Abstract
Background
Low adherence to pharmacological treatments is one of the factors associated with poor blood pressure control. Questionnaires are an indirect measurement method that is both economic and easy to use. However, questionnaires should meet specific criteria, to minimize error and ensure reproducibility of results. Numerous studies have been conducted to design questionnaires that quantify adherence to pharmacological antihypertensive treatments. Nevertheless, it is unknown whether questionnaires fulfil the minimum requirements of validity and reliability. The aim of this study was to compile validated questionnaires measuring adherence to pharmacological antihypertensive treatments that had at least one measure of validity and one measure of reliability.
Methods
A literature search was undertaken in PubMed, the Excerpta Medica Database (EMBASE), and the Latin American and Caribbean Health Sciences Literature database (Literatura Latino-Americana e do Caribe em Ciências da Saúde [LILACS]). References from included articles were hand-searched. The included papers were all that were published in English, French, Portuguese, and Spanish from the beginning of the database’s indexing until July 8, 2013, where a validation of a questionnaire (at least one demonstration of the validity and at least one of reliability) was performed to measure adherence to antihypertensive pharmacological treatments.
Results
A total of 234 potential papers were identified in the electronic database search; of these, 12 met the eligibility criteria. Within these 12 papers, six questionnaires were validated: the Morisky–Green–Levine; Brief Medication Questionnaire; Hill-Bone Compliance to High Blood Pressure Therapy Scale; Morisky Medication Adherence Scale; Treatment Adherence Questionnaire for Patients with Hypertension (TAQPH); and Martín–Bayarre–Grau. Questionnaire length ranged from four to 28 items. Internal consistency, assessed by Cronbach’s α, varied from 0.43 to 0.889. Additional statistical techniques utilized to assess the psychometric properties of the questionnaires varied greatly across studies.
Conclusion
At this stage, none of the six questionnaires included could be considered a gold standard. However, this revision will assist health professionals in the selection of the most appropriate tool for their individual circumstances.
Introduction
Hypertension is a major public health concern worldwide. It is one of the leading risk factors for cardiovascular disease and is associated with a decrease in a patient’s quality of life and an increase in the probability of health complications.Citation1 An estimated 14% of all deaths worldwide are attributable to high blood pressure (systolic blood pressure >140 mmHg; diastolic blood pressure >90 mmHg).Citation2 In Spain, 54% of cardiovascular-related deaths in people older than 50 years are due to hypertension.Citation3
It is widely accepted that there are relationships between poor blood pressure control and a patient’s lack of adherence to antihypertensive treatment, as well as lack of effectiveness to antihypertensive treatments.Citation4–Citation8 Research has been conducted in an attempt to reduce nonadherence.Citation6,Citation8,Citation9 In spite of this, a study showed the mean prevalence of nonadherence to antihypertensive treatments to be 30%.Citation10
Several terms are found in biomedical literature to explain the degree to which patients follow prescribed medication directions, including the terms “adherence” and “compliance”. Haynes et al defined “compliance” in 1979 as the extent to which a person’s behavior (in terms of taking medications, following diets or executing lifestyle changes) coincides with medical care or health advice; thus, noncompliance is the extent to which these instructions are not accomplished.Citation11 The World Health Organization (WHO) combined the definitions developed by Haynes et al with one by RandCitation12 to obtain their definition of adherence to long-term treatment as
the extent to which a person’s behavior (in terms of taking medications, following diets or executing lifestyle changes) corresponds to the recommendations agreed by a health care provider.Citation13
Later, Osterberg and Blaschke stated the term “adherence”
… is preferred by many health care providers, because compliance suggests that the patient is passively following the doctor’s orders and that the treatment plan is not based on a therapeutic alliance or contract established between the patient and the physician.Citation14
Regardless of the definition used, health discipline, or health problem, measuring adherence has been complicated. Both direct (biological measures) and indirect (pill counts, patient-kept diaries of medication-taking, and questionnaires) methods have been used.Citation14,Citation15 Biological measures are considered a gold standard due to their objectivity, but their high cost is prohibitive. On the other hand, pill counts are simple, but they do not guarantee patient collaboration, and as such, results of pill counts may be inaccurate. Questionnaires are an alternative. Even though questionnaires have the disadvantage of overestimating patient adherence or nonadherence,Citation14 their advantage is they are easy to use and are relatively inexpensive.Citation16 Moreover, questionnaires are the most common method used in clinical settings because they have the ability to provide information about a patient’s reasons for not adhering to prescribed treatments.Citation14 A number of scales of adherence to antihypertensive medication have been developed, but a questionnaire considered to be a gold standard does not exist. The most widely usedCitation17–Citation20 questionnaire is that designed by Morisky–Green–Levine (MGL)Citation21 in 1986. This is a unidimensional questionnaire containing four items.
