Abstract
As a determining factor in various diseases and the leading known cause of preventable mortality and morbidity, tobacco use is the number one public health problem in developed countries. Facing this health problem requires authorities and health professionals to promote, via specific programs, health campaigns that improve patients’ access to smoking cessation services. Pharmaceutical care has a number of specific characteristics that enable the pharmacist, as a health professional, to play an active role in dealing with smoking and deliver positive smoking cessation interventions. The objectives of the study were to assess the efficacy of a smoking cessation campaign carried out at a pharmaceutical care center and to evaluate the effects of pharmaceutical care on patients who decide to try to stop smoking. The methodology was an open, analytical, pre–post intervention, quasi-experimental clinical study performed with one patient cohort. The results of the study were that the promotional campaign for the smoking cessation program increased the number of patients from one to 22, and after 12 months into the study, 43.48% of the total number of patients achieved total smoking cessation. We can conclude that advertising of a smoking cessation program in a pharmacy increases the number of patients who use the pharmacy’s smoking cessation services, and pharmaceutical care is an effective means of achieving smoking cessation.
Introduction
As a determining factor in various diseases and the leading known cause of preventable mortality and morbidity, tobacco use is the number one public health problem in developed countries. In Spain, it is estimated that 55,000 people die every year from diseases directly linked to tobacco use.Citation1 We are facing a significant public health problem that requires authorities and health professionals to promote, via specific programs, health campaigns that improve patients’ access to smoking cessation services.Citation2,Citation3
Based on the available evidence, it is recommended that health professionals’ involvement in smoking cessation interventions be based on criteria such as access to smokers, appropriate professional training, and each professional’s interest and experience, rather than on the field in which they work.Citation4 Community pharmacies can deliver regular interventions to a large number of healthy and ill people. Pharmacists, therefore, have an excellent opportunity to help promote good health and take part in disease prevention activities as part of their service to patients. Pharmacists’ role as a provider of nicotine replacement therapy (NRT), available without prescription in Spain, means that they are ideally placed to support patients who want help to stop smoking, and they can ensure that patients have access to these kinds of programs. Their role should go further than the provision of advice on the correct use of pharmacological products to include providing appropriate advice and guidelines aimed at helping patients achieve abstinence and, when necessary, referring patients to the relevant health care providers.Citation5
Pharmaceutical care has a number of specific characteristicsCitation6 that enable the pharmacist, as a health professional, to play an active role in dealing with smoking and to deliver positive smoking cessation interventions. The studies performed to date indicate that interventions by suitably trained community pharmacy personnel can have a positive effect on smoking cessation rates.Citation5
The objectives of this study were: 1) to assess the efficacy of a smoking cessation campaign carried out at a pharmaceutical care center; and 2) to evaluate the effects of pharmaceutical care on patients who decide to try to stop smoking.
Materials and methods
This was an open, analytical, pre–post intervention, quasi-experimental clinical study performed with one patient cohort. It was carried out in a pharmacy in the city of Murcia, Spain. The study population consisted of smokers who were patients of the pharmacy where the study on smoking cessation took place, and all smokers were contacted via the campaign promoting the pharmacy’s smoking cessation service. All patients who came to the pharmacy over a period of 4 months (April–July, 2011) to ask for help to stop smoking were included.
To assess the primary objective, the pharmacy’s smoking cessation service was offered to all patients who came to the pharmacy to ask for help to stop smoking during the study period. During the last 2 months of this period, a promotional campaign for a smoking cessation program was carried out. This campaign involved passing out leaflets to all the patients who visited the pharmacy () and displaying posters inside and outside the pharmacy (). The promotional campaign was launched to coincide with “World No Tobacco Day” (May 31, 2011), and the contents of the leaflets and posters were taken from awareness campaigns created by Spain’s Ministry of Health and Social Services.
The dependent variable was the number of patients who enrolled in the program before and after the campaign. The Dader methodCitation7,Citation8 was used to assess the second objective, and seven patient follow-up visits were planned: the first between the first and third day after the patient stopped smoking; the second between days 7 and 15; and then subsequent visits at months 1, 2, 3, 6, and 12.
