Abstract
Background
Many female breast cancer (FBC) patients take Chinese herbal medicine (CHM) and Western medication (WM) concurrently in Taiwan. Despite the possibility of interactions between the CHM and WM mentioned in previous studies, the pattern of these coprescriptions in FBC patients remains unclear. Hence, the aim of the present study is to investigate the utilization of coprescriptions of CHM and WM among the FBC patients in Taiwan.
Methods
The study was a cross-sectional survey using the sampled cohort in 2009 obtained from the National Health Insurance Research Database in Taiwan. There were 3,507 FBC patients identified from the registry for catastrophic illness patients. Ambulatory visit records, corresponding prescriptions, and the data of beneficiaries belonging to the FBC patients were further extracted. A total of 1,086 FBC patients used CHM at least once. CHM and WM prescribed within any overlapping duration were defined as coprescriptions.
Results
There were 868 (80.0%) patients simultaneously receiving CHM and WM. A total of 4,927 CHM prescriptions and 6,358 WM prescriptions were prescribed concurrently. Among these coprescriptions, the most frequently used CHM was jia-wei-xiao-yao-san (21.2%), and the most frequently coprescribed WM was acetaminophen (38.9%), followed by tamoxifen (25.5%). There were 346 patients using systemic adjuvant therapy and CHM concurrently. The most commonly coprescribed CHM with chemotherapy, endocrine therapy, and trastuzumab was xiang-sha-liu-jun-zi-tang, jia-wei-xiao-yao-san, and zhi-gan-cao-tang, respectively.
Conclusion
The combined use of CHM with WM is prevalent. The main purpose of combining CHM with systemic cancer treatment is to alleviate the treatment-related adverse effects. However, the combination may result in the potential risk of drug–herb interactions. Further clinical studies are needed to evaluate the efficacy and safety of the CHM and WM coprescriptions for FBC patients.
Introduction
Breast cancer is the most frequent cancer and the chief cause of cancer death among women in Taiwan and worldwide.Citation1,Citation2 The mortality rate of breast cancer has declined, due to the popularly used screening mammography and the greater use of adjuvant therapies in recent decades.Citation3 Modern systemic adjuvant treatments, including cytotoxic chemotherapy, endocrine therapy, and anti-human epidermal growth factor receptor 2 (anti-HER2) therapy, should be selected based on the tumor size, grade, hormone-receptor content, and HER2 status.Citation4–Citation7 However, treatment-related adverse effects, such as fatigue, nausea, vomiting, hot flashes and other menopausal symptoms, cardiac toxicity, etc, make patients unable to tolerate treatment and lead them to seek additional help.Citation8
Many breast cancer patients use complementary and alternative medicine for cancer treatment, immune system enhancement, and symptom relief.Citation9,Citation10 Traditional Chinese medicine (TCM), including Chinese herbal medicine (CHM) and acupuncture, is the most popular complementary and alternative medicine modality and plays an important role in the Chinese population.Citation11–Citation13 A previous study, conducted in Shanghai, the People’s Republic of China, showed that 76.8% of breast cancer patients used CHM therapy after a diagnosis of breast cancer.Citation9 In Taiwan, Lai et al also found that 81.5% of female breast cancer (FBC) patients used TCM services and received CHM in 76.8% of visits during the 10-year study period.Citation14 In the survey conducted by Lin and Chiu, 35.6% of breast cancer patients used TCM during the 1-year observation period in Taiwan, and CHM (80.5%) was the most commonly used therapy.Citation15 All the studies revealed that the vast majority of the CHM users with breast cancer concurrently used Western medication (WM). This common practice raises concerns about the potential problems of drug–herb interactions.
TCM service is popular in the modern health system covered by the National Health Insurance (NHI) in Taiwan.Citation11,Citation16 Despite the possibility of interactions between CHM and WM mentioned in previous reports, the pattern of coprescribed CHM and WM in breast cancer patients remains unclear. Hence, the aim of the present study is to investigate the utilization of coprescriptions of CHM and WM among patients with breast cancer in Taiwan.