In order to be a useful tool, a questionnaire must be valid and reliable.Citation22 Scale validity refers to
the degree of confidence we can have that the measurement corresponds to the reality of the phenomenon that is being measured,Citation23
the degree to which an instrument measures what it is supposed to measure.Citation24
Whereas reliability is
the extent to which a measure yields the same number or score each time it is administered when the construct being measured has not changed.Citation25
A number of questionnaires used in daily clinical practice do not reach the minimum standards for validity and reliability.Citation26 Given this, it is necessary to compile an exhaustive list of the available validated tools measuring adherence to pharmacological antihypertensive treatment.
The aim of this work was to compile the questionnaires designed to measure adherence to pharmacological antihypertensive treatment that have at least one test of validity and one test of reliability.
Materials and methods
A literature search was undertaken in three electronic databases: US National Library of Medicine (PubMed), Excerpta Medica Database (EMBASE), and the Latin American and Caribbean Health Sciences Literature database (Literatura Latino-Americana e do Caribe em Ciências da Saúde [LILACS]). The search terms were: “patient adherence”, “patient compliance [Medical Subject Headings {MeSH}]”, “compliance”, “predictive validity”, “content validity”, “concurrent validity”, “convergent validity”, “discriminant validity”, “construct validity”, “psychometric properties”, “clinimetric properties”, “test-retest reliability”, “temporal stability”, “interobserver agreement”, “internal homogeneity”, “internal consistency”, “questionnaires [MeSH]”, “reproducibility of results [MeSH]”, “cronbach’s alpha”, and “hypertension [MeSH]”. Keywords were truncated in the LILACS database to avoid the loss of papers that could be of interest. In addition, the search was complemented by reference lists from the included papers.
Inclusion criteria
Articles needed to include the validation of a questionnaire to measure patient adherence to pharmacological antihypertensive treatments, published from the beginning of the database’s indexing until July 8, 2013. The articles had to include at least one validity test (content-, construct-, or criterion-related) and one reliability test (stability, equivalence, or homogeneity) of the questionnaire. Language limits were English, Spanish, French, and Portuguese.
Article selection
Duplications were removed. Two independent authors read the titles and abstracts to select the articles that met the inclusion criteria. If doubts appeared, the whole article was read. If there was disagreement, a third author arbitrated the debate between the two first authors.
Data extraction
Two authors extracted data independently. These data referred to the characteristics of the studied population and to the psychometric properties of the questionnaire being tested.Citation26 Afterwards, extracted data was checked for any disagreement. When there was disagreement, the third author resolved the conflict.
Results
A total 234 articles were retrieved. Three were not available. From the remaining 231, 52 were duplicated. Of these,104 articles were removed by title, 46 by abstract, and finally, 17 were removed after reading the whole article. Ultimately 12 articles were included in the revision ().
Figure 1 Flow of information through the different phases of the systematic review.
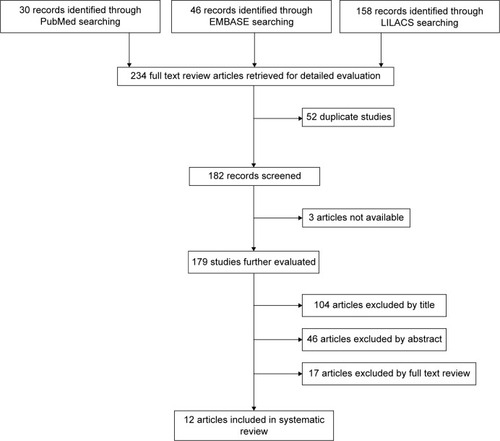
Articles were removed if they did not meet the inclusion criteria, that is, they did not validate a questionnaire for measuring adherence to antihypertensive treatments or demonstrate the required psychometric properties. For example, some articles did not measure treatment adherence but rather, reported the patients’ understanding about hypertension treatment, while others measured quality of life in hypertensive patients, patient knowledge about the disease, or patient satisfaction with their medication. Furthermore, there were articles that measured adherence in diseases other than hypertension and in other fields, such as nutrition. Articles in which adherence was the subject but measured related factors (aptitudes, beliefs, etc) instead of classifying patients as adherent or nonadherent, were similarly eliminated.
