, two adverse events consistent with ONJ were reported. In one woman, healing occurred within the 6-month dosing interval, and she continued to receive denosumab (two further doses) without any additional oral events. For the other woman, healing occurred after the 6-month dosing interval, and denosumab was subsequently discontinued.
In a Phase II study, denosumab treatment was administered for up to 8 years to postmenopausal women with low bone mass.Citation60 For the subjects who received continuous administration of denosumab over that period, BMD for the lumbar spine (n=88) and for the total hip (n=87) increased by 16.5% and 6.8%, respectively, compared with the baseline of the parent study, and increased by 5.7% and 1.8%, respectively, compared with the baseline of the extension study. At the end of year 8, serum levels of C-terminal telopeptide of type I collagen (CTX) and BSAP remained below the parent study baseline, and median reductions of 65% and 44%, respectively, were observed. Overall, the results of this Phase II study and its extension demonstrate that denosumab therapy mediated a progressive and substantial increase in BMD over 8 years for postmenopausal women with low bone mass. In addition, treatment was well tolerated and the adverse event profile was similar to what has been reported previously.
Effects of discontinuing denosumab on BMD and levels of BTM
For 256 postmenopausal women, 60 mg denosumab or a placebo was administered every 6 months for 2 years, followed by 2 years of discontinued treatment.Citation61 After this 4 year period, the group that initially received denosumab was found to maintain a higher BMD than the placebo group (P≤0.05). Furthermore, levels of BTM were found to increase above baseline within 3 months (for the serum C-terminal telopeptide of type 1 collagen) or 6 months (for the N-terminal propeptide of type 1 procollagen) of the initial 2 year treatment period. By the end of the 4 year period, the levels of the BTM had returned to baseline. Adverse event rates during the nontreatment phase were found to be similar between the two groups. For the 60 mg denosumab dose that was administered for 24 months, levels of BMD and BTM were found to be reversible upon discontinuation, thereby reflecting the biological mechanism of action for denosumab. However, residual BMD measurements did remain greater than those of the placebo group.Citation61
Effects of denosumab on bone histomorphometry
Iliac crest bone biopsies were collected 24 and/or 36 months from the first diagnosis of osteoporosis for 45 postmenopausal women who received a placebo and 47 postmenopausal women who received denosumab in the FREEDOM study.Citation57 Biopsies were also collected from postmenopausal women who had been treated for 12 months with alendronate in the STAND (Study of Transitioning from AleNdronate to Denosumab) study.Citation62 Of this latter group, 21 continued to receive alendronate while 15 received denosumab upon entry into the FREEDOM trial. Indices of bone turnover tended to be lower for the women who received denosumab compared to alendronate alone. Moreover, the women who received denosumab maintained normal bone microarchitecture and there were no adverse effects associated with the mineralization or formation of lamellar bone. In future studies, a longer follow-up period will be necessary to determine the duration during which such low turnover is safe.
A cohort study was also conducted to evaluate the effects of discontinuing denosumab at the tissue level.Citation63 The mean period of discontinued osteoporosis treatment was 25.1 months (range, 21–29 months). Bone histomorphometry studies showed normal histology and bone remodeling similar to that observed for untreated postmenopausal women with osteoporosis. Furthermore, all of the biopsy specimens from women who had discontinued treatment showed evidence of tetracycline labels. Assays of biochemical markers also found levels to be comparable with pretreatment levels. Taken together, these data confirm that the effects of denosumab on bone turnover at the tissue level are reversible.
Renal function does not significantly affect the PK or PD of denosumab
Chronic kidney disease (CKD) has been identified as a potential independent risk factor for bone loss.Citation64–Citation66 Correspondingly, CKD is also more common among older adults. To evaluate whether treatment with denosumab affects renal function, subjects were enrolled in one of five renal function groups based on glomerular filtration rates (GFRs) as follows: normal renal function (GFR >80 mL/minute/1.73 m2) (n=12); mild CKD (GFR 50–80 mL/minute/1.73 m2) (n=13); moderate CKD (GFR 30–49 mL/minute/1.73 m2) (n=13); severe CKD (GFR <30 mL/minute/1.73 m2) (n = 9); or kidney failure requiring hemodialysis (n=8).Citation67 Data collected for these groups indicated that renal function did not have a significant effect on the PK or PD of denosumab, and dose adjustments were not needed for these patients. However, the potential for developing hypocalcemia was found to be higher for subjects with severe CKD and kidney failure compared with subjects with mild or moderate CKD or subjects with normal renal function. Two subjects who experienced kidney failure (one symptomatic and one asymptomatic) were hospitalized for intravenous calcium gluconate treatment. Thus, it is recommended that patients with impaired renal function who receive denosumab, particularly those with severe kidney disease (GFR <30 mL/minute/1.73 m2), should receive calcium and vitamin D supplements and should be monitored for secondary hyperparathyroidism.
