Abstract
Little is known about the factors associated with patient compliance with nucleos(t)ide analog (NUC) treatment for chronic hepatitis B (CHB). The purpose of this study was to examine the association between sociodemographic and clinical characteristics and adherence to NUCs among patients with CHB. A total of 211 CHB patients receiving NUC monotherapy were asked to report the number of prescribed doses of medication they had taken during the last 90 days. A total of four 3-month adherence scores were averaged to obtain a combined rate of NUC adherence during a 1-year follow up period. The mean age of the patients was 29.6 years, 79% were men, and 68% had no prior NUC treatment for CHB. Females, patients without a previous NUC treatment, and those who had NUC drug resistance showed better adherence to NUC treatment, and compliance was better with telbivudine than with lamivudine and entecavir.
Introduction
Hepatitis B virus (HBV) infection affects approximately 400 million people worldwide.Citation1 In the People’s Republic of China, it is estimated that 20 million people suffer from chronic hepatitis B (CHB).Citation2 Five nucleos(t)ide analogs (NUCs) approved to treat CHB include lamivudine, telbivudine, entecavir, adefovir dipivoxil, and tenofovir disoproxil fumarate.Citation3 Although NUCs are potent medications that achieve long-term viral suppression, it is difficult to eradicate the virus.Citation4 A recent study indicated that adults with CHB need more than 2 years of treatment with NUCs to reduce the risk of cirrhosis, CHB-related death, and hepatocellular carcinoma.Citation5 In order to achieve virologic suppression and to avoid virologic breakthrough, medication adherence is very important.Citation6 However, a study conducted by Ha et alCitation7 found that nonadherence rates increased over time, with cumulative nonadherence rates at year 4 reaching 10% and 12% for patients taking adefovir and entecavir, respectively.
Optimal adherence to medication is necessary to achieve undetectable levels of HBV DNA. Consequently, the concern is that patients will not adhere to treatment over long periods and risk virologic failure.
Lieveld et alCitation8 searched PubMed, Embase, the Cochrane Library, and the Web of Knowledge for articles on compliance with NUCs in the treatment of CHB and only found six that met their criteria. Mean adherence to various NUC treatment protocols varied from 81% to 91%, as reported in three studies.Citation3,Citation4,Citation9 Two studies showed significant associations between good or perfect adherence and complete virologic suppression.Citation10,Citation11 Older age, a history of treatment for CHB using NUCs, use of NUCs other than lamivudine, and high income were shown to be associated with better NUC adherence.Citation3,Citation4,Citation9,Citation10 However, Giang et alCitation12 found no association between age and adherence, and researchers dispute whether sex or baseline viral load are predictors of adherence.Citation3,Citation4,Citation10–Citation12
We feel that further study is warranted to help clarify these disputes. In the current study, we examined factors associated with adherence to NUC treatment for CHB prospectively in a Chinese population. NUCs are well tolerated, with minimal side effects,Citation13–Citation16 and we controlled for the influence of cost by providing medications at no charge. We show that female sex, no previous NUC treatment, and a history of NUC resistance are factors that promote adherence to NUCs. The difference between our findings and previous reports is discussed.
Materials and methods
Participating patients
A total of 222 CHB patients between 17 and 56 years of age who were either hepatitis B e antigen (HBeAg) positive or negative were recruited from the Nan-Fang Hospital of the Southern Medical University. Among them eleven patients were excluded because of coinfection with hepatitis C, hepatitis D, or human immunodeficiency virus; evidence of hepatic decompensation; cirrhosis or hepatocellular carcinoma; autoimmune hepatitis; and pregnancy. Patients with renal dysfunction and chronic renal failure were also excluded from the study. Because tenofovir was not approved for treatment of hepatitis B in Mainland China during the study period, a total of 211 CHB patients received NUC monotherapy of telbivudine, lamivudine, entecavir, or adefovir dipivoxil. There were 31 patients who developed drug resistance to lamivudine prior to the current study.
Study design
The study was conducted between November 2002 and July 2008. The protocol was approved by the Institutional Review Board of the Nan-Fang Hospital of the Southern Medical University. All patients signed informed consent forms before participating in the study. They were randomly assigned to receive 600 mg of telbivudine, 100 mg of lamivudine, 0.5 mg of entecavir, or 10 mg of adefovir dipivoxil once daily as oral treatment. At enrollment, demographic characteristics (age and sex) were collected and height and body weight were assessed. Information such as current and previous HBV treatments, family history of CHB, and HBV markers were examined. Patients were asked to return for a follow-up visit every 3 months for 1 year to assess adherence to treatment. Pills were counted at each visit, and patients were asked about the numbers of tablets taken and missed in the last 90 days.