The 12 articles selected included 15 validation processes for six questionnaires that measured adherence to pharmacological antihypertensive treatments (). The questionnaires were the following:
– MGL (two validations)Citation21,Citation27
– Brief Medication Questionnaire (BMQ) (one validation)Citation27
– Hill-Bone Compliance to High Blood Pressure Therapy Scale (HB Comp Scale) (six validations)Citation7,Citation28–Citation30
– Morisky Medication Adherence Scale (MMAS-8) (three validations)Citation31–Citation33
– Treatment Adherence Questionnaire for Patients with Hypertension (TAQPH) (one validation)Citation34
– Martín–Bayarre–Grau (MBG) (two validations).Citation35,Citation36
Table 1 Characteristics of the articles included
Content validity
Content validity was assessed in four questionnaires: HB Comp Scale,Citation28 TAQPH,Citation34 MMAS-8 (Urdu version), Citation31 and MBG (validated in Cuba).Citation35 In all four validation studies, the authors used an expert panel. In addition, for item generation, two questionnaires (HB Comp Scale and TAQPH) included a narrative review, and one (TAQPH) performed a focus group.
Construct validity
Construct validity was assessed in eleven of 15 validation processes (). The Urdu version of the MMAS-8Citation33 evaluated both convergent validity, by comparing the results to the MGL,Citation21 and known group validity, comparing the results of the questionnaire to blood pressure control. The remaining construct validity assessments were performed by factor analysis. In the MGL,Citation21 HB Comp Scale,Citation28 Hill-Bone Medication Adherence-Korean version (HBMA-K) scale (a validation of HB Comp Scale),Citation30 and MMAS-8 (EnglishCitation32 and FrenchCitation31 versions) the factor analysis yielded a one-dimensional solution. On the other hand, the TAQPHCitation34 questionnaire yielded six factors, and the Colombian MBGCitation36 yielded five.
Table 2 Validity and reliability assessment
Total variance ranged from 68.7%, in the MBG validated in Cuba,Citation35 to 27%, in the HB Comp Scale validated in young African American males.Citation28
Criterion validity
Four validations (MGL,Citation21 HBMA-K,Citation30 MMAS-8Citation32 (English), and TAQPHCitation34) assessed concurrent validity, and six evaluated predictive validity (MGL,Citation21 Xhosa HB Comp Scale,Citation29 HB Comp ScaleCitation28 [two validations], and the MMAS-8Citation32 [English]). The comparison tests are detailed in .
Reliability
Cronbach’s α was used to estimate internal consistency as a reliability measure in every validation. The MBG validated in CubaCitation35 obtained the highest coefficient (α=0.889), while the lowest (α=0.43) was obtained in the HB Comp ScaleCitation7 validated in an elderly population, published by Krousel-Wood et al. Item-total scale correlation was also used in all but three validations ().
The MMAS scale in UrduCitation33 and French,Citation31 the TAQPH,Citation34 and the BMQCitation27 evaluated temporal stability with the test–retest method. In each of these validations, temporal stability was demonstrated with a moderate to high correlation, except for the TAQPHCitation34 questionnaire, which had an intraclass correlation coefficient of 0.82.
Discussion
This review provides health professionals with a report summarizing the evidence across different questionnaires according to the psychometric properties evaluated. The review did not intend to determine or indicate an optimum questionnaire but rather, to compile validated scales that measure antihypertensive treatment adherence. Consequently, health professionals can choose the most appropriate questionnaire, depending on their circumstances. To our knowledge there is no similar published study.
