Abstract
Background
Little is known about weekly variability in medication nonadherence both between and within persons.
Purpose
To characterize medication nonadherence across repeated, closely spaced occasions.
Methods
This prospective cohort study comprised four unannounced telephone assessment occasions, each separated by approximately 2 weeks. On each occasion, adult outpatients taking at least a single antihypertensive medication completed a measure of extent of, and reasons for, nonadherence.
Results
Two hundred and sixty-one participants completed 871 (83%) of 1,044 occasions. Nonadherence was reported on 152 (17.5%) of 871 occasions by 93 (36%) of 261 participants. The most commonly endorsed reasons for nonadherence were forgetting (39.5%), being busy (23.7%), and traveling (19.7%). Among 219 participants completing at least three occasions, 50% of the variability in extent of nonadherence was a result of within-person fluctuations, and 50% was a result of between-person differences.
Conclusion
Interventions to reduce nonadherence should be informed by variability in the extent of nonadherence and specific reasons for nonadherence.
Introduction
Nonadherence to antihypertensive medications is common, occurs for a number of different reasons, and results in suboptimal blood pressure control, cardiovascular events, mortality, and increased health care costs.Citation1 Several interventions have been developed to reduce antihypertensive medication nonadherence. These interventions consisted of simplified dosing, education, motivational techniques, and skills training.Citation2–Citation4 Although some of these interventions improved adherence relative to usual care, effect sizes were small, leading to calls for more effective interventions.Citation1
Interventions could be more effective if they considered both between- and within-patient variability in nonadherence. Such knowledge can be obtained by repeatedly assessing individuals over the course of relatively closely spaced occasions. When such data are collected, they are often analyzed using a mean summary score (eg, electronic drug monitoring data are averaged across days), which masks any variability in medication nonadherence.Citation5 Nonadherence could fluctuate between individuals, as well as within individuals across measurement occasions as various contextual or personal challenges become more or less salient. Understanding variability in medication nonadherence between and within patients would allow better matching of interventions to patient circumstances, thereby improving outcomes.
Intraindividual (ie, within-person) variability is defined as “relatively short-term changes that are construed as more or less reversible”.Citation6 These short-term changes occur over minutes, hours, days, or weeks, depending on the construct of interest, study design, and measurement. In a study of medication nonadherence among older adults with arthritis, 68% of the variability in monthly pill count data was a result of within-person fluctuations across occasions.Citation7 We sought to extend such prior work using a different measure of nonadherence (self-report) in the context of a different disease (hypertension) and during a different time period (weekly). To our knowledge, this is the first attempt to specifically characterize intraindividual variability in weekly self-reported antihypertensive medication nonadherence.
In addition to characterizing between- and within-person variability in nonadherence, we examined reasons for nonadherence. Such information can inform the design and content of interventions to improve antihypertensive medication adherence. For instance, a different intervention approach would be warranted if participants tended to miss medications repeatedly for the same reason than if they missed medications for different reasons across time. Many previously tested interventions to improve antihypertensive medication adherence took a one-size-fits-all approach, such as blister packs,Citation8,Citation9 reminders,Citation10 copayment reduction,Citation11 education and/or psychological intervention,Citation2,Citation12 self-monitoring,Citation13 or regimen simplification.Citation14 Even multifactorial interventions comprised a limited menu of intervention strategies that were provided to all participants.Citation15 If a few reasons for nonadherence (eg, regimen complexity or cost) were dominant across nonadherence occasions for all participants, then this approach would be sensible. In our previous cross-sectional study on antihypertensive nonadherence, however, reasons for nonadherence varied across individuals, with no single reason being endorsed by more than 27% of participants.Citation16 Here we extend our previous research by examining the prevalence of reasons for nonadherence across repeated occasions.
Methods
Design overview, setting, and participants
This 8 week prospective cohort study conducted in 2011 involved four telephone assessments, each separated by approximately 2 weeks. Participants were recruited from the Durham Veterans Affairs Medical Center in North Carolina, where institutional review board approval was obtained. Inclusion criteria determined by an electronic medical record data pull were age older than 40 years and documented diagnosis of hypertension. Inclusion criteria determined during the screening telephone call included prescription of at least a single antihypertensive medication, receiving the current antihypertensive regimen for at least 3 months before enrollment, and receiving antihypertensive medications from the Durham Veterans Affairs Medical Center. Exclusion criteria assessed during the screening telephone call included cognitive impairment based on a six-item screen,Citation17 unable to communicate in English or by telephone, resident in nursing home or receiving home health care, and health problem that would make it difficult to participate (as defined by patients).
