Abstract
Purpose
Several randomized controlled clinical trials have been conducted to investigate the role of carvedilol and propranolol on the effect of portal pressure in patients with cirrhosis, leading to controversial results. Current meta-analysis was performed to compare the efficacy of the two drugs on portal pressure.
Patients and methods
Two-hundred and ninety eligible patients were recruited. Published studies were selected based on PubMed, the Cochrane Library, Chinese Journal Full-text Database, and Wanfang Database. The outcome measurements included the mean difference (MD) in the percentage of hepatic vein pressure gradient reduction (%HVPG reduction), the risk ratio (RR) of nonresponders in hemodynamic assessment, and the percentage of mean arterial pressure reduction (%MAP reduction). Subgroup analysis was performed.
Results
Seven trials were identified (including five acute and three long-term drug administration randomized controlled trials). A summary of pooled MD between the %HVPG reduction is as follows: overall −8.62 (confidence interval [CI] −11.76, −5.48, P<0.00001), acute −10.05 (CI −14.24, −5.86, P<0.00001), and long term −6.80 (CI −11.53, −2.07, P=0.005), while summary of pooled RR of hemodynamic nonresponders with carvedilol was as follows: overall 0.64 (CI 0.51, 0.81, P=0.0002), acute 0.63 (CI 0.47, 0.85, P=0.002), and long term 0.67 (CI 0.47, 0.97, P=0.03). Both of the outcome measurements favored carvedilol. Significant heterogeneity (P<0.1, I2=92%) existed between the two treatment groups in %MAP reduction. No considerable difference could be observed in the %MAP reduction through the poor overlapping CI boundaries.
Conclusion
Carvedilol has a greater portal hypertensive effect than propranolol. Further comparative trials of the two drugs are required to identify the effect of MAP reduction.
Introduction
It has already been acknowledged that portal hypertension is an unavoidable complication of cirrhosis, and variceal bleeding is the most serious consequence, resulting in a significant mortality of 7%–15%.Citation1–Citation3 Hepatic vein pressure gradient (HVPG) is regarded as the gold standard for the assessment of treatment effects of portal hypertension. Measurement of HVPG can accurately reflect the pressure in patients with portal hypertension.Citation4 It has been shown that the HVPG should increase to above 12 mmHg for variceal bleeding.Citation5 A good hemodynamic response is defined as the reduction of HVPG to a value <12 mmHg or by ≥20% from baseline, which can significantly decrease the risk of bleeding or mortality, presenting a better outcome than hemodynamic nonresponders.Citation6,Citation7
The nonselective beta-blockers (especially propranolol and nadolol) or the endoscopic variceal ligation (EVL) is currently recommended as the first-line therapy to prevent variceal bleeding.Citation8,Citation9 However, only a few patients show a substantial HVPG reduction after propranolol administration. When it comes to EVL, its invasiveness and costs have resulted in variable application. Carvedilol, having been proposed to be used in clinical practice in recent years, is a promising nonselective beta-blocker with intrinsic anti-alpha1 adrenergic activity (one-tenth of its beta-blocker activity),Citation10 giving the drug a potential for a higher portal pressure reduction compared to propranolol. There have been several randomized controlled clinical trials exploring the efficacy of carvedilol and propranolol in patients with portal hypertension, yielding controversial results.
The aim of the present meta-analysis was to assess the hemodynamic effectiveness of carvedilol and propranolol on the effect of portal hypertension in patients with cirrhosis. We have made extensive efforts to find all relevant studies by means of computerized and manual search, and the treatment effect is combined across all studies.
Methods
Data retrieval
Pertinent studies were retrieved between January 1995 and December 2013 in the Pubmed, The Cochrane Library, Chinese Journal Full-text Database, and Wanfang Database with the keywords carvedilol, propranolol, portal hypertension, randomized controlled clinical trials in both English (from the index Medicus Medical Subject Headings list) and Chinese. A manual search of the reference lists of pertinent studies was also performed. When the results of a single study were reported in more than one publication, only the most recent and complete data were included. Unpublished studies were not referred to in this meta-analysis.