The inclusion criteria were as follows: all patients who smoked and were over 18 years old;Citation9 who asked for help to stop smoking; and who agreed to register for the smoking cessation program.
The exclusion criterion included patients who smoked, but who did not want to take part in the study.
The independent variables included:
Patient data
○ Age
○ Sex
Initial condition
○ Number of cigarettes per day: discrete, categorical, numerical variable referring to the number of cigarettes consumed per day. Patients were categorized as smokers of ten or fewer cigarettes, 11–20 cigarettes, 21–30 cigarettes, and 31 or more cigarettes per day.
○ CO-oximetry value:Citation10 discrete, categorical, numerical variable measured in parts per million (ppm) indicating the quantity of carbon monoxide (CO) in the air exhaled by a subject. A Smoke Check Monitor® CO-oximeter was used to measure this parameter. Patients who were found to have 20 or more ppm of CO in exhaled air were classified as heavy smokers; patients with 11–20 ppm CO were classified as moderate smokers; patients with 7–10 ppm CO were classified as occasional smokers; and those with values of 6 ppm CO or less were classified as nonsmokers.Citation9,Citation11,Citation12
○ Nicotine dependence (Fagerstrom test):Citation13 discrete, categorical, numerical variable to measure smokers’ levels of physical dependence on nicotine. This is a six-question test with multiple choice answers (). A certain number of points are attributed to each question depending on the answer given by the smoker. The points for all of the questions are then added together to give a total of between 0 and 10 points. If the patient has a score of between 0 and 3 points, his level of dependence is judged to be low; a score of between 4 and 6 indicates moderate dependence; and a score of 7 or more indicates a high level of dependence.
Smoking history
○ Starting age: continuous, numerical variable indicating how old the patient was in years when he started smoking.
○ Previous attempts: discrete, numerical variable indicating how many times the patient tried to stop smoking in the past.
○ Treatment: nominal, qualitative variable indicating the therapy used by the patient during his previous attempts to stop smoking. The options were: the patient has not received any treatment; the patient has used NRT; the patient has used non-nicotine-based pharmaceutical treatments; the patient has used self-help resources; or the patient has used other treatments (such as acupuncture, homeopathy, and herbal medicines).
○ Perception of difficulties: nominal, qualitative variable indicating the situations in which the patient found it difficult not to smoke. Smokers could choose from the following situations: social functions; work or study environment; leisure time and bars; smoking while alone; having a cigarette with coffee; or other.
The dependent variables included:
Acceptance of pharmaceutical care: nominal, dichotomous, qualitative variable expressing the results as a percentage of patients who accepted help to stop smoking.
Smoking cessation: qualitative, ordinal variable indicating abstinence as reported by the patient. Cessation could be positive or negative. If positive, it was categorized as either total or partial (the latter indicating a reduction in the number of cigarettes consumed). Partial cessation was recorded if the patient moved into a lower category. Four follow-up visits were planned to assess smoking cessation (at 1, 3, 6, and 12 months). The value of this variable was confirmed by measuring CO in exhaled air during each follow-up visit.
Patients were referred to a doctor in the following situations:Citation9,Citation14 if they were pregnant or breastfeeding; if they had been suffering from ischemic heart disease for less than 8 weeks; if they suffered from uncontrolled arrhythmias or uncontrolled high blood pressure; if they suffered from an uncontrolled chronic disease (neuropathic disorder, liver disease, heart disease, and so on); or if they had any psychiatric disorders.
All referred patients received both medical and pharmaceutical care. The pharmaceutical care comprised two components: psychological support and pharmacological treatment. Psychological support was offered at all visits and consisted of providing advice to patients to help them get ready to give up smoking, stay motivated, and avoid relapse. This involved the use of cognitive–behavioral techniques to develop the necessary skills and coping strategies, problem-solving techniques, and appropriate social support both during and after therapy sessions.Citation15,Citation16 This process was aimed at obtaining the results required for the smoking cessation variable.