Patients identified as eligible in the electronic data pull received by mail a recruitment letter that described the study and included a toll-free number to opt out. If patients did not opt out within 2 weeks of mailing recruitment letters, then a research assistant telephoned the patients to describe the study and further assess eligibility. Eligible patients were then consented verbally. Consented participants were told that they would receive the first assessment telephone call within 30 days, followed by three additional assessment telephone calls, each separated by approximately 2 weeks. The target assessment frequency was every 14 days; a window of 11–17 days was used to accommodate participant scheduling. Telephone calls were made unannounced to reduce expectancy effects (participants adhering better to their medication regimen because they knew they would be called). Participants received $10 for each completed assessment.
Outcomes
During call 1, we collected demographic data (age, sex, race/ethnicity, and education) and clinical data (number of years since diagnosis, drug names, and dosing instructions). During calls 1–4, we assessed the extent of nonadherence and reasons for nonadherence, using a self-report measure.Citation16 The three extent-of-nonadherence items assess how often participants missed doses of their antihypertensive medications during the last 7 days. In a previous study, this measure had a single-factor structure in confirmatory factor analysis and was reliable (α=0.84). Furthermore, the measure demonstrated discriminant validity via small correlations with social desirability,Citation18 beliefs about medications,Citation19 and conscientiousness and convergent validity via strong correlations with self-efficacy to take medications,Citation20 habit strength, and the 8-item Morisky measure.Citation21 Furthermore, our measure was significantly associated with blood pressure, whereas the Morisky measure was not. Although we originally validated this measure with an agreement response scale (strongly disagree, disagree, neutral, agree, and strongly agree), we used a frequency response scale (never, rarely, sometimes, often, and always) in the present study because frequency may correspond more closely to actual behavior.Citation22 The instructions and item stems did not change. The 21-item reasons measure assessed 21 distinct contributing factors for participants missing any of their antihypertensive medication during the last 7 days on a 5-point scale anchored by “not at all” and “very much”. As this portion of the measure includes causal indicators, internal consistency reliability and factor analysis are inappropriate, and the primary concern is content validity, which can be established via literature review or qualitative methods.Citation16,Citation23
Statistical analyses
All analyses were performed with SPSS (version 20; IBM Corporation, Armonk, NY, USA) or SAS (PROC NLMIXED, version 9.2; SAS Institute Inc., Cary, NC, USA). We first provide baseline descriptive information for the full analytic sample. For each occasion for each participant, a summary nonadherence score was created by averaging responses across the three extent items. Internal consistency reliability (Cronbach’s α) was calculated for the extent of nonadherence scale at each occasion. Then, because the majority of responses were “never”, responses at each occasion were dichotomized as nonadherent (any response other than never for at least one extent of nonadherence item) or adherent (response of never for all three items) for each occasion. We also calculated the percentage of nonadherence occasions that each reason was endorsed (where a response other than “not at all” was provided) and provide descriptive information on the total number of reasons endorsed for each occasion for each participant.
To characterize intraindividual variability in nonadherence, the remaining analyses were limited to participants completing 3 or 4 assessments, as such variability cannot be estimated with fewer observations.Citation24 To decompose the variance in nonadherence into between- and within-person components, we estimated a “null” (fully unconditional) logistic multilevel model (generalized linear mixed model) that included only fixed and random (subject-specific) intercepts. This modeling approach was employed because occasions were nested within individuals and the outcome was dichotomous.Citation25–Citation28 Between-person variability is represented by the random effect variance component, denoted σ2bw. This component captures differences between persons in the probability of nonadherence, with higher values implying greater heterogeneity between persons.