Inclusion and exclusion criteria
To be included in this meta-analysis, a study had to meet the following criteria. 1) The published study was randomized controlled trial (RCT). 2) The percentage of HPVG reduction (%HVPG reduction) was a main outcome measure of the treatment effect. Secondary outcome measures included number of responders in hemodynamic evaluation and the percentage of mean arterial pressure reduction (%MAP reduction). 3) The participants were patients with clinically significant portal hypertension. Randomized trials were included irrespective of blinding or language.
The criteria for the selection of a study were established before this meta-analysis was designed.
Data collection
Data were extracted by two independent authors (Liu and Yang), and disagreements were solved by discussion. For each study, the following data were extracted: 1) patients’ characteristics, including number of patients, mean age, sex, etiology of cirrhosis, Child-Pugh score, and proportion of patients with previous variceal bleeding and 2) outcome measures, including the %HVPG reduction, the number of hemodynamic responders, and the %MAP reduction.
Outcome measures
The main outcome measure was %HVPG reduction; secondary outcome measures included the number of patients achieving a hemodynamic response and the outcome related to main adverse events, such as %MAP reduction.
Statistical analysis
Studies and patient characteristics were reported as number of observations, proportions, or means ± standard deviations.
Analyses were performed by fixed-effects model and RevMan software (Cochrane Collaboration, Canada, Version 5.2.7). Inverse variance was used to calculate the mean difference (MD) of the %HVPG reduction and the %MAP reduction. The Mantel–Haenszel was used to assess the hemodynamic responders using the risk ratio (RR). Statistical data were expressed as 95% confidence interval (CI). A level of P<0.05 was considered statistically significant. Adverse effect of the meta-analysis was performed by random-effects model.
Heterogeneity was evaluated by a chi-square test and was reported using I2 values. If pronounced heterogeneity was found, potential reasons were explored and the combinability of trials was reconsidered. The significant level for heterogeneity test was P=0.1. Methodological quality assessment was conducted using a validated table derived from the Cochrane Handbook, version 5.1.0, and Fisher’s exact probability test was conducted to calculate the difference levels between qualitative variables. The studies might be measured by acute and long-term hemodynamic effects under clinical treatment, so subgroup analysis was implemented. However, if the combinability of trials was inappropriate, separate analysis was also undertaken.
Analyses were conducted following the intention-to-treat principle and included all patients randomized as they were reported in each single trial.
Results
Analysis of retrieval results
Overall, 44 pertinent studies were identified. Thirty-one were excluded by reading the abstract because they did not include randomized clinical trials. The remaining eleven were RCTs and were assessed for inclusion in the present meta-analysis ().
Figure 1 Study flow diagram of the identification process of RCTs for inclusion in this meta-analysis.
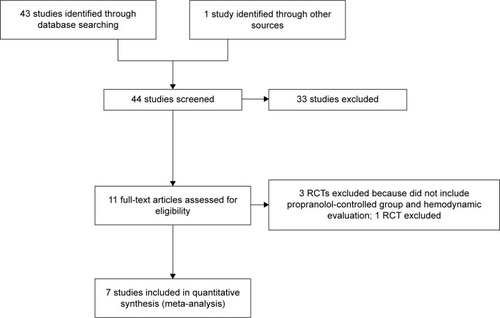
Among these eleven, three were excluded as they did not compare carvedilol with propranolol and did not include hemodynamic measurements;Citation11–Citation13 another randomized controlled trial incorporated the HVPG as a main measurementCitation14 but was excluded because the %HVPG reduction was given in a way that made it difficult to assess the pooled effect using current software. The remaining seven studiesCitation15–Citation21 corresponded to our inclusion criteria and were included in the present study. All included studies had been published. The characteristics of them are summarized in . Among the seven, fourCitation15,Citation18 assessed the acute hemodynamic effect and twoCitation16,Citation19 evaluated the long-term effect. In oneCitation17 study, both the acute hemodynamic and long-term effects were assessed after initial trial drug administration. As a result, five acute and three long-term RCTs were available for the present meta-analysis.