Materials and methods
Data resources
The NHI program in Taiwan was implemented in 1995 and has covered approximately 98% of the total population of Taiwan in recent years (http://nhird.nhri.org.tw/en/index.htm). Both Western medicine and TCM services are covered by NHI. TCM is only reimbursed by NHI for ambulatory care, not for inpatient care. Both claims of Western medicine and TCM visits are required to record diagnoses based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) coding system for reimbursement.
All electronic claim data under the NHI Research Database (NHIRD) project are released by the Bureau of NHI (BNHI) for research and are further managed by the National Health Research Institutes (NHRI).Citation17 The identification numbers of patients and care providers are deidentified before being sent to the NHRI and are further encrypted before being released to each researcher by the NHRI. Thus, the researchers cannot identify the patients or the care providers. However, the cryptographically scrambled identification numbers remain unique for record linking within the datasets. The present study was exempted from a full review by the Institutional Review Board of the Taipei Veterans General Hospital.
We obtained the database of the complete registry for catastrophic illness patients (RCIP) since 1997 (HV1997–HV2009) and the sampled cohort file (Longitudinal Health Insurance Database 2005, LHID2005) in 2009, including the registry for beneficiaries (ID2009), ambulatory visit records (CD2009), and corresponding prescription files (OO2009). The RCIP includes all approved cases of catastrophic illness, including breast cancer. Patients with catastrophic illness should submit the pathology and related laboratory reports for registration with RCIP. The approved cases can waive the copayment for each ambulatory visit and hospitalization.
Study sample
This was a cross-sectional study. Recruited subjects were FBC patients. Initially, we identified the FBC patients who had ever been registered with a malignant neoplasm of female breast (ICD-9-CM code: 174) from the RCIP. Ambulatory visit records, corresponding prescriptions, and the data of beneficiaries belonging to the FBC patients among the sampled cohort (LHID2005) in 2009 were further extracted by linking with the cryptographic identification numbers.
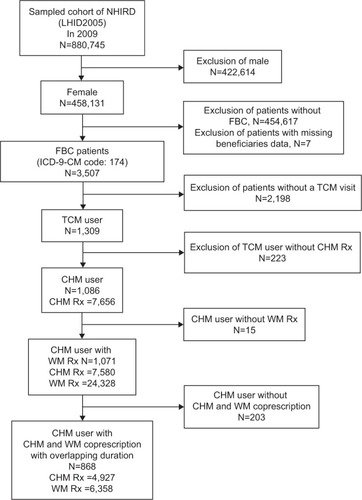
The FBC patients with at least one TCM visit in 2009 were defined as TCM users. Among TCM users, those who had ever received CHM prescriptions were defined as CHM users. All diagnoses of each CHM visit were considered. We tried to find the most frequently coprescribed CHM and WM and the most commonly coprescribed CHM with systemic adjuvant therapy among FBC patients in 2009. CHM and WM prescribed within any overlapping duration were defined as coprescriptions. illustrates the framework of the CHM users among FBC patients and corresponding prescription selection.
Figure 1 Framework of CHM users among FBC patients and corresponding prescriptions selection.
Study drugs
In Taiwan, CHM generally presents as Chinese herbal products (CHP), which were concentrated herbal extract, or as a traditional form (crude drug slices processed for decoction). However, only CHPs produced by the Good Manufacturing Practice-certified pharmaceutical companies and prescribed by licensed TCM physicians were reimbursed by the NHI. CHPs include single herbs or herbal formulas composed of two or more herbs. We downloaded all the items of the reimbursed CHPs and WM from the BNHI website.Citation18 Detailed information of CHPs, including constituents, was obtained from the website of the Committee on Chinese Medicine and Pharmacy.Citation19 In addition, WM that was composed of more than one drug was classified by the Anatomical Therapeutic Chemical (ATC) classification system.Citation20
Systemic adjuvant agents for breast cancer in the study were specified, based on reimbursement. Chemotherapeutic drugs included cisplatin, carboplatin, cyclophosphamide, fluorouracil, capecitabine, gemcitabine, methotrexate, doxorubicin, epirubicin, mitoxantrone, vinorelbine, vinblastine, docetaxel, paclitaxel, and etoposide. Drugs for endocrine therapies included selective estrogen receptor modulators (SERMs), aromatase inhibitors (AIs), luteinizing hormone-releasing hormone agonists, fluoxymesterone, and megestrol. SERMs included tamoxifen and toremifene. AIs included anastrozole, letrozole, and exemestane. The luteinizing hormone-releasing hormone agonists included goserelin and leuprolide. Trastuzumab was the only included HER2-directed agent.