For logistic models, no within-person variance component could be estimated from the data. Rather, the within-person variance is assumed constant and equal to the variance of an underlying standard logistic distribution (ie, π2/3≈3.29). Thus, the proportion of the total variance resulting from between-person differences is Propbw = σ2bw/(σ2bw+3.29), and the proportion resulting from within-person variation across occasions is 1-Propbw. Propbw is also known as the intraclass correlation coefficient (ICC) and ranges from 0 to 1. An ICC of 0 implies that all persons have the same probability of nonadherence, and therefore, variability is entirely a result of random fluctuations in the binomial response, which occur with a fixed probability for all persons and occasions. As the ICC increases, more of the total variability is explained by between-person differences in the likelihood of nonadherence. At the upper limit, ICC =1, implying that all variability is a result of between-person differences and there is perfect correlation (no variability) in nonadherence across occasions within persons. Finally, an ICC ≈0.50 implies that the variability in the data is explained equivalently by between-person differences in the propensity to not adhere and by within-person fluctuations in nonadherence across occasions. We also qualitatively identified and described participant nonadherence across completed occasions (always adherent; sometimes nonadherent, defined as nonadherent on at least one occasion; and always nonadherent).
Our a priori sample size of 250 patients and four time points were considered sufficient for variance decomposition, as this is consistent with previous studies (eg, Almeida et alCitation29). The mixed model uses all available cases and yields unbiased parameter estimates under a missing-at-random assumption. Missing data were reduced, given our limitation of this analysis to individuals with 3 or 4 completed occasions.
Results
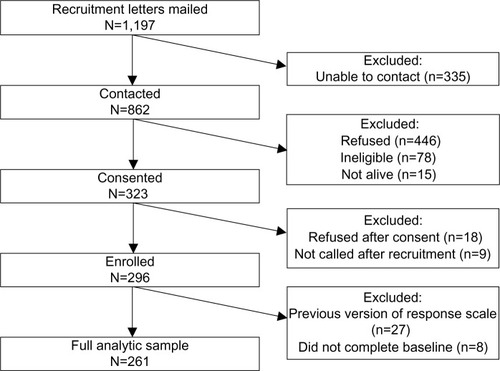
Of the 1,197 patients to whom recruitment letters were mailed, 862 were contacted. Of those, 323 provided verbal consent by telephone. Reasons for refusal included family issues, health reasons, lack of time, and lack of interest. Eighteen patients refused participation after consent, and nine were never called because of errors in the tracking database early in the study. Of the 296 participants who received at least one assessment telephone call, 27 were administered a version of the extent scale that used a different response scale; eight additional participants did not receive the first assessment telephone call because of errors in the tracking database. Data from these 35 participants were excluded from analyses, resulting in a final analytic sample of 261 participants ().
The 261 participants were 64 years old on average, and 93% were men (). The sample was 51% white and 48% black. Participants ranged in educational and financial status, with nearly 67% reporting some education beyond high school and 40% reporting that they had sufficient income to buy special things after paying bills.
Table 1 Demographic characteristics of participants
Of the 1,044 possible occasions (261 participants multiplied by four time points), 871 (83.4%) were completed; 142 participants (54.4%) had data from all four occasions, 77 (29.5%) from three occasions, 30 (11.5%) from two occasions, and 12 (4.5%) from one occasion, indicating good compliance to the study protocol. The sample size differed across occasions because of the inability to reach patients by telephone within the 11–17 day assessment window.
Characterizing occasions: extent of nonadherence and reasons for nonadherence
Across the 871 completed occasions from 261 participants, the mean of extent of nonadherence (before dichotomizing) was 1.19 (standard deviation =0.48; median =1.0), skewness was 3.44, and kurtosis was 16.84, indicating that participants reported a low degree of nonadherence. The internal consistency reliability for the three-item extent of nonadherence scale was 0.86 for occasion 1 (n=261), 0.78 for occasion 2 (n=162), 0.94 for occasion 3 (n=222), and 0.90 for occasion 4 (n=226). These values from the frequency response scale are comparable to the value of 0.84 obtained in prior work using an agreement response scale.Citation16
Nonadherence (any response other than “never” on any extent of nonadherence item) was reported on 152 (17.5%) of 871 occasions by 93 (35.6%) of 261 participants. provides descriptive statistics for each reason for nonadherence across the 152 occasions on which nonadherence was reported. Forgetting was the most commonly endorsed reason, reported on 60 (39.5%) of the 152 occasions, followed by being busy (n=36, 23.7%), traveling (n=30, 19.7%), running out of medication (n=23, 15.1%), and coming home late (n=22, 14.5%). The remaining reasons were cited on 0.7%–13.8% of nonadherence occasions. Across all nonadherence occasions, the number of reasons endorsed by a participant ranged from 0 to 21 (mean =2.09; standard deviation =2.53; median =1.0). On 31 nonadherence occasions (20.4%), no reason for nonadherence was endorsed, despite the patient reporting nonadherence on the extent scale.