Adherence was measured by patient self-reports. The patients were asked how many prescribed doses of medication they had taken during the last 90 days. Because the rate of adherence is usually expressed as the percentage of prescribed dosages the patient consumed,Citation17 the rates of four periods were averaged for each participant.
Statistical analyses
The data analyses were done with SAS (version 9.2), and a P-value <0.05 was considered statistically significant. We used generalized estimating equations (GEEs)Citation18 to test the relationship of interest because GEEs have better control than other statistical measures for within-subject correlations over time as a result of multiple visits. In the GEE model, the dependent variable was the percentage of consumed dosages of medication prescribed (adherence to NUCs), and the independent variables were age, sex, HBeAg status, types of NUC medication (telbivudine, lamivudine, entecavir, or adefovir dipivoxil), history of NUC treatment, and history of drug resistance.
Results
Sociodemographic and clinical characteristics of the study patients
outlines the baseline characteristics of all patients. A total of 211 CHB patients who participated in the NUC monotherapy were included in the analysis. Of the 211 patients, the mean age was 29.6±7.8 years. Overall, 137 (65%) of the 211 patients were positive for HBeAg. A total of 143 (68%) patients were NUC-naïve, and 68 (32%) patients had received prior NUC treatment. There were 63 (30%), 27 (13%), 20 (9%), and 101 (48%) patients taking telbivudine, lamivudine, entecavir, and adefovir, respectively.
Table 1 Characteristics of the study patients (N=211)
Factors associated with adherence
Results of the regression analysis using GEEs are presented in . Female sex (β=0.96, P<0.001), the absence of prior NUC treatment (β=1.19, P<0.01), and a history of NUC drug resistance were significantly associated with better adherence to NUCs (β =2.07, P<0.001). Further analysis showed the adherence rate of NUC-naïve patients was 1.28% higher than of those with a history of NUC treatment. Compared to patients taking telbivudine, those who took lamivudine or entecavir showed worse adherence (β=−1.21, P=0.02; β=−1.05, P=0.01, respectively). Although adherence to adefovir was lower than to telbivudine, the difference did not achieve statistical significance (β=−0.29, P=0.31). shows the mean adherence rates across the four time periods (12, 24, 36, 48 weeks) separately for the four different NUC medications.
Figure 1 Mean adherence rates across the four time periods of the four different NUC medications.
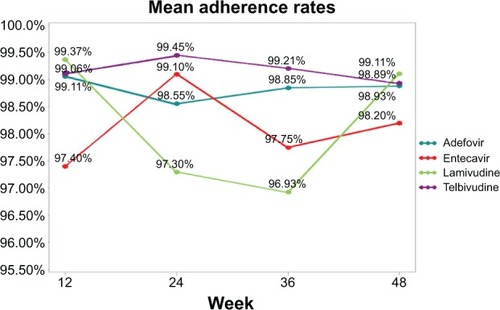
Table 2 Estimates from GEE models of indicators of adherence to NUC for CHB patients
Discussion
Although NUCs have been shown to be efficacious in the treatment of CHB,Citation19,Citation20 the concern remains that nonadherence may lead to treatment failure.Citation11 With one out of 20 CHB sufferers worldwide living in the People’s Republic of China and drugs available to treat the disease, it is important to determine the factors associated with adherence in this population.Citation1 Here we report that female sex, the absence of previous NUC treatment, and a history of NUC drug resistance are factors associated with greater NUC adherence, and patients adhere to telbivudine better than to lamivudine and entecavir.
In studies of adherence to antihypertensive medications, it was found that female patients have higher medication adherence rates than males.Citation21,Citation22 However, according to a systematic review on the factors affecting therapeutic compliance, inconsistent results have been obtained regarding sex-related adherence.Citation23 Chotiyaputta et alCitation3,Citation4 reported that men and women did not differ in adherence rates. The reasons for the disagreement between their findings and our results are not known. Possible contributing factors are different ages, cultural and educational background, and household income level. Moreover, in the studies by Chotiyaputta et al there were near perfect adherence rates, which might be inflated in the patients’ self-reporting to health providers.Citation3,Citation4 This high rate may have some masking effect on the actual adherence rate. Another possible factor is that we provided free medicines to the participating patients. In future studies, these factors need to be considered and carefully controlled.
Chotiyaputta et alCitation4 suggested that the history of HBV treatment was not correlated with adherence. However, we found that new patients without a NUC treatment history showed better adherence rates than previously treated patients. This result is in agree with a report by Sevim et alCitation24 who found that tuberculosis patients with a history of treatment had less adherence than new patients. Again, the variances between different studies may be explained by the different geographical and cultural background, the level of medical education, and the different experimental designs and strategies in different studies.