A large number of authors have designed questionnaires in order to measure adherence to antihypertensive treatments. Nevertheless, no questionnaire could be considered a gold standard. Any tool to be used as a measure must demonstrate validity and reliability, including questionnaires.Citation22 Furthermore, validity and reliability must be measured in each sample or have been conducted in a comparable sample since it is not possible to extrapolate results among different populations. Differences in validity and reliability results obtained across versions of the MMAS-8Citation31–Citation33 can be seen as an example.
A sensitive search strategy was designed to ensure the collection of all papers validating questionnaires to measure adherence to pharmacological antihypertensive treatment. Inclusion criteria in this review required that articles have at least one validity test and one of reliability. Taking into account these criteria, such well-known questionnaires as the BMQ (English version),Citation44 the Haynes–SackettCitation11 and Batalla questionnaires,Citation45 etc were not included.
The validity of a questionnaire (the confirmation that it measures what it is supposed to measure) is evaluated in several ways, as: content validity, construct validity, and criterion validity.
The methods most commonly used to assess content validity are expert opinion and systematic review. However, researchers often choose only one of these options. From the 15 validations found, only four studied content validity, and even these did not explain in detail the process followed to assess it. It is suspected that in the remaining questionnaires, authors assumed, without testing, content validity was present. In the four validations that assessed content validity, the technique utilized was an expert panel. None of the authors based content validity on a systematic review. This method could be of great interest, but it can increase costs and research time.
The most common technique to assess construct validity was factor analysis. A one-dimensional solution emerged in the majority of the scales, but the factor explained only a small percentage of the total response variability. As an example, the HB Comp ScaleCitation28 and the HBMA-KCitation30 explained up to 35% of the total variance. Consequently questionnaires should consider including further dimensions and items to explain more of the total response variance. This could be observed in the TAQPH,Citation34 which had 28 items and six factors, and was able to explain 62.5% of the variance. The total explained variance is considered an indicator of how factors extracted from a questionnaire actually correspond to patient answers. When little variance is explained, the scale shows a deficiency that indicates lack of variables or a poor initial theoretical construct. However, it is also important that a questionnaire does not have too many items, to minimize patient resistance to being interviewed and interview duration. As such, the number of items needs to be balanced between explaining variance and user acceptance.
Construct validity assessment by convergent validity was completed in two questionnaires. The correlation between the Urdu version of the MMAS-8Citation33 and the same language version of the MGLCitation21 was checked. A high correlation was found between the scales (ρ=0.765, P<0.001), which in theory confirmed that both questionnaires measured the same construct. However such a correlation is not surprising as the MMAS-8 was based on the MGL four-item questionnaire, onto which more questions were added.Citation32
Criterion validity is traditionally defined as
the correlation of a scale with some other measure of the trait or disorder under study, ideally a gold standard which has been used and accepted in the field.Citation46
Criterion validity consists of two types: concurrent and predictive. In concurrent validity, both the new scale and a criterion measure are applied simultaneously and subsequently correlated. On the other hand, when using predictive validity, the criterion measure will not be available until sometime in the future. This implies that testing predictive validity may extend the time of study.
With the exception of six validations, the remainder tested criterion validity. The validation of HBMA-KCitation30 obtained a low correlation to systolic blood pressure control (r=0.18; P<0.01) and diastolic blood pressure (r=0.24; P<0.01). This low correlation could be due to the fact that medication adherence is not perfectly correlated to blood pressure control. That is, it is possible for a patient to have high medication adherence and to not have controlled blood pressure due to another factor, such as lack of effectiveness of the treatment. Nevertheless, it is assumed that there is a direct relationship between adherence and blood pressure control.Citation47 On the other hand, the MMAS-8,Citation32 compared with the MGLCitation21 to assess concurrent validity, obtained a moderate to high correlation (r=0.64; P>0.05). It is necessary to keep in mind that the development of the MMAS-8 was based on the MGL, as stated previously.
In addition to verifying the validity of a questionnaire, it is necessary to check the results are reproducible. That is, it is necessary to assess its reliability. From a technical point of view, it could be said that reliability is
a ratio of the variability between individuals to the total variability in the scores; in other words, the reliability is a measure of the proportion of the variability in scores which was due to true differences between individuals.Citation46
One of the methods used to measure reliability is internal consistency. Internal consistency describes reliability estimations based on the average correlation among items in a test.Citation46 A Cronbach’s α value is considered acceptable when above 0.70.Citation48 Only two validations obtained Cronbach’s α values well below 0.70 (the HB Comp Scale [elderly]Citation7 [0.43] and MMAS-8 [French]Citation31 [0.54]), indicating the items were not highly related. In the remaining questionnaires, Cronbach’s α varied from 0.61 (in the MGL)Citation21 to 0.889 (in the MGB).Citation35 However, it should be noted that Cronbach’s α is sensitive to the number of items and the sample’s variance.