Table 1 Characteristics of seven included studies
A total of 290 eligible patients were recruited in the seven trials, 145 in the carvedilol group and 145 in the propranolol group. The characteristics of patients in the seven studies are summarized in , and none of the parameters differed significantly between the two groups across the seven studies.
Table 2 Characteristics of patients in seven included studies
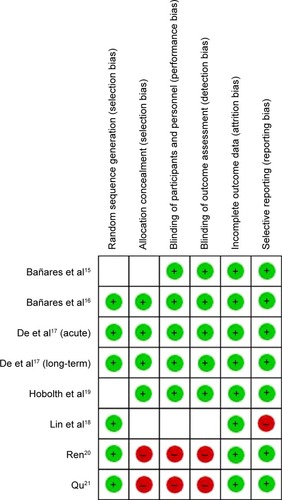
Methodological quality assessment
Methodological quality of each selected RCT was assessed in terms of the following six domainsCitation22,Citation23: 1) random sequence generation; 2) allocation concealment; 3) blinding of participants and personnel; 4) blinding of outcome assessment; 5) incomplete outcome data; and 6) selective reporting. The quality of seven studies included was relatively unsatisfactory (). The risk of bias is summarized in .
Table 3 Methodological quality assessment of included studies
Meta-analysis
Main outcome
%HVPG reduction
Mean baseline HVPG was 16.4 mmHg (median 16.6 mmHg) in the propranolol-treated group and 16.8 mmHg (median 18.9 mmHg) in the carvedilol-treated group. The mean %HVPG reduction was 12.5% (median 12.4%) with propranolol and 23.9% (median 26.4%) with carvedilol (including patients with both acute and long-term assessments) ().
Table 4 Main results of the included studies
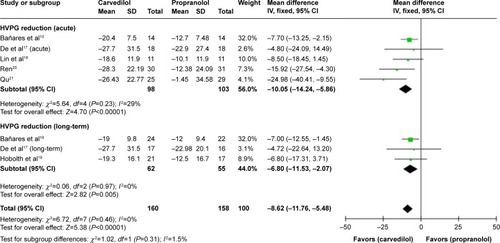
The pooled MD %HVPG reductions with the two drugs was −8.62 (CI −11.76, −5.48; fixed-effect model), denoting superiority of carvedilol (Z=5.38, P<0.00001) (). No heterogeneity was found (P=0.46; I2=0%).
Figure 3 Subgroup analysis (fixed-effect model) of the percentage of hepatic vein pressure gradient reduction, representing the MDs (rectangles) and 95% CI (horizontal lines) for trials that compared carvedilol and propranolol.
Abbreviations: MD, mean difference; CI, confidence interval; SD, standard deviation; IV, inverse variance; HVPG, hepatic vein pressure gradient.
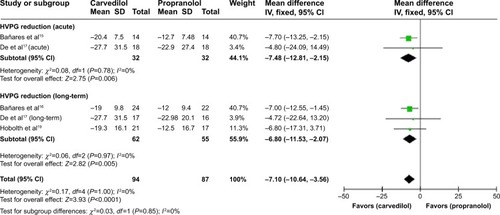
A sensitivity analysis demonstrated that %HVPG reduction is significantly higher in carvedilol-treated group for either the acute or the long-term treatment () and superiority of carvedilol hold true by excluding the studiesCitation18 with high-risk factors (MD −7.1, CI −10.64, −3.56; fixed-effect model) ().
Figure 4 Subgroup analysis (fixed-effect model) of the percentage of hepatic vein pressure gradient reduction.
Abbreviations: MD, mean difference; CI, confidence interval; SD, standard deviation; IV, inverse variance; HVPG, hepatic vein pressure gradient.
Secondary outcome
Number of hemodynamic responders
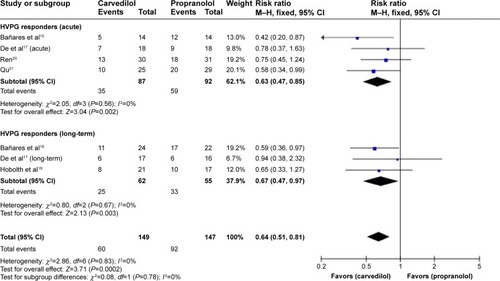
The total number of hemodynamic responders was 55 out of 147 evaluable patients in propranolol as compared to 89 out of 149 with carvedilol (including patients with both acute and long-term assessments) ().