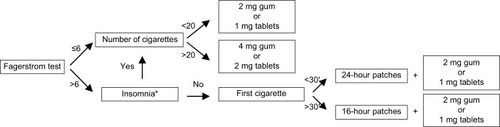
The pharmacological treatment for those patients who needed it was NRT.Citation17,Citation18 In Spain, only chewing gum, patches, and tablets are currently available. If the Fagerstrom test score was ≤6, the pharmacist recommended chewing gum or tablets as required for the patient to use before he experienced the need or intense desire to smoke. The patient was advised to limit the number of doses taken because nicotine is addictive in all forms of administration. The maximum daily dose was 20 pieces of 2 mg gum or 15 pieces of 4 mg gum, or 20 1 mg tablets or 15 2 mg tablets. The minimum recommended dose was one piece of gum or one tablet every 2–3 hours, with a usual dose being 8–12 pieces of gum or tablets. In order to provide personalized treatment, the pharmacist measured the quantity of nicotine generally consumed by each patient. If the patient smoked more than 20 cigarettes per day, 4 mg gum or 2 mg tablets were recommended. If the patient smoked less than 20 cigarettes per day, 2 mg gum or 1 mg tablets were recommended.
If the Fagerstrom test score was >6, the pharmacist recommended the use of nicotine patches, except in cases of insomnia.Citation9 The pharmacist based the recommended patch type on the length of time between waking up and smoking the first cigarette. If this period was greater than 30 minutes, the 16-hour patch was recommended. If this period was less than 30 minutes, the 24-hour patch was recommended. The use of patches was combined with low-dose gum or tablets to control cravings (). The patients gave their consent to the choice of treatment.
Results
Overall, 25 patients were invited to participate in the study on smoking cessation, of which 23 (92%) accepted the pharmacy’s service and two (8%) declined. During the first 2 months in which the pharmacy’s service was offered to patients who asked for help to stop smoking, only one patient asked for help, and that one patient was enrolled in the study. During the following 2 months in which the promotional campaign for the smoking cessation program was running, 24 patients asked for help, of which 22 patients were enrolled in the study and two declined the offer of the pharmacy’s service. This represents a 24-fold increase in the number of smokers who asked for help to stop smoking and a 22-fold increase in the number of patients who were enrolled in the study.
In the final study population, 69.57% of participants were female and 30.43% were male. The average age of the participants was 41.61 years, with a standard deviation of 8.56 years and an age range of 23–60 years. The average age of the patients who achieved total smoking cessation at 12 months was 43.6 years, with a standard deviation of 8.14 years and an age range of 33–60 years. The average age of the patients who achieved partial cessation at 12 months was 43 years, with a standard deviation of 7.07 years and an age range of 38–48 years.
The smoking history data were as follows: average starting age of 16.48 years with a standard deviation of 2.94 years, and an age range of 13–25 years; 82.61% of patients had made at least one previous attempt to stop smoking, and 17.39% had made five or more attempts; almost half, 47.83%, had used no particular method to stop smoking in the past; and no patients had tried any pharmacological treatment other than NRT. In addition, 43.48% of patients identified three or more situations in which they found it difficult not to smoke.
The initial condition data revealed that 60.87% of patients smoked between eleven and 20 cigarettes per day; 26.09% of patients had a CO-oximetry level of between 7 ppm and 10 ppm, 39.13% between 11 ppm and 20 ppm, and 34.78% of more than 20 ppm; and 43.48% presented low nicotine dependence, 30.43% moderate dependence, and 26.09% high dependence.
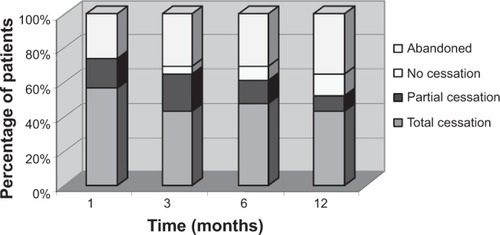
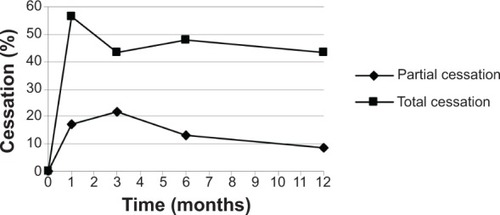
shows the smoking cessation results obtained at the 1, 3, 6, and 12 months follow-up visits. One month into the study, 73.91% of patients had achieved smoking cessation, and 26.09% abandoned the study. Of the patients who obtained a positive result, 76.47% achieved total smoking cessation (56.52% of the total number of patients) and 23.53% achieved partial smoking cessation (17.39% of the total). None of the patients who completed the follow-up failed to comply with the treatment.