Data analysis
Microsoft SQL Server 2008 (Microsoft Corporation, Redmond, WA, USA) was used for data management and computing. The statistical analysis of the data in this study was performed using PASW Statistics for Windows, version 18.0 (SPSS, Inc, Chicago, IL, USA). Descriptive statistics were used for the utilization of CHM and WM coprescriptions in FBC patients.
Results
Patient demographics
A total of 3,507 FBC patients had ambulatory visits in the sampled cohort (LHID2005) in 2009. Of these, 1,309 (37.3%) were TCM users, and 1,086 (31.0%) were CHM users. The detailed demographics of the CHM users with FBC are presented in . Among CHM users with FBC, the highest proportion of patients was 50–59 years of age (38.7%). The majority lived in the northern area (49.2%) and the highly urbanized towns (35.6%) of Taiwan. The most frequent insurance amount range was about 20,000–39,999 New Taiwan Dollars (41.2%). More than 60% of CHM users had been diagnosed with breast cancer <5 years. About 43.9% of CHM users were under systemic adjuvant treatment, and 32.6% of the patients received endocrine therapy alone during 2009. Young patients were more likely to use CHM only.
Table 1 Demographics of CHM users among FBC patients in Taiwan, 2009
Diagnoses
The most common diagnosis for CHM users was “general symptoms” (24.7%); the second was “malignant neoplasm of female breast” (21.8%). Approximately one-half of CHM users looked for relieving “symptoms, signs, and ill-defined conditions” (49.6%), and the others used CHM to treat respiratory diseases (31.5%), musculoskeletal diseases (27.7%), digestive diseases (27.6%), and neoplasms (23.0%) ().
Table 2 Main reasons for CHM use among FBC patients in Taiwan, 2009
Coprescriptions of CHM and WM
Among 1,086 FBC patients who were CHM users, 1,071 were under treatment with WM. There were 868 (80.0%) patients simultaneously receiving CHM and WM. A total of 4,927 CHM prescriptions and 6,358 WM prescriptions were prescribed concurrently during the study period. presents the most commonly used CHM and WM in coprescriptions among FBC patients in Taiwan during 2009. In these coprescriptions, the most frequently used CHM was jia-wei-xiao-yao-san (JWXYS) (21.2%), and the most frequently coprescribed WM was acetaminophen (38.9%), followed by tamoxifen (25.5%).
Table 3 Most commonly used CHM and WM in coprescriptions among FBC patients in Taiwan, 2009
According to the ATC classification system, analgesics, cough and cold preparations, psycholeptics (including anxiolytics and hypnotics), drugs for acid-related disorders, and endocrine therapy were the most common WM used concurrently with CHM.
There were 346 CHM users receiving systemic adjuvant therapy concurrently. shows the most frequently coprescribed CHM with different types of systemic adjuvant therapy. Xiang-sha-liu-jun-zi-tang (27.3%) and zhi-gan-cao-tang (40.0%) were the most commonly used CHM with chemotherapy and anti-HER2 therapy, respectively. In addition, JWXYS was the most popular CHM coprescribed with SERMs or AIs. The ingredients of CHPs mentioned in the text are shown in and .
Table 4 Most commonly used CHM coprescribed with systemic adjuvant treatment among FBC patients in Taiwan, 2009
Discussion
To our knowledge, this study is the first population-based pharmacoepidemiology survey of CHM and WM coprescriptions among patients with FBC in Taiwan. Our results highlight the importance of further studies required to evaluate the clinical impact of these coprescriptions for FBC patients.