Safety
Although denosumab has been shown to be safe in the collective data from Phase II and III clinical trials,Citation68 the clinical concern was the potential risk for infections or neoplasms due to the ubiquitous presence of RANKL throughout many tissues. In a meta-analysis of randomized placebo-controlled trials involving denosumab, including the large FREEDOM registration trial,Citation57 a borderline increased risk of serious infection was observed (risk ratio =1.25, 95% confidence interval: 1.00–1.54) for women with postmenopausal osteoporosis when intention-to-treat analysis was used.Citation69 However, a nonsignificant risk ratio of 2.1 was observed when a per-protocol analysis was employed.Citation70 Thus, the incidence of infection and neoplasms in ongoing larger Phase III trials will be of interest.
While accumulating evidence indicates that denosumab is a safe treatment, there remains the potential for side effects from this treatment. For bisphosphonate therapy, ONJ has recently emerged as an adverse side effect,Citation71,Citation72 although the nature and cause of ONJ remains controversial. Given the capacity for denosumab to strongly inhibit osteoclastic bone resorption similar to bisphosphonates, it will be important for future studies of denosumab to monitor the incidence and clinicopathologic characteristics of ONJ. Another potential side effect to consider is the so-called frozen bone process, whereby complete inhibition of remodeling leads to an accumulation of microfractures and an increased risk for atypical femoral fractures. This complication was considered in an analysis of postmenopausal women receiving bisphosphonate therapy based on the findings of animal studies.Citation73 In trials that have continuously administered denosumab for up to 5 years, there have been no reports of atypical femoral fractures.Citation57,Citation59 However, two cases of atypical femoral fracture have been confirmed in patients receiving denosumab 60 mg for 2.5 years or more participating in the ongoing open-label extension study of the pivotal Phase III fracture trial in postmenopausal osteoporosis (FREEDOM).Citation74 It is possible that differences in shorter half-life of denosumab compared with bisphosphonates (5 years or longer) can account for less incidence of atypical femoral fractures. Tsai et al also recently reported that a combination treatment of teriparatide and denosumab increased BMD to a greater extent than either agent alone.Citation75 Teriparatide is an effective anabolic (bone growing) agent that might help prevent frozen bone caused by denosumab-induced oversuppression of bone turnover. Furthermore, although denosumab has been found to be completely cleared from the body following its discontinuation, the frozen bone process may still be an issue for long-term denosumab treatments.
Since RANK and RANKL are also expressed by endothelial cells and lymphocytes,Citation76 additional studies are needed to evaluate the potential effects of denosumab therapy on the cardiovascular and immune systems of the body. Continued documentation and quantification of the efficacy of denosumab for large numbers of patients will also be important. Recently, RANKL signaling was implicated in the pathogenesis of hepatic insulin resistance and type 2 diabetes mellitus.Citation77 This may provide a link between inflammation and disrupted glucose homeostasis, and may also contribute to pharmacological strategies being developed for the treatment of RANKL-related diseases.
Finally, Freemantle et al reported that postmenopausal women with osteoporosis were more adherent, compliant, and persistent with subcutaneous injections of denosumab every 6 months than with once-weekly alendronate tablets in a 2-year randomized crossover study.Citation78 In addition, the women expressed greater satisfaction with injectable denosumab and preferred it over oral alendronate. Thus, preferences in the administration of denosumab may influence patient persistence and adherence to therapy, and this represents an important consideration for the treatment of chronic conditions that require long-term therapy.
Conclusion
The inhibition of RANKL by denosumab has been shown to significantly affect bone metabolism. Correspondingly, this highly specific antibody for RANKL appears to be a promising treatment for osteoporosis and other bone diseases characterized by increased bone turnover. Freemantle et al showed that denosumab was more effective at reducing the occurrence of vertebral fractures than raloxifene, risedronate, and alendronate.Citation79 The cost-effectiveness of denosumab in postmenopausal osteoporotic women has been evaluated by estimating expected cost and quality-adjusted life-years. Analyses have shown that denosumab represented good value-for-money in postmenopausal women with low bone mass compared with no treatment Citation80 or treatment with oral bisphosphonates,Citation81–Citation83 and, therefore, has the potential to be a first-line treatment for postmenopausal osteoporotic women. In addition, the cost-effectiveness of denosumab is favorable, particularly for patients at high risk of fracture and low expected adher ence to oral treatments.Citation84 The long-term efficacy and toxicity of denosumab remains to be confirmed with studies that include longer follow-up periods. This is particularly relevant since postmenopausal women are increasingly experiencing a longer life expectancy, and, thus, the potential for anti-osteoporosis therapy to span multiple decades is a growing consideration.