Cronbach’s α is assessed from a single administration of the test, not taking into account variations in time or between administrators, and as such, it can provide an optimistic interpretation of the reliability of a questionnaire. For this reason, it is advisable that internal consistency is accompanied by a stability measure (test–retest) or by interobserver equivalence.Citation46 In the present study, six validations provided stability data and none checked interobserver verifications.
As previously stated, the intent of this article was to gather all questionnaires measuring patient adherence to antihypertensive treatments that contain at least one validity measure and one reliability measure. Furthermore the authors do not consider any of the questionnaires to have sufficient reliability or validity to be highly recommended. Nevertheless, if one had to choose a questionnaire, the authors offer these considerations.
For research, the TAQPH may be advisable. The TAQPH contains the greatest number of items, potentially providing more information to the research investigation, explains a high percentage of variance, and has sound indicators for both stability and criterion validity. The MBG also contains a large number of items but cannot be endorsed as it lacks criterion validity, stability, and item-total correlations.
In clinical practice it is necessary that a questionnaire is acceptable to both health care professionals and patients (containing the fewest possible items), is quick and easy to use, and shows good sensitivity, specificity, positive predictive value, and negative predictive value. The MGL, BMQ, HB Comp Scale, and MMAS-8 have similar indicators of reliability and validity; however, only the MMAS-8 and MGL show the probability of a clinician deciding that a non-adherent patient is not controlled (positive predictive value) or that an adherent patient is controlled (negative predictive value). As such, in clinical practice, both the MMAS-8 and MGL may be recommended. However as previously noted, the MMAS-8 was developed by Morisky et al in 2008Citation32 based on the MGL and as such, has an improved capacity to collect information (by having four additional items), sensitivity, specificity, positive predictive value, and negative predictive value, while maintaining acceptable validity and reliability. Consequently in clinical practice, the MMAS-8 may be an appealing option.
Limitations
This study could be influenced by publication bias as a consequence of the general tendency to publish only positive results. Language bias could also have appeared. In spite of including studies published in four languages, it is possible that some studies were not included because of being published in other languages. Moreover, despite the strict methodology followed by reviewers, it is possible that selection bias appeared as a consequence of the lack of availability of some articles.
Conclusion
This review provides information of great relevance to daily clinical practice. In spite of the number of studies performed to measure adherence, not all of the questionnaires used in the studies reach the requirements to be considered valid and reliable tools. Six questionnaires were identified to measure adherence to pharmacological antihypertensive treatment that had at least one validity test and one test of reliability. While some of these questionnaires had evidence to demonstrate acceptable validity, they failed in reliability and vice versa. Therefore although none of the questionnaires can be considered as a gold standard, this revision will assist health professionals in the selection of the most appropriate tool, depending on their circumstances. In the future, the design and validation of a questionnaire to measure adherence to antihypertensive treatments reaching the following requirements is fundamental: a) to be succinct enough to avoid patient and/or administrator fatigue (acceptability) but comprehensive enough to explain variance; b) include explicit content validity, have construct validity in accordance with a logical and reasoned theoretical structure that justifies items and subscales, and/or demonstrate criterion validity and; c) be a tool that provides reproducible results (reliable).
Disclosure
The authors report no conflicts of interest in this work.