CHM utilization
From the present study, the high prevalence (37.3%) of TCM usage among FBC patients in Taiwan is in accordance with the survey conducted by Lin and Chiu.Citation15 Breast cancer patients are more likely to use TCM than patients with other cancers.Citation21,Citation22 CHM is the dominant type of TCM used by FBC patients and the general population.Citation11,Citation16 In our study, 83.0% (1,086/1,309) TCM users received CHM treatment. We also found that 1,071 (98.6%) CHM users of FBC patients received both CHM and WM during 2009.
Among them, 868 CHM users took CHM and WM concurrently. In the present study, the most common diagnoses for CHM users of FBC patients were “malignant neoplasm of female breast” and “general symptoms”, such as nausea, vomiting, fatigue, etc. However, treatment for breast cancer accounted for only 21.8%, which is in accordance with the findings of the previous survey in Taiwan.Citation14,Citation15 This is significantly lower than the results from Shanghai, which found that more than 90% of FBC patients used CHM for fighting cancer.Citation9 The different results may also relate to the different methods of data collection used by our study and the study from Shanghai.
Because the study from Shanghai used direct-participant interviews, it may not directly compare to our results. In addition, our study found that diseases of the digestive, respiratory, musculoskeletal, and genitourinary systems were the most common reasons for using CHM. The purposes of CHM usage are consistent with the results of the general population surveys in Taiwan.Citation11,Citation16 Obviously, CHM usage among FBC patients in Taiwan may be regarded as an add-on therapy rather than a substitute modality.
Possible reasons for coprescriptions
Menopausal syndrome is the major complication among breast cancer patients under chemotherapy and endocrine therapy.Citation8 Menopausal complications – including hot flashes, night sweats, insomnia, etc – seriously impact the quality of life of breast cancer patients. Therefore, most of them search for management strategies. According to the study conducted in Shanghai, about 29.4% of breast cancer patients use CHM to lessen menopausal symptoms. Patients with menopausal symptoms or who had used tamoxifen in the past were prone to use CHM.Citation9 In coprescriptions, we found that JWXYS was the most frequently used CHM, and tamoxifen was the most commonly prescribed WM associated with FBC treatment. Furthermore, our study revealed a high coprescription rate between JWXYS and chemotherapy or JWXYS and endocrine therapy. JWXYS, also named dan-zhi-xiao-yao-san, is the principal CHM for the relief of menopausal syndrome.Citation23–Citation26 The clinical effects of JWXYS were documented in the classics of traditional medicine and reported by clinical studies.Citation27,Citation28 This implies that many FBC patients under modern systemic cancer treatment suffered from menopausal symptoms and sought CHM therapy concurrently. The results of the present study are compatible with the Shanghai survey.Citation9 Our results also found that a considerable proportion of CHM was likely to be used for insomnia when combined with SERMs. These CHM include suan-zao-ren-tang, tian-wang-bu-xin-dan, suan-zao-ren (Ziziphi spinosae semen) and shou-wu-teng (Polygoni multiflori caulis), etc.Citation29–Citation34
Musculoskeletal problems, such as arthralgia and osteoporosis, that result from AI treatment are significantly more than those caused by tamoxifen treatment.Citation35 Joint pain and musculoskeletal pain are the most commonly reported troublesome adverse effects from AI users.Citation36,Citation37 Our results found that most CHM coprescribed with AIs was used for arthritis and pain relief. For example, du-huo-ji-sheng-tang and xu-duan (Dipsaci radix) are used to treat osteoarthritis, osteoporosis, and other bone diseases.Citation38–Citation41 Shao-yao-gan-cao-tang is used to relieve dysmenorrhea and muscle pain.Citation42