Table 2 Endorsement of reasons for nonadherence on nonadherence occasions
Intraindividual variability in extent of nonadherence
We examined the extent of nonadherence among the 219 participants completing 3 or 4 measurement occasions. In this subsample, any nonadherence was reported on 136 (17.0%) of 799 completed occasions by 82 participants (37.4%). Only 7 of these participants reported nonadherence on all completed occasions (“always nonadherent”); the other 75 participants were nonadherent on some of their completed occasions (“sometimes nonadherent”). Results from the logistic multilevel model indicated that 50% of the variability in extent of nonadherence was from between-person differences (ICC =0.50; standard error =0.05), with 50% of the variability resulting from within-person fluctuations across occasions. We were unable to examine intraindividual variability in the reasons for nonadherence further, given the small number of nonadherence occasions and few participants with a sufficient number of nonadherence occasions.
Discussion
In this study of self-reported antihypertensive medication nonadherence, the likelihood of nonadherence was explained as much by within-person variability across occasions as by between-person differences. Taken together, these findings suggest that important information about self-reported nonadherence is lost if repeated assessments are not conducted or when repeated assessments are averaged to create a summary estimate. These findings also underscore the need to examine reasons for within-person variability in nonadherence across occasions.
In this study, and in our prior cross-sectional study,Citation16 no single reason was endorsed among the majority of patients. This might suggest that one-size-fits-all approaches (eg, value-based insurance design, as implemented in the Affordable Care Act to address cost, which was rarely endorsed) would have limited effectiveness. In contrast, offering a menu of intervention approaches for all possible reasons for nonadherence may not be feasible or cost-effective. Accordingly, researchers, providers, and insurers may need to prioritize which reasons for nonadherence are addressed in multifactorial interventions, offering solutions for reasons that are more commonly endorsed across repeated occasions such as forgetting, traveling, and running out of medication. Variability in reasons could be addressed if an intervention involved frequent assessments of extent of, and reasons for, nonadherence.
The current findings have implications for the design of interventions to reduce antihypertensive nonadherence. In studies evaluating such interventions, nonadherent patients are targeted so that there is room for improvement in outcomes. As seen in this study, identification of nonadherent patients is complicated by the reporting of nonadherence on some occasions and not others. Therefore, a run-in period including multiple assessments of nonadherence could be used to identify individuals who would benefit from intervention using some criterion (eg, nonadherent on at least 20% of occasions), as has been done with electronic drug monitoring.Citation30–Citation32 The eligible patients could be further classified on the basis of the extent of nonadherence into consistently nonadherent or variably nonadherent, which could suggest different intervention strategies.
Observational data on intraindividual variability in extent of nonadherence may also inform intervention dosing. With a large number of occasions, one could calculate the mean lag between nonadherence occasions within persons, as well as variability about this mean, to determine how frequently to intervene and whether intervention frequency should be tailored (in the case of great variability around the lag) or untailored (in the case of less variability around the lag).
Our results also have important clinical implications. Because patients may be adherent at one time point and not another, it is important to assess and monitor nonadherence across time to inform clinical decision making, as is done with clinical parameters such as blood pressure.Citation33 Including a valid, reliable measure of medication nonadherence in the electronic medical record, a goal of the National Cancer Institute’s Grid-Enabled Measures Database,Citation34 could facilitate repeated assessments at the point of care. Indeed, self-reported medication nonadherence could be considered an additional vital sign.
This study has some limitations. First, the small number and spacing of occasions may have led to biased estimates of intraindividual variability. In addition, nonadherence was reported infrequently, limiting assessment of variability. Second, the frequency response scale for extent of nonadherence yielded few positive values, compelling us to dichotomize the data. However, given that there were few observed values greater than 1 (“never”), collapsing the data into binary categories should not result in a major loss of information. Third, the amount of intraindividual variability observed in this sample of veterans with hypertension may not generalize to other patient populations with hypertension or to other populations taking other medications. Similarly, the prevalence of reasons for nonadherence to antihypertensive medications across occasions may not generalize to other medications. Generalizability may also be limited by the response rate if participants differ in significant ways from non-participants. Finally, there were 31 nonadherence occasions on which no reason for nonadherence was endorsed. This could suggest that the list of reasons for nonadherence is incomplete or could reflect patient misunderstanding of instructions. The potential for error may be reduced by introducing a skip pattern and by using an open-ended question to capture additional reasons not included in the list.