Disclosure
The authors report no conflicts of interest in this work.
References
- EriksenEFNormal and pathological remodeling of human trabecular bone: three dimensional reconstruction of the remodeling sequence in normals and in metabolic bone diseaseEndocr Rev1986743794083536460
- ParfittAMOsteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human boneJ Cell Biochem19945532732867962158
- KarsentyGWagnerEFReaching a genetic and molecular understanding of skeletal developmentDev Cell20022438940611970890
- SudaTTakahashiNUdagawaNJimiEGillespieMTMartinTJModulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand familiesEndocr Rev199920334535710368775
- RobbinsJASchottAMGarneroPDelmasPDHansDMeunierPJRisk factors for hip fracture in women with high BMD: EPIDOS studyOsteoporos Int200516214915415185066
- DufresneTEChmielewskiPAManhartMDJohnsonTDBorahBRisedronate preserves bone architecture in early postmenopausal women in 1 year as measured by three-dimensional microcomputed tomographyCalcif Tissue Int200373542343212964065
- GlothFM3rdGundbergCMHollisBWHaddadJGJrTobinJDVitamin D deficiency in homebound elderly personsJAMA199527421168316867474272
- LipsPVitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implicationsEndocr Rev200122447750111493580
- JohnellOKanisJAOdénAFracture risk following an osteoporotic fractureOsteoporos Int200415317517914691617
- GullbergBJohnellOKanisJAWorld-wide projections for hip fractureOsteoporos Int1997754074139425497
- No authors listedEffects of hormone therapy on bone mineral density: results from the postmenopausal estrogen/progestin interventions (PEPI) trial. The Writing Group for the PEPIJAMA199627617138913968892713
- DelmasPDBjarnasonNHMitlakBHEffects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal womenN Engl J Med199733723164116479385122
- McClungMRWasnichRDHoskingDJPrevention of postmenopausal bone loss: six-year results from the Early Postmenopausal Intervention Cohort StudyJ Clin Endocrinol Metab200489104879488515472179
- DelmasPDTreatment of postmenopausal osteoporosisLancet200235993222018202612076571
- WattsNBDiabDLLong-term use of bisphosphonates in osteoporosisJ Clin Endocrinol Metab20109541555156520173017
- ReckerRRGallagherRMacCosbePEEffect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of womenMayo Clin Proc200580785686116007889
- CaroJJIshakKJHuybrechtsKFRaggioGNaujoksCThe impact of compliance with osteoporosis therapy on fracture rates in actual practiceOsteoporos Int200415121003100815167989
- PadhiDJangGStouchBFangLPosvarESingle-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibodyJ Bone Miner Res2011261192620593411
- CostaAGBilezikianJPSclerostin: therapeutic horizons based upon its actionsCurr Osteoporos Rep2012101647222234741
- TakahashiNYamanaHYoshikiSOsteoclast-like cell formation and its regulation by osteotropic hormones in mouse bone marrow culturesEndocrinology19881224137313823345718
- UdagawaNTakahashiNAkatsuTThe bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cellsEndocrinology19891254180518132676473
- UdagawaNTakahashiNAkatsuTOrigin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cellsProc Natl Acad Sci U S A19908718726072642169622
- SudaTTakahashiNMartinTJModulation of osteoclast differentiationEndocr Rev199213166801555533
- AndersonDMMaraskovskyEBillingsleyWLA homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell functionNature199739066561751799367155
- YasudaHShimaNNakagawaNOsteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKLProc Natl Acad Sci U S A1998957359736029520411
- LaceyDLTimmsETanHLOsteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activationCell19989321651769568710
- WongBRRhoJArronJTRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cellsJ Biol Chem19972724025190251949312132
- KongYYYoshidaHSarosiIOPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesisNature199939767173153239950424
- NakashimaTKobayashiYYamasakiSProtein expression and functional difference of membrane-bound and soluble receptor activator of NF-kappaB ligand: modulation of the expression by osteotropic factors and cytokinesBiochem Biophys Res Commun2000275376877510973797
- SchlöndorffJLumLBlobelCPBiochemical and pharmacological criteria define two shedding activities for TRANCE/OPGL that are distinct from the tumor necrosis factor alpha convertaseJ Biol Chem200127618146651467411278735