Cardiac toxicity is one of the major concerns for patients with anthracycline-based chemotherapy and trastuzumab treatment.Citation43,Citation44 In contrast to anthracycline-related cardiomyopathy and heart failure, cardiotoxicity of trastuzumab is often manifested by asymptomatic left ventricular ejection fraction reduction, palpitations, and – less often – by heart failure. Sheng-mai-san is a famous herbal formula used for the treatment of heart failure and ischemic heart disease.Citation45 The present study found that many FBC patients treated with chemotherapy used sheng-mai-san concurrently. On the other hand, the study revealed that zhi-gan-cao-tang was one of the commonly coprescribed CHM with trastuzumab. Zhi-gan-cao-tang is traditionally used for reversing irregular cardiac rhythm.Citation46
In the present study, we also found that many CHM coprescribed with chemotherapy were used for relieving gastrointestinal symptoms. The most frequently coprescribed xiang-sha-liu-jun-zi-tang is a famous formula for the treatment of functional dyspepsia.Citation47 Ban-xia-xie-xin-tang can mitigate nausea and vomiting caused by systemic cancer therapy.Citation48,Citation49 The other single herbs such as sha-ren (Amomi fructus) and bai-zhu (Atractylodis macrocephalae rhizoma) can modulate gastrointestinal symptoms.Citation50,Citation51
Besides relieving treatment-related symptoms, other commonly coprescribed CHM are also applied to fight cancer. For example, pu-gong-ying (Taraxaci herba), bai-hua-she-she-cao (Hedyotis diffusae herba), yu-jin (Curcumae radix), and zhen-ren-huo-ming-yin all have shown antitumor effects.Citation52–Citation54 Huang-qin (Scutellariae radix) can enhance the antitumor effect.Citation55 Huang-qi (Astragali radix) has an anticancer effect and boosts the immune function.Citation56 Overall, one of the main purposes of CHM when combined with systemic cancer treatment was to alleviate the treatment-related adverse effects.
Possible interactions between CHM and SERM/anti-HER2 therapy
Recently, there was a small cohort study, and a case report found that si-wu-tang (SWT), one of the popular Chinese herbal formulas for the regulation of menstruation, might have mitogenic potential side effects on breast duct cells in long-term use.Citation57
Moreover, SWT and its constituents, Angelicae sinensis radix, Paeoniae radix alba, Chuanxiong rhizome, and Rehmanniae radix preparata, have been proven to stimulate mammary duct cell growth by the activation of estrogen receptor α and HER-2 signaling in cell line studies.Citation58,Citation59 Another study demonstrated that, based on in vivo and in vitro studies, SWT respectively reversed tamoxifen- and trastuzumab-induced antiproliferative effects.Citation60 Therefore, it is necessary to cautiously evaluate the coprescriptions of CHM and WM for breast cancer patients.
Our study found that some CHM coprescribed with SERM or with trastuzumab contained parts of the constituents of SWT. Examples are: JWXYS contains Angelicae sinensis radix and Paeoniae radix alba; suan-zao-ren-tang contains Chuanxiong rhizoma; and tian-wang-bu-xin-dan contains Angelicae sinensis radix and Rehmanniae radix praparata. It is still unknown whether these formulas containing these single herbs have the same potential risk to reverse tamoxifen- or trastuzumab-induced antiproliferative effects.
Furthermore, the observed results from in vitro or in vivo studies cannot be extrapolated to those in humans. It is better to remind patients to pay more attention when using CHM and tamoxifen or trastuzumab treatment concurrently. Further clinical studies are required to explore the risk of these interactions.
Limitation
Our study has several potential limitations.
First, breast cancer in situ, including ductal carcinoma in situ and lobular carcinoma in situ, was not included as a catastrophic illness in the Taiwan NHI program. In addition, some CHM services were self-paid and not covered by the NHI program, including CHM services provided by non-NHI-contracted health care institutions, or self-paid traditional-form Chinese herbal remedies, etc. The lack of above data leads to the underestimation of CHM utilization for FBC patients.
Second, due to the lack of information, including cancer stages, lab data, clinical symptoms, and survival data in NHIRD, the study was neither to evaluate the relationship between disease severities and CHM usage nor to elucidate the effects of CHM therapies.
Third, we defined coprescriptions as CHM and WM prescribed within any overlapping duration. However, we cannot verify the concurrent use of CHM and WM.
Finally, the clinical effects of interactions between CHM and SERM or CHM and anti-HER2 therapy cannot be confirmed.