Strengths of this study include the dual conceptualization of extent of, and reasons for, nonadherence; the use of a reliable and valid measure of extent of nonadherence; and the use of a comprehensive measure of reasons for nonadherence. In addition, although a handful of participants did not receive a scheduled call because of database errors early in the study, compliance with the study was good, as indicated by a large proportion of participants who received 3 or 4 calls.
The findings suggest several directions for future research. For one, studies should include a larger number of assessments of extent of, and reasons for, nonadherence to determine the effects on the ICC. Second, measures of time-varying covariates, such as daily stressors or mood, should be assessed to aid identification of circumstances that are associated with greater propensity for episodic nonadherence. Such measures could explain differences in between-person variability. Third, studies should include a larger number of participants to yield a sufficient number of nonadherence occasions for examining intraindividual variability in reasons for nonadherence. Finally, the recall period and response scale might be varied to determine the effects both on the proportion of individuals identified as nonadherent and on the ICC.
In summary, our data highlight that antihypertensive medication nonadherence varies just as much within patients as between patients, underscoring the need to assess and analyze nonadherence across repeated occasions. Such data, when coupled with information about reasons for nonadherence, could improve clinical decision making, such as when and whether to adjust or switch medications. Such data could also lead to comprehensive, multifactorial interventions that match patient experiences with medication taking.
Acknowledgments
We are grateful to the many individuals who participated in data collection, including Jamiyla Bolton, Tamika Brown, Jahdai Dawes, Terry Ervin, Leslie Gaillard, Kymeiria Ingram, and Cherisa Williams. We are also grateful to Jamiyla Bolton and Jennifer Lindquist for study database development and maintenance. This study was funded by a grant from the National Institute on Aging (R21 AG035233) to CIV and WSY. HAK was supported by a postdoctoral fellowship from the Department of Veterans Affairs, Office of Academic Affiliations, Health Services Research and Development (TPP 21-020). MLM was supported by a Research Career Scientist award from the Department of Veterans Affairs (RCS 10-391). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Disclosure
The authors report no conflicts of interest in this work.
References
- HaynesRBAcklooESahotaNMcDonaldHPYaoXInterventions for enhancing medication adherenceCochrane Database Syst Rev20082CD00001118425859
- OgedegbeGOBoutin-FosterCA randomized controlled trial of positive-affect intervention and medication adherence in hypertensive African AmericansArch Intern Med2012172432232622269592
- OgedegbeGChaplinWSchoenthalerAA practice-based trial of motivational interviewing and adherence in hypertensive African AmericansAm J Hypertens200821101137114318654123
- SchroederKFaheyTEbrahimSHow can we improve adherence to blood pressure-lowering medication in ambulatory care? Systematic review of randomized controlled trialsArch Intern Med2004164772273215078641
- KnaflGJBovaCAFennieKPO’MalleyJPDieckhausKDWilliamsABAn analysis of electronically monitored adherence to antiretroviral medicationsAIDS Behav201014475576819107587
- NesselroadeJRThe warp and woof of the developmental fabricDownsRLibenLPalermoDVisions of Development, the Environment, and Aesthetics: The Legacy of Joachim F. WohlwilHillsdale, NJLawrence Erlbaum Associates1991213240
- McDonald-MiszczakLNeupertSDGutmanGDoes cognitive ability explain inaccuracy in older adults’ self-reported medication adherence?J Appl Gerontol2009285560581
- ZedlerBKJoyceAMurrelleLKakadPHarpeSEA pharmacoepidemiologic analysis of the impact of calendar packaging on adherence to self-administered medications for long-term useClin Ther201133558159721665043