It has been suggested that adherence increases with increasing ageCitation25,Citation26 and that the elderly (over 55 years old) might have higher adherence rates.Citation3,Citation4,Citation9,Citation27,Citation28 In contrast, we found that age was not a factor affecting adherence to NUCs. Participating patients in our study had younger average age and smaller age range (17–56 years, with only one case over 55 years) than those of other studies that included larger numbers of elderly patients.Citation4,Citation9 Thus, it is possible that in our study, the age range is too small to reveal a difference in NUC adherence in different age groups.
In spite of the widely acknowledged notion that side effects are associated with nonadherence for many drugs,Citation29,Citation30 the four NUCs have minimal side effects and have similar adverse events records.Citation13–Citation16 Nevertheless, we found that lamivudine and entecavir were less adhered to than telbivudine. Therefore, the different adherence rates of different NUCs may not be attributable to their side effects. Our results partially agree with those of Chotiyaputta et alCitation4 who showed that lamivudine was less adhered to than other NUCs. The mechanisms of this difference remain to be elucidated.
We report for the first time that a history of NUC resistance conferred better adherence. Thus, it appears that patients with previous NUC treatment are less adherent to NUCs in the absence of drug resistance, but are better adherent when resistance is developed. It should be mentioned that our data only show the influence of resistance to lamivudine on adherence. Whether resistance to other NUCs has a similar impact on adherence remains to be investigated.
Results are greatly diversified in studies of factors associated with NUC adherence. The diversity may be explained by the differences in experimental designs and strategies; different study population; different cultural, educational, and economic background; and different definitions of “good adherence rate.” Some studies used the most stringent method of pill counts to assess adherence and reported low adherence rate,Citation3,Citation4,Citation8–Citation10,Citation12,Citation31 while in other studies, physician assessment or patient self-reporting to health providers adopted less stringent methods and may have led to overestimation of adherence and inadequate recognition of poor adherence.Citation4,Citation7
Our study used a moderately stringent method. We found a less than perfect rate of adherence, and the assessment scores are less likely to be inflated. In addition, a study by Giang et alCitation12 showed that NUC adherence is vulnerable to a variety of factors, such as forgetfulness, change of routine, being too busy, etc. Therefore, application of the results of NUC adherence investigations has certain geographical limitations and should be taken into consideration by doctors in different parts of the world. We did not collect disease severity data in this study. However, all of the patients had CHB and those with hepatic dysfunction, liver cirrhosis, and liver cancer were excluded. For determination of disease severity, the most objective standard is liver biopsy, and unfortunately, our patients did not have liver biopsy before treatment.
In conclusion, our data suggest that in the People’s Republic of China, female sex, no previous NUC treatment, and NUC resistance are factors associated with adherence to NUCs for CHB treatment. We report that CHB patients had better adherence to telbivudine than lamivudine and entecavir. To our knowledge, this is the first report to have investigated the factors associated with adherence to NUCs in CHB treatment in the People’s Republic of China, and the first to show NUC resistance as a factor associated with NUC adherence. Our findings should potentially benefit CHB patients’ through better control of adherence to NUCs and thus improve the treatment outcomes.
Conclusion
Key messages
Optimal adherence to NUC medications is necessary to achieve undetectable levels of HBV DNA in patients with CHB and is necessary to prevent the sequelae of liver cirrhosis and hepatocellular carcinoma common in this disease.
The factors associated with adherence to treatment regimens remain unclear.
We found that adherent patients were more likely to be female and were more likely to be NUC-naïve.
With approximately 400 million patients worldwide with CHB, it is important to understand the factors associated with medication adherence in order to be able to assist patients in improving their adherence rates.
Disclosure
The authors report no conflicts of interest in this work.