- MiyamotoTAraiFOhnedaOTakagiKAndersonDMSudaTAn adherent condition is required for formation of multinuclear osteoclasts in the presence of macrophage colony-stimulating factor and receptor activator of nuclear factor kappa B ligandBlood200096134335434311110710
- HikitaAYanaIWakeyamaHNegative regulation of osteoclastogenesis by ectodomain shedding of receptor activator of NF-kappaB ligandJ Biol Chem200628148368463685517018528
- BucayNSarosiIDunstanCRosteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcificationGenes Dev1998129126012689573043
- SimonetWSLaceyDLDunstanCROsteoprotegerin: a novel secreted protein involved in the regulation of bone densityCell19978923093199108485
- HofbauerLCKhoslaSDunstanCRLaceyDLSpelsbergTCRiggsBLEstrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cellsEndocrinology199914094367437010465311
- LocklinRMKhoslaSTurnerRTRiggsBLMediators of the bipha-sic responses of bone to intermittent and continuously administered parathyroid hormoneJ Cell Biochem200389118019012682918
- HofbauerLCGoriFRiggsBLStimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosisEndocrinology1999140104382438910499489
- NakashimaTHayashiMFukunagaTEvidence for osteocyte regulation of bone homeostasis through RANKL expressionNat Med201117101231123421909105
- DanksLTakayanagiHImmunology and boneJ Biochem20131541293923750028
- TakayanagiHOsteoimmunology: shared mechanisms and crosstalk between the immune and bone systemsNat Rev Immunol20077429230417380158
- KongYYFeigeUSarosiIActivated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligandNature1999402675930430910580503
- BachmannMFWongBRJosienRSteinmanRMOxeniusAChoiYTRANCE, a tumor necrosis factor family member critical for CD40 ligand-independent T helper cell activationJ Exp Med199918971025103110190893
- DesantiGECowanJEBaikSDevelopmentally regulated availability of RANKL and CD40 ligand reveals distinct mechanisms of fetal and adult cross-talk in the thymus medullaJ Immunol2012189125519552623152561
- MoschenARKaserAEnrichBThe RANKL/OPG system is activated in inflammatory bowel disease and relates to the state of bone lossGut200554447948715753532
- AshcroftAJCruickshankSMCroucherPIColonic dendritic cells, intestinal inflammation, and T cell-mediated bone destruction are modulated by recombinant osteoprotegerinImmunity200319684986114670302
- StolinaMGuoJFaggioniRBrownHSenaldiGRegulatory effects of osteoprotegerin on cellular and humoral immune responsesClin Immunol2003109334735414697750
- KostenuikPJNguyenHQMcCabeJDenosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKLJ Bone Miner Res200924218219519016581
- KostenuikPJSmithSYJoletteJSchroederJPyrahIOminskyMSDecreased bone remodeling and porosity are associated with improved bone strength in ovariectomized cynomolgus monkeys treated with denosumab, a fully human RANKL antibodyBone201149215116121457806
- BekkerPJHollowayDNakanishiAArrighiMLeesePTDunstanCRThe effect of a single dose of osteoprotegerin in postmenopausal womenJ Bone Miner Res200116234836011204435
- McClungMRole of RANKL inhibition in osteoporosisArthritis Res Ther20079Suppl 1S3
- BekkerPJHollowayDLRasmussenASA single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal womenJ Bone Miner Res20041971059106615176987
- KumagaiYHasunumaTPadhiDA randomized, double-blind, placebo-controlled, single-dose study to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of denosumab administered subcutaneously to postmenopausal Japanese womenBone20114951101110721871589
- NakamuraTMatsumotoTSugimotoTShirakiMDose-response study of denosumab on bone mineral density and bone turnover markers in Japanese postmenopausal women with osteoporosisOsteoporos Int20122331131114021927920
- McClungMRLewieckiEMCohenSBDenosumab in postmenopausal women with low bone mineral densityN Engl J Med2006354882183116495394
- LewieckiEMMillerPDMcClungMRTwo-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMDJ Bone Miner Res200722121832184117708711
- MillerPDBologneseMALewieckiEMEffect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trialBone200843222222918539106
- CummingsSRSan MartinJMcClungMRDenosumab for prevention of fractures in postmenopausal women with osteoporosisN Engl J Med2009361875676519671655
- McClungMRBoonenSTorringOEffect of denosumab treatment on the risk of fractures in subgroups of women with postmenopausal osteoporosisJ Bone Miner Res201227121121821976367