References
- CôtéIMoisanJChabotIGrégoireJPHealth-related quality of life in hypertension: impact of a pharmacy intervention programmeJ Clin Pharm Ther200530435536215985049
- HuxJEIvisFFlintoftVBicaADiabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithmDiabetes Care200225351251611874939
- Gascón CánovasJJSaturno HernándezPJLlor EstebanBGrupo de Investigación del Proyecto EMCA sobre Evaluación y Mejora de la Adhesión Terapéutica en la Hipertensión. [Evaluation and improvement of therapy adherence of hypertensive patients]Aten Primaria2001289615619 Spanish11747776
- BerrySDQuachLProcter-GrayEPoor adherence to medications may be associated with fallsJ Gerontol A Biol Sci Med Sci201065555355820231214
- Espinosa GarciaJMartell ClarosNLlerena RuizAFernández Bergés GurreaDPharmacological compliance in the treatment of arterial hypertension. A review of studies published between 1975 and 2011Semergen2012385292300 Spanish23544776
- Fikri-BenbrahimNFausMJMartínez-MartínezFSabater-HernándezDImpact of a community pharmacists’ hypertension-care service on medication adherence. The AFenPA studyRes Social Adm Pharm20139679780523391845
- Krousel-WoodMMuntnerPJannuADesalvoKReRNReliability of a medication adherence measure in an outpatient settingAm J Med Sci2005330312813316174996
- SchroederKFaheyTEbrahimSInterventions for improving adherence to treatment in patients with high blood pressure in ambulatory settingsCochrane Database Syst Rev20042CD00480415106262
- JingSNaliboffAKaufmanMBChoyMDescriptive analysis of mail interventions with physicians and patients to improve adherence with antihypertensive and antidiabetic medications in a mixed-model managed care organization of commercial and Medicare membersJ Manag Care Pharm201117535536621657805
- CramerJABenedictAMuszbekNKeskinaslanAKhanZMThe significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a reviewInt J Clin Pract2008621768717983433
- HaynesRBTaylorDLSackettDCompliance in Health CareBaltimore, MDJohn Hopkins University Press1979
- RandCSMeasuring adherence with therapy for chronic diseases: implications for the treatment of heterozygous familial hypercholesterolemiaAm J Cardiol1993721068D74D
- World Health OrganizationAdherencia a los Tratamientos a Largo Plazo. [Adherence to long-term therapies]GenevaWorld Health Organization2004 Available from: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&gid=18722&Itemid=>Accessed, July 2, 2013 Spanish
- OsterbergLBlaschkeTAdherence to medicationN Engl J Med2005353548749716079372
- VermeireEHearnshawHVan RoyenPDenekensJPatient adherence to treatment: three decades of research. A comprehensive reviewJ Clin Pharm Ther200126533134211679023
- GozumSHacihasanogluRReliability and validity of the Turkish adaptation of medication adherence self-efficacy scale in hypertensive patientsEur J Cardiovasc Nurs20098212913619046930
- Gutiérrez-AnguloMLLopetegi-UrangaPSánchez-MartinIGaraigordobil-LandazabalMTherapeutic compliance in patients with arterial hypertension and type 2 diabetes mellitusRev Calid Asist20122727277 Spanish22138203
- Amado GuiradoEPujol RiberaEPacheco HuergoVBorrasJMADIEHTA GroupKnowledge and adherence to antihypertensive therapy in primary care: results of a randomized trialGac Sanit2011251626721354671
- GascónJJSánchez-OrtuñoMLlorBSkidmoreDSaturnoPJTreatment Compliance in Hypertension Study GroupWhy hypertensive patients do not comply with the treatment: results from a qualitative studyFam Pract200421212513015020377
- Morales Suárez-VarelaMTGEMECORStudy on the use of a smart pillbox to improve treatment complianceAten Primaria2009414185191 Spanish19328598
- MoriskyDEGreenLWLevineDMConcurrent and predictive validity of a self-reported measure of medication adherenceMed Care198624167743945130
- LampreaJAMGómez RestrepoCValidez en la evaluación de escalas [Validity in scale-testing]Rev Colomb Psiquiat2007362340348
- Sánchez PedrazaRGómez RestrepoCIConceptos básicos sobre validación de escalas. [Basic concepts on validation]Rev Colomb Psiquiat1998272121130 Spanish
- PolitDFBeckCTAssessing Data QualityNursing Research Principles and Methods7th edPhiladelphia, PALippincott Williams & Wilkins1995413448