Three months into the study, 65.22% of patients achieved smoking cessation, 4.35% had not achieved it, and 30.43% had abandoned the study. Of the patients who obtained a positive result, 66.67% achieved total smoking cessation (43.48% of the total number of patients) and 33.33% achieved partial cessation (21.74% of the total).
Six months into the study, 60.87% of patients achieved smoking cessation, 8.70% had not achieved it, and 30.43% abandoned the study. Of the patients who obtained a positive result, 78.57% achieved total smoking cessation (47.83% of the total number of patients) and 21.43% achieved partial cessation (13.04% of the total).
Twelve months into the study, 52.18% of patients achieved smoking cessation, 13.04% had not achieved it, and 34.78% abandoned the study. Of the patients who obtained a positive result, 83.33% achieved total smoking cessation (43.48% of the total number of patients) and 16.67% achieved partial cessation (8.70% of the total; ). The rate of smoking cessation achieved by patients during the study period is shown in .
Table 1 Smoking cessation results
Discussion
A very high percentage of the patients in our study accepted the offer of support from the pharmaceutical care service to help them stop smoking (92%). In other studies, the number of patients declining the support of other pharmaceutical care services, such as medical follow-up, has been much higher.Citation19 There are many intervention methods designed to improve patient health,Citation20 but in our field, the most commonly used method is health campaigns.Citation21 Promoting the service through an awareness campaign significantly increased the number of patients who enrolled in the pharmaceutical care program (from one patient to 22 patients).
The average rates of abstinence achieved after 6 and 12 months of treatment with NRT in other studies carried out in primary care and other health clinics are between 25% and 35%.Citation16,Citation22 In our study, the total abstinence rate was 47.83% at 6 months and 43.48% at 12 months, indicating that the inclusion of pharmacies in smoking cessation programs may improve health outcomes. Pharmaceutical care may be responsible for this result, thanks to the continuous, active intervention on the part of the pharmacist, achievable due to the pharmacist’s proximity and access to the patient.
There were no significant differences in smoking cessation between the sexes, although the percentage of women participating in the study (69.57%) was higher than that of men. As other authors have described, women are more likely to seek advice from pharmacies than men.Citation23 The majority of studies carried out in primary care settings include similar numbers of men and women,Citation17 and the rates of cessation are similar in both sexes in the published literature.
The possible limitations of this study are the number of study participants, and to avoid this limitation, an additional, multicenter study should be performed to include a number of different pharmacies in order to corroborate the results. In addition, the level of abstinence was obtained by measuring CO in exhaled air. Elevated CO values can only be detected by the CO-oximeter if the measurement is taken within 24 hours of tobacco consumption. Some patients may have tried to cover up a relapse by stopping smoking 48 hours prior to the test. This also represents a limitation of our study.
Conclusion
Advertising a smoking cessation program in a pharmacy increases the number of patients who use the pharmacy’s smoking cessation service. Pharmaceutical care is an effective means of achieving smoking cessation, with total abstinence after 12 months observed in 43% of participating patients, representing an improvement over the results obtained in other health care settings. Given that smoking is a public health issue, pharmacy smoking cessation services should be systematically promoted at an institutional level in order to reduce the problems associated with smoking.
Supplementary material
Table S1 Fagerstrom test for nicotine dependence
Reference
- FagerstromKOSchneiderNGMeasuring nicotine dependence: a review of the Fagerstrom Tolerance QuestionnaireJ Behav Med19891221591822668531
Disclosure
The authors report no conflicts of interest in this work.