- PapapoulosSChapurlatRLibanatiCFive years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extensionJ Bone Miner Res201227369470122113951
- McClungMRLewieckiEMGellerMLEffect of denosumab on bone mineral density and biochemical markers of bone turnover: 8-year results of a phase 2 clinical trialOsteoporos Int201324122723522776860
- BoneHGBologneseMAYuenCKEffects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone massJ Clin Endocrinol Metab201196497298021289258
- ReidIRMillerPDBrownJPEffects of denosumab on bone histomorphometry: the FREEDOM and STAND studiesJ Bone Miner Res201025102256226520533525
- BrownJPDempsterDWDingBBone remodeling in postmenopausal women who discontinued denosumab treatment: off-treatment biopsy studyJ Bone Miner Res201126112737274421735475
- JassalSKvon MuhlenDBarrett-ConnorEMeasures of renal function, BMD, bone loss, and osteoporotic fracture in older adults: the Rancho Bernardo studyJ Bone Miner Res200722220321017059370
- IshaniAPaudelMTaylorBCRenal function and rate of hip bone loss in older men: the Osteoporotic Fractures in Men StudyOsteoporos Int200819111549155618392664
- JamalSASwanVJBrownJPKidney function and rate of bone loss at the hip and spine: the Canadian Multicentre Osteoporosis StudyAm J Kidney Dis201055229129920042259
- BlockGABoneHGFangLLeeEPadhiDA single-dose study of denosumab in patients with various degrees of renal impairmentJ Bone Miner Res20122771471147922461041
- MillerPDA review of the efficacy and safety of denosumab in postmenopausal women with osteoporosisTher Adv Musculoskelet Dis20113627128222870485
- ToulisKAAnastasilakisADIncreased risk of serious infections in women with osteopenia or osteoporosis treated with denosumabOsteoporos Int201021111963196420012939
- von KeyserlingkCHopkinsRAnastasilakisAClinical efficacy and safety of denosumab in postmenopausal women with low bone mineral density and osteoporosis: a meta-analysisSemin Arthritis Rheum201141217818621616520
- WooSBHellsteinJWKalmarJRNarrative [corrected] review: bisphosphonates and osteonecrosis of the jawsAnn Intern Med20061441075376116702591
- CapsoniFLonghiMWeinsteinRBisphosphonate-associated osteonecrosis of the jaw: the rheumatologist’s roleArthritis Res Ther20068521917049069
- AllenMRIwataKPhippsRBurrDBAlterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronateBone200639487287916765660
- MHRADenosumab 60 mg (Prolia▾): rare cases of atypical femoral fracture with long-term use. [webpage on the Internet]LondonMHRA2013 Available from: http://www.mhra.gov.uk/Safetyinformation/DrugSafetyUpdate/CON239411Accessed March 31, 2014
- TsaiJNUihleinAVLeeHTeriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trialLancet20133829886505623683600
- Collin-OsdobyPRotheLAndersonFNelsonMMaloneyWOsdobyPReceptor activator of NF-kappa B and osteoprotegerin expression by human microvascular endothelial cells, regulation by inflammatory cytokines, and role in human osteoclastogenesisJ Biol Chem200127623206592067211274143
- KiechlSWittmannJGiaccariABlockade of receptor activator of nuclear factor-kappaB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitusNat Med201319335836323396210
- FreemantleNSatram-HoangSTangETFinal results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal womenOsteoporos Int201223131732621927922
- FreemantleNCooperCDiez-PerezAResults of indirect and mixed treatment comparison of fracture efficacy for osteoporosis treatments: a meta-analysisOsteoporos Int201324120921722832638
- HiligsmannMReginsterJYPotential cost-effectiveness of denosumab for the treatment of postmenopausal osteoporotic womenBone2010471344020303422
- HiligsmannMReginsterJYCost effectiveness of denosumab compared with oral bisphosphonates in the treatment of post-menopausal osteoporotic women in BelgiumPharmacoeconomics2011291089591121692551
- ParthanAKruseMYurginNHuangJViswanathanHNTaylorDCost effectiveness of denosumab versus oral bisphosphonates for postmenopausal osteoporosis in the USAppl Health Econ Health Policy201311548549723868102
- ChauDBeckerDLCoombesMEIoannidisGAdachiJDGoereeRCost-effectiveness of denosumab in the treatment of postmenopausal osteoporosis in CanadaJ Med Econ201215Suppl 131423035625
- JönssonBStrömOEismanJACost-effectiveness of Denosumab for the treatment of postmenopausal osteoporosisOsteoporos Int201122396798220936401