Conclusion
CHM use in FBC patients is popular in Taiwan. The main purpose of CHM combined with systemic cancer treatment is to alleviate the treatment-related adverse effects. A high prescription rate of CHM combined with WM may lead to possible drug–herb interactions. Further research is needed to evaluate the clinical impact of CHM and WM coprescriptions for FBC patients.
Acknowledgments
This study is based, in part, on data from the NHIRD provided by the BNHI, Department of Health, and managed by the NHRI. The interpretations and conclusions contained herein do not represent those of the BNHI, Department of Health, or the NHRI. This study was supported by grants from Taipei Veterans General Hospital (V100A-058) in Taiwan. Sponsors had no role in the design, analysis, or presentation of this study.
Supplementary materials
Table S1 Chinese herbal products
Table S2 Chinese herbal products of herbal formulas
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalABrayFCenterMMFerlayJWardEFormanDGlobal cancer statisticsCA Cancer J Clin2011612699021296855
- Bureau of Health Promotion, Department of HealthCancer registry annual report, 2010, Taiwan2013 Available from: http://www.hpa.gov.tw/BHPNet/Portal/File/StatisticsFile/201305061037065219/99%E5%B9%B4%E7%99%8C%E7%97%87%E7%99%BB%E8%A8%98%E5%A0%B1%E5%91%8A.pdfAccessed April 29, 2014
- BerryDACroninKAPlevritisSKCancer Intervention and Surveillance Modeling Network (CISNET) CollaboratorsEffect of screening and adjuvant therapy on mortality from breast cancerN Engl J Med2005353171784179216251534
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG)Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trialsLancet200536594721687171715894097
- HowellACuzickJBaumMATAC Trialists’ GroupResults of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancerLancet20053659453606215639680
- SwainSMJeongJHGeyerCEJrLonger therapy, iatrogenic amenorrhea, and survival in early breast cancerN Engl J Med2010362222053206520519679
- HudisCATrastuzumab – mechanism of action and use in clinical practiceN Engl J Med20073571395117611206
- ShapiroCLRechtASide effects of adjuvant treatment of breast cancerN Engl J Med2001344261997200811430330
- ChenZGuKZhengYZhengWLuWShuXOThe use of complementary and alternative medicine among Chinese women with breast cancerJ Altern Complement Med20081481049105518928393
- WanchaiAArmerJMStewartBRComplementary and alternative medicine use among women with breast cancer: a systematic reviewClin J Oncol Nurs2010144E45E5520682492
- ChenFPChenTJKungYYUse frequency of traditional Chinese medicine in TaiwanBMC Health Serv Res200772617319950
- ChungVWongEWooJLoSVGriffithsSUse of traditional Chinese medicine in the Hong Kong special administrative region of ChinaJ Altern Complement Med200713336136717480138
- HeskethTZhuWXHealth in China. Traditional Chinese medicine: one country, two systemsBMJ199731571001151179240055
- LaiJNWuCTWangJDPrescription pattern of Chinese herbal products for breast cancer in Taiwan: a population-based studyEvid Based Complement Alternat Med2012201289189322685488
- LinYHChiuJHUse of Chinese medicine by women with breast cancer: a nationwide cross-sectional study in TaiwanComplement Ther Med201119313714321641518
- ChangLCHuangNChouYJLeeCHKaoFYHuangYTUtilization patterns of Chinese medicine and Western medicine under the National Health Insurance Program in Taiwan, a population-based study from 1997 to 2003BMC Health Serv Res2008817018691428
- National Health Insurance Research Database [database on the Internet]Taipei, TaiwanNational Health Research Institutes2003 Available from: http://nhird.nhri.org.tw/Accessed January 5, 2012