References
- BanegasJRDíez GañanLGonzález EnríquezJVillar AlvarezFRodríguez-ArtalejoFRecent decrease in smoking attributable mortality in SpainMed Clin (Barc)200512420769771 Spanish15927102
- International Union for Health Promotion and EducationThe Evidence of Health Promotion Effectiveness: A Report for the European Commission by the International Union for Health Promotion and Education Shaping Public Health in a New Europe. Report by International Union for Health Promotion and Education for the European Commission. Part Two. Evidence BookMadrid, Spain2000 Available from: http://www.iuhpe.org/images/PUBLICATIONS/THEMATIC/EFFECTIVENESS/HPE_Evidence-2_EN.pdfAccessed November 25, 2014
- Council for National Health ServiceFormation in Health Promotion and Education Report by the Working Group for the Promotion of Public Health of the Council for National Health ServiceMadrid, SpainMinistry of Health and Consumer Affairs2003
- RawMMcNeillAWestRSmoking cessation guidelines for health professionals. A guide to effective smoking cessation interventions for the health care system. Health education authorityThorax199853Suppl 5 Pt 1S1S19
- SinclairHKBondCMSteadLFCommunity pharmacy personnel interventions for smoking cessationCochrane Database Syst Rev20041CD00369814974031
- Forum of Pharmaceutical CareConsensus document 2008. MSC, RANF, CGCOF, SEFAP, SEFAC, SEFH, FPCE, GIAFUGMadrid, SpainForum of Pharmaceutical Care2008 Available from: http://www.portalfarma.com/inicio/serviciosprofesionales/forodeattfarma/Documents/FORO_At_farma.pdfAccessed February 24, 2010
- MachucaMFernández-LlimosFFausMJMétodo Dáder. Dader method. Guide to pharmaceutical monitoring. GIAF-UGRGranada2003
- MachucaMBaenaMIFausMJGuide to pharmaceutical indication. Pharmaceutical care investigation group(CTS-131) University of Granada and Abbott Fundation2005
- U.S. Department of Health and Human ServicesPublic Health ServiceClinical Practice Guideline: Treating Tobacco Use and DependenceWashington, DC2000
- JarvisMJRussellMASaloojeeYExpired air carbon monoxide: a simple breath of tobacco smoke intakeBr Med J198028162384844857427332
- ClarkKDWardrobe-WongNElliottJJGillPTTaitNPSnashallPDCigarette smoke inhalation and lung damage in smoking volunteersEur Respir J19981223953999727791
- ZacnyJPStitzerMLBrownFJYinglingJEGriffithsRRHuman cigarette smoking: effects of puff and inhalation parameters on smoke exposureJ Pharmacol Exp Ther198724025545643806411
- FagerstromKOSchneiderNGMeasuring nicotine dependence: a review of the Fagerstrom Tolerance QuestionnaireJ Behav Med19891221591822668531
- Torrecilla GarcíaMDominguez GrandalFTorres LanaARecomendaciones en el abordaje diagnostico y terapeutico del fumador. Documento de consensoMedifam200212484492 Spanish
- Pérez TrullenAClemente JiménezMLMorales BlánquezCTerapias psicologicas en la deshabituacion tabaquicaPsiquis2001226251263 Spanish
- Camarelles GuillemFSalvador LlivinaTRamón TorellJMConsensus on health assistance for smoking control in SpainRev Esp Salud Publica2009832175200 Spanish19626247
- SteadLFPereraRBullenCMantDLancasterTNicotine replacement therapy for smoking cessationCochrane Database Syst Rev20081CD00014618253970
- AubinHJKarilaLReynaudMPharmacotherapy for smoking cessation: present and futureCurr Pharm Des201117141343135021524268
- BarrisDFausMJAn initiation for Dader methodology of pharmaceutical monitoring in a community pharmacyArs Pharm2003443225237
- DíezEJuárezOVillamarínFHealth promotion interventions based on theoretical modelsMed Clin (Barc)20051255193197 Spanish16153363
- DentLAHarrisKJNoonanCWTobacco interventions delivered by pharmacists: a summary and systematic reviewPharmacotherapy20072771040105117594210
- SilagyCLancasterTSteadLMantDFowlerGNicotine replacement therapy for smoking cessationCochrane Database Syst Rev20043CD00014615266423
- BaixauliVJSalar IbáñezLBarbero GonzálezADemanda de información en al Farmacia ComunitariaPharmaceutical Care España200463136144 Spanish