- SchneiderPJMurphyJEPedersenCAImpact of medication packaging on adherence and treatment outcomes in older ambulatory patientsJ Am Pharm Assoc (2003)2008481586318192132
- ChristensenAChristrupLLFabriciusPEThe impact of an electronic monitoring and reminder device on patient compliance with antihypertensive therapy: a randomized controlled trialJ Hypertens201028119420019770778
- ChernewMEShahMRWeghAImpact of decreasing copayments on medication adherence within a disease management environmentHealth Aff (Millwood)200827110311218180484
- FriedmanRHKazisLEJetteAA telecommunications system for monitoring and counseling patients with hypertension. Impact on medication adherence and blood pressure controlAm J Hypertens199694 Pt 12852928722429
- Márquez-ContrerasEMartell-ClarosNGil-GuillénVCompliance Group of the Spanish Society of Hypertension (SEE)Efficacy of a home blood pressure monitoring programme on therapeutic compliance in hypertension: the EAPACUM-HTA studyJ Hypertens200624116917516331115
- GirvinBMcDermottBJJohnstonGDA comparison of enalapril 20 mg once daily versus 10 mg twice daily in terms of blood pressure lowering and patient complianceJ Hypertens199917111627163110608477
- Márquez ContrerasEVegazo GarcíaOMartel ClarosNEfficacy of telephone and mail intervention in patient compliance with antihypertensive drugs in hypertension. ETECUM-HTA studyBlood Press200514315115816036495
- VoilsCIMaciejewskiMLHoyleRHInitial validation of a self-report measure of the extent of and reasons for medication nonadherenceMed Care201250121013101922922431
- CallahanCMUnverzagtFWHuiSLPerkinsAJHendrieHCSix-item screener to identify cognitive impairment among potential subjects for clinical researchMed Care200240977178112218768
- PaulhusDPaulhus Deception ScalesPearson AssessmentLondon1998
- HorneRWeinmanJHankinsMThe beliefs about medicines questionnaire: The development and evaluation of a new method for assessing the cognitive representation of medicationPsychol Health1999141124
- OgedegbeGMancusoCAAllegranteJPCharlsonMEDevelopment and evaluation of a medication adherence self-efficacy scale in hypertensive African-American patientsJ Clin Epidemiol200356652052912873646
- MoriskyDEAngAKrousel-WoodMWardHJPredictive validity of a medication adherence measure in an outpatient settingJ Clin Hypertens (Greenwich)200810534835418453793
- TourangeauRRipsLJRasinskiKThe Psychology of Survey ResponseNew YorkCambridge University Press2000
- VoilsCIHoyleRHThorpeCTMaciejewskiMLYancyWSJrImproving the measurement of self-reported medication nonadherenceJ Clin Epidemiol201164325025421194887
- RamNGerstorfDTime-structured and net intraindividual variability: tools for examining the development of dynamic characteristics and processesPsychol Aging200924477879120025395
- SnijdersTBoskerRJMultilevel Analysis: An Introduction to Basic and Advanced Multilevel ModelingThousand Oaks, CASage Publications1999
- HedekerDGibbonsRLongitudinal Data AnalysisHoboken, NJJohn Wiley & Sons2006
- HoxJMultilevel Analysis: Techniques and ApplicationsMahwah, NJLawrence Erlbaum Associated2002
- HedekerDGeneralized linear mixed modelsEverittBHowellHEncyclopedia of Statistics in Behavioral ScienceNew YorkWiley2005
- AlmeidaDMPiazzaJRStawskiRSInterindividual differences and intraindividual variability in the cortisol awakening response: an examination of age and genderPsychol Aging200924481982720025398
- WagnerGJGhosh-DastidarBElectronic monitoring: adherence assessment or intervention?HIV Clin Trials200231455111819185
- van OnzenoortHAMengerFENeefCParticipation in a clinical trial enhances adherence and persistence to treatment: a retrospective cohort studyHypertension201158457357821825228
- BergKMArnstenJHPractical and conceptual challenges in measuring antiretroviral adherenceJ Acquir Immune Defic Syndr200643Suppl 1S79S8717133207
- PowersBJOlsenMKSmithVAWoolsonRFBosworthHBOddoneEZMeasuring blood pressure for decision making and quality reporting: where and how many measures?Ann Intern Med20111541278178821690592
- EstabrooksPABoyleMEmmonsKMHarmonized patient-reported data elements in the electronic health record: supporting meaningful use by primary care action on health behaviors and key psychosocial factorsJ Am Med Inform Assoc201219457558222511015