- National Health Insurance Administartion Ministry of Health and Welfare [webpage on the Internet]Bureau of National Health Insurance: List of reimbursed drugs Available from: http://www.nhi.gov.tw/webdata/webdata.aspx?menu=17&menu_id=879&webdata_id=873Accessed January 5, 2012 Chinese
- Department of Chinese Medicine and PharmacyDatabase of registered Chinese herbal medicines-current and withdrawn Available from: http://www.mohw.gov.tw/cht/DOCMAP/DM1.aspx?f_list_no=499Accessed April 23, 2014 Chinese
- World Health Organization Collaborating Centre for Drug Statistics MethodologyGuidelines for ATC classification and DDD assignment 2013Oslo, NorwayWorld Health Organization2012 Available from: http://www.whocc.no/filearchive/publications/1_2013guidelines.pdfAccessed January 5, 2012
- LinYHChenKKChiuJHCoprescription of Chinese Herbal Medicine and Western Medications among Prostate Cancer Patients: A Population-Based Study in TaiwanEvid Based Complement Alternat Med2012201214701521792368
- LinYHChiuJHUse of Chinese Medicine among patients with liver cancer in TaiwanJ Altern Complement Med201016552752820804364
- ChenHYLinYHWuJCPrescription patterns of Chinese herbal products for menopausal syndrome: analysis of a nationwide prescription databaseJ Ethnopharmacol201113731261126621824510
- YangYHChenPCWangJDLeeCHLaiJNPrescription pattern of traditional Chinese medicine for climacteric women in TaiwanClimacteric200912654154719905906
- ScheidVWardTChaWSWatanabeKLiaoXThe treatment of menopausal symptoms by traditional East Asian medicines: review and perspectivesMaturitas201066211113020079585
- ScheidVWardTTuffreyVComparing TCM textbook descriptions of menopausal syndrome with the lived experience of London women at midlife and the implications for Chinese medicine researchMaturitas201066440841620444560
- ChenLCTsaoYTYenKYChenYFChouMHLinMFA pilot study comparing the clinical effects of Jia-Wey Shiau-Yau San, a traditional Chinese herbal prescription, and a continuous combined hormone replacement therapy in postmenopausal women with climacteric symptomsMaturitas2003441556212568736
- LaiJNHwangJSChenHJWangJDFinished herbal product as an alternative treatment for menopausal symptoms in climacteric womenJ Altern Complement Med20051161075108416398600
- ChenFPJongMSChenYCPrescriptions of Chinese Herbal Medicines for Insomnia in Taiwan during 2002Evid Based Complement Alternat Med2011201123634119339485
- YehCHArnoldCKChenYHLaiJNSuan zao ren tang as an original treatment for sleep difficulty in climacteric women: a prospective clinical observationEvid Based Complement Alternat Med2011201167381321660310
- YeungWFChungKFPoonMMPrescription of Chinese herbal medicine and selection of acupoints in pattern-based traditional Chinese medicine treatment for insomnia: a systematic reviewEvid Based Complement Alternat Med2012201290257823259001
- PengWHHsiehMTLeeYSLinYCLiaoJAnxiolytic effect of seed of Ziziphus jujuba in mouse models of anxietyJ Ethnopharmacol200072343544110996283
- JiangJGHuangXJChenJSeparation and purification of saponins from Semen Ziziphus jujuba and their sedative and hypnotic effectsJ Pharm Pharmacol20075981175118017725862
- YeRYuanZZDaiCXIntervention of tianwang buxin decoction combined with dormancy hygiene education for treatment of sub-healthy insomnia patients of yin deficiency fire excess syndromeZhongguo Zhong Xi Yi Jie He Za Zhi2011315618621 Chinese [with English abstract]21812260
- SmithIEDowsettMAromatase inhibitors in breast cancerN Engl J Med2003348242431244212802030
- FelsonDTCummingsSRAromatase inhibitors and the syndrome of arthralgias with estrogen deprivationArthritis Rheum20055292594259816142740
- MaoJJChungABentonAOnline discussion of drug side effects and discontinuation among breast cancer survivorsPharmacoepidemiol Drug Saf201322325626223322591