- FrostMHReeveBBLiepaAMStaufferJWHaysRDMayo/FDA Patient-Reported Outcomes Consensus Meeting GroupWhat is sufficient evidence for the reliability and validity of patient-reported outcome measures?Value Health200710Suppl 2S94S10517995479
- NunnallyJCBernsteinIHTeoría Psicométrica [Psychometric theory]3rd edMexico DFMcGraw-Hill Inc1995 Spanish
- BenAJNeumannCRMengueSSThe Brief Medication Questionnaire and Morisky-Green test to evaluate medication adherenceRev Saude Publica201246227928922331180
- KimMTHillMNBoneLRLevineDMDevelopment and testing of the Hill-Bone Compliance to High Blood Pressure Therapy ScaleProg Cardiovasc Nurs2000153909610951950
- LambertEVSteynKStenderSEverageNFourieJMHillMCross-cultural validation of the hill-bone compliance to high blood pressure therapy scale in a South African, primary healthcare settingEthn Dis200616128629116599385
- SongYHanHRSongHJNamSNguyenTKimMTPsychometric evaluation of hill-bone medication adherence subscaleAsian Nurs Res (Korean Soc Nurs Sci)20115318318825030368
- Korb-SavoldelliVGillaizeauFPouchotJValidation of a French version of the 8-item Morisky medication adherence scale in hypertensive adultsJ Clin Hypertens (Greenwich)201214742943422747615
- MoriskyDEAngAKrousel-WoodMWardHJPredictive validity of a medication adherence measure in an outpatient settingJ Clin Hypertens (Greenwich)200810534835418453793
- SaleemFHassaliMAShafieAATranslation and validation study of Morisky Medication Adherence Scale (MMAS): the Urdu version for facilitating person-centered healthcare in PakistanInt J Pers Cent Med201223384390
- MaCChenSYouLLuoZXingCDevelopment and psychometric evaluation of the Treatment Adherence Questionnaire for Patients with HypertensionJ Adv Nurs20126861402141321954893
- Martin AlfonsoLBayarre VeaHDGrau ÁbaloJAValidation of MBG Questionnaire (Martin-Bayarre-Grau) for the evaluation of therapy adherence in blood hypertension patientsRev Cuba Salud Publica2008341 Spanish
- MartínezJWVilla PereaJAJaramilloJQuintero BetancurAMCalderónVValidación del cuestionario de adherencia al tratamiento anti hipertensivo Martín Bayarré Grau. [Validation of a scale of adherence treatment antihypertensive: Martin Bayarre Grau Test]Rev Med Risaralda2011172101105 Spanish
- NormanSAMarconiKMSchezelGWSchechterCFStolleyPDBeliefs, social normative influences, and compliance with antihypertensive medicationAm J Prev Med19851310173870899
- StamlerRStamlerJCivinelliJAdherence and blood-pressure response to hypertension treatmentLancet1975279471227113053721
- SchoenbergNEThe relationship between perceptions of social support and adherence to dietary recommendations among African-American elders with hypertensionInt J Aging Hum Dev199847427929710198806
- SvenssonSKjellgrenKIAdverse events and patients’ perceptions of antihypertensive drug effectivenessJ Hum Hypertens2003171067167514504624
- BittarNMaintaining long-term control of blood pressure: the role of improved complianceClin Cardiol1995186 Suppl 3III 12III 16
- KleinLECompliance and blood pressure controlHypertension1988113 Pt 2II 61II 64
- ScholzUGutiérrez DoñaBSudSSchwarzerRIs general self-efficacy a universal construct? Psychometric findings from 25 countriesEur J Psychol Assess2002183242251
- SvarstadBLChewningBASleathBLClaessonCThe Brief Medication Questionnaire: a tool for screening patient adherence and barriers to adherencePatient Educ Couns199937211312414528539
- Batalla MartínezCBlanquer LaguartaACiurana MisolRCumplimiento de la prescripción farmacológica en pacientes hipertensos. [Compliance with drug prescriptions in hypertensive patients]Aten Primaria198412185191 Spanish
- StreinerDNormanGHealth Measurement Scales. A Practical Guide to Their Development and Use2nd edNew York, NYOxford University Press1991
- Quintana SetiénCFernández-Britto RodríguezJEAdherencia terapéutica farmacológica antihipertensiva en adultos de atención primaria y factores relacionados con su incumplimiento. [Antihypertensive drug therapy compliance by adults in the primary health care and factors related to non-compliance]Rev Cubana Invest Bioméd2009282 Spanish
- BlandJMAltmanDGCronbach’s alphaBMJ199731470805729055718