References
- KoseSTurkenMCavdarGAkkocluGThe effectiveness of nucleoside analogues in chronic hepatitis B patients unresponsive to interferon therapy: our clinical trials for one yearHepat Mon201010428929322312395
- LiangXBiSYangWEpidemiological serosurvey of hepatitis B in China – declining HBV prevalence due to hepatitis B vaccinationVaccine200927476550655719729084
- ChotiyaputtaWPetersonCDitahFAGoodwinDLokASPersistence and adherence to nucleos(t)ide analogue treatment for chronic hepatitis BJ Hepatol2011541121820888661
- ChotiyaputtaWHongthanakornCOberhelmanKFontanaRJLicariTLokASAdherence to nucleos(t)ide analogues for chronic hepatitis B in clinical practice and correlation with virological breakthroughsJ Viral Hepat201219320521222329375
- ZhangQQAnXLiuYHLong-term nucleos(t)ide analogues therapy for adults with chronic hepatitis B reduces the risk of long-term complications: a meta-analysisVirol J201187221324130
- HongthanakornCChotiyaputtaWOberhelmanKVirological breakthrough and resistance in patients with chronic hepatitis B receiving nucleos(t)ide analogues in clinical practiceHepatology20115361854186321618260
- HaNBHaNBGarciaRTMedication nonadherence with long-term management of patients with hepatitis B e antigen-negative chronic hepatitis BDig Dis Sci20115682423243121327918
- LieveldFIvan VlerkenLGSiersemaPDvan ErpecumKJPatient adherence to antiviral treatment for chronic hepatitis B and C: a systematic reviewAnn Hepatol201312338039123619254
- BergATBerkovicSFBrodieMJRevised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009Epilepsia201051467668520196795
- HilleretMNLarratSStanke-LabesqueFLeroyVDoes adherence to hepatitis B antiviral treatment correlate with virological response and risk of breakthrough?J Hepatol201155614681469 author reply 146921703170
- SogniPCarrieriMPFontaineHThe role of adherence in virological suppression in patients receiving anti-HBV analoguesAntivir Ther201217239540022293326
- GiangLSelingerCPLeeAUEvaluation of adherence to oral antiviral hepatitis B treatment using structured questionnairesWorld J Hepatol201242434922400085
- KhungarVHanSHA systematic review of side effects of nucleoside and nucleotide drugs used for treatment of chronic hepatitis BCurr Hepatitis Rep2010927590
- LiawYFRaptopoulou-GigiMCheinquerHEfficacy and safety of entecavir versus adefovir in chronic hepatitis B patients with hepatic decompensation: a randomized, open-label studyHepatology20115419110021503940
- SuQMYeXGEffects of telbivudine and entecavir for HBeAg-positive chronic hepatitis B: a meta-analysisWorld J Gastroenterol201218436290630123180951
- JiangHWangJZhaoWLamivudine versus telbivudine in the treatment of chronic hepatitis B: a systematic review and meta-analysisEur J Clin Microbiol Infect Dis2013321111822898727
- OsterbergLBlaschkeTAdherence to medicationN Engl J Med2005353548749716079372
- LiangKYZegerSLLongitudinal data analysis using generalized linear modelsBiometrika19867311322
- LaiCLGaneELiawYFGlobe Study GroupTelbivudine versus lamivudine in patients with chronic hepatitis BN Engl J Med2007357252576258818094378
- WiensALenziLVensonRComparative efficacy of oral nucleoside or nucleotide analog monotherapy used in chronic hepatitis B: a mixed-treatment comparison meta-analysisPharmacotherapy201333214415123359454
- Choi-KwonSKwonSUKimJSCompliance with risk factor modification: early-onset versus late-onset stroke patientsEur Neurol200554420421116401893
- FodorGJKotrecMBacskaiKIs interview a reliable method to verify the compliance with antihypertensive therapy? An international central-European studyJ Hypertens20052361261126615894903
- JinJSklarGEMin Sen OhVChuen LiSFactors affecting therapeutic compliance: a review from the patient’s perspectiveTher Clin Risk Manag20084126928618728716
- SevimTAksoyEAtaçGTreatment adherence of 717 patients with tuberculosis in a social security system hospital in Istanbul, TurkeyInt J Tuberc Lung Dis200261253111931397
- CaspardHChanAKWalkerAMCompliance with a statin treatment in a usual-care setting: retrospective database analysis over 3 years after treatment initiation in health maintenance organization enrollees with dyslipidemiaClin Ther200527101639164616330301
- LacasseYArchibaldHErnstPBouletLPPatterns and determinants of compliance with inhaled steroids in adults with asthmaCan Respir J200512421121716003458
- SeniorVMarteauTMWeinmanJGenetic Risk Assessment for FH Trial (GRAFT) Study GroupSelf-reported adherence to cholesterol-lowering medication in patients with familial hypercholesterolaemia: the role of illness perceptionsCardiovasc Drugs Ther200418647548115770435
- HertzRPUngerANLustikMBAdherence with pharmacotherapy for type 2 diabetes: a retrospective cohort study of adults with employer-sponsored health insuranceClin Ther20052771064107316154485
- HovingaCAAsatoMRManjunathRAssociation of non-adherence to antiepileptic drugs and seizures, quality of life, and productivity: survey of patients with epilepsy and physiciansEpilepsy Behav200813231632218472303
- HoPMBrysonCLRumsfeldJSMedication adherence: its importance in cardiovascular outcomesCirculation2009119233028303519528344
- LeifeldJAngersDAChenuCFuhrerJKättererTPowlsonDSOrganic farming gives no climate change benefit through soil carbon sequestrationProc Natl Acad Sci U S A201311011E98423431207