- LiuMXiaoGGRongPTherapeutic effects of radix dipsaci, pyrola herb, and Cynomorium songaricum on bone metabolism of ovariectomized ratsBMC Complement Altern Med2012126722639966
- ChenCWSunJLiYMShenPAChenYQAction mechanisms of du-huo-ji-sheng-tang on cartilage degradation in a rabbit model of osteoarthritisEvid Based Complement Alternat Med2011201157147921792361
- LaiJNChenHJChenCCLinJHHwangJSWangJDDuhuo jisheng tang for treating osteoarthritis of the knee: a prospective clinical observationChin Med20072417394666
- ShihWTYangYHChenPCPrescription patterns of Chinese herbal products for osteoporosis in Taiwan: a population-based studyEvid Based Complement Alternat Med2012201275283723093986
- HinoshitaFOguraYSuzukiYEffect of orally administered shao-yao-gan-cao-tang (Shakuyaku-kanzo-to) on muscle cramps in maintenance hemodialysis patients: a preliminary studyAm J Chin Med200331344545312943175
- SingalPKIliskovicNDoxorubicin-induced cardiomyopathyN Engl J Med1998339139009059744975
- PerezEARodehefferRClinical cardiac tolerability of trastuzumabJ Clin Oncol200422232232914722042
- ZhengHChenYChenJKwongJXiongWShengmai (a traditional Chinese herbal medicine) for heart failure [review]Cochrane Database Syst Rev20112CD00505221328272
- ChenWGBaZMProf. ZHANG Yi’s experience in treating severe arrhythmiaJ Tradit Chin Med2010301475020397463
- XiaoYLiuYYYuKQOuyangMZLuoRZhaoXSChinese herbal medicine liu jun zi tang and xiang sha liu jun zi tang for functional dyspepsia: meta-analysis of randomized controlled trialsEvid Based Complement Alternat Med2012201293645923304226
- XuHZhangWXModern Applications of Modified Ban Xia Xie Xin Tang and Their DevelopmentAustralian Journal of Acupuncture and Chinese Medicine2008312530
- ZhaoLZhangSWangZEfficacy of modified ban xia xie xin decoction on functional dyspepsia of cold and heat in complexity syndrome: a randomized controlled trialEvid Based Complement Alternat Med2013201381214323589722
- YamazakiTMatsushitaYKawashimaKSomeyaMNakajimaYKurashigeTEvaluation of the pharmacological activity of extracts from amomi semen on the gastrointestinal tractsJ Ethnopharmacol2000711–233133510904182
- BoseSKimHEvaluation of In Vitro Anti-Inflammatory Activities and Protective Effect of Fermented Preparations of Rhizoma Atractylodis Macrocephalae on Intestinal Barrier Function against Lipopolysaccharide InsultEvid Based Complement Alternat Med2013201336307623573125
- KimSHAhnBZRyuSYAntitumour effects of ursolic acid isolated from Oldenlandia diffusaPhytother Res1998128553556
- LuBXuLYuLZhangLExtract of radix curcumae prevents gastric cancer in ratsDigestion2008772879118376129
- LuBYuLXuLChenHZhangLZengYThe effects of radix curcumae extract on expressions of VEGF, COX-2 and PCNA in gastric mucosa of rats fed with MNNGCurr Pharm Biotechnol201011331331720210736
- KumagaiTMüllerCIDesmondJCImaiYHeberDKoefflerHPScutellaria baicalensis, a herbal medicine: anti-proliferative and apoptotic activity against acute lymphocytic leukemia, lymphoma and myeloma cell linesLeuk Res200731452353017007926
- KurashigeSAkuzawaYEndoFEffects of astragali radix extract on carcinogenesis, cytokine production, and cytotoxicity in mice treated with a carcinogen, N-butyl-N’-butanolnitrosoamineCancer Invest1999171303510999046
- ChangCJChiuJHWuCWLuiWYHerb-related aneuploidy in breast fibroadenomaBMJ Case Rep Epub2009512
- ChangCJChiuJHTsengLMSi-Wu-Tang and its constituents promote mammary duct cell proliferation by up-regulation of HER-2 signalingMenopause200613696797617075435
- ChangCJChiuJHTsengLMModulation of HER2 expression by ferulic acid on human breast cancer MCF7 cellsEur J Clin Invest200636858859616893382
- ChenJLWangJYTsaiYFIn vivo and in vitro demonstration of herb-drug interference in human breast cancer cells treated with tamoxifen and trastuzumabMenopause201320664665423340260