The pooled RR of hemodynamic nonresponse with carvedilol was 0.64 (CI 0.51–0.81; fixed-effect model), underlining superiority of carvedilol in responder rate (Z=3.71, P=0.0002). No heterogeneity was found (P=0.83; I2=0%) ().
Figure 5 Subgroup analysis (fixed-effect model) of hemodynamic nonresponders, representing the RRs (rectangles) and 95% CI (horizontal lines) for trials that compared carvedilol and propranolol.
Abbreviations: RRs, risk ratios; CI, confidence interval; M–H, Mantel–Haenszel; HVPG, hepatic vein pressure gradient.
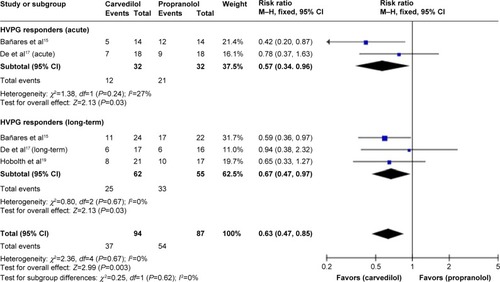
A sensitivity analysis suggested that responder rate is significantly higher in carvedilol-treated group with either the acute or the long-term treatment () and superiority of carvedilol hold true by excluding the studiesCitation18 with high-risk factors (RR 0.63, CI 0.47, 0.85; fixed-effect model) ().
Figure 6 Subgroup analysis (fixed-effect model) of hemodynamic nonresponders.
Abbreviations: RRs, risk ratios; CI, confidence interval; M–H, Mantel–Haenszel; HVPG, hepatic vein pressure gradient.
Adverse event
%MAP reduction
The test of heterogeneity (P<0.00001, I2=94%, random-effect model) for the reduction of %MAP () in all studies indicated that important differences might exist. But when considering the subgroups separately, heterogeneity was significant, precluding combining the results of two drugs in %MAP reduction ().
Figure 7 Subgroup analysis (random-effect model) of the percentage of mean arterial pressure reduction, representing the MDs (rectangles) and 95% CI (horizontal lines) for trials that compared carvedilol and propranolol.
Abbreviations: MD, mean difference; CI, confidence interval; SD, standard deviation; IV, inverse variance; MAP, mean arterial pressure reduction.
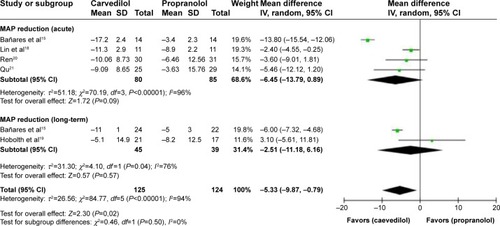
Table 5 %MAP reduction of the included studies
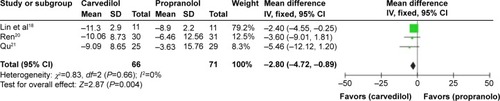
However, in the acute assessment, if we exclude one studyCitation15 with unclear risk, the heterogeneity would be eliminated (P=0.66, I2=0%), favoring carvedilol in decreasing MAP over propranolol (MD −2.80, CI −4.72, −0.89) (). Nevertheless, the remaining three studies estimated were all high-risk trials. Therefore, this result should be considered with caution.
Figure 8 MD (fixed-effect model) of the percentage of mean arterial pressure reduction (%MAP reduction) between carvedilol and propranolol in acute treatment trials, excluding one study with unclear risk. The heterogeneity test is also performed.
Discussion
Propranolol has been utilized for the prevention of variceal bleeding in cirrhotic patients with portal hypertension for almost 30 years.Citation24 Recently, carvedilol, which causes beta-blockade as well as anti-alpha1 adrenergic activities, has been proved to decrease portal pressure both by lowering the portal blood flow (beta-blocker effect) and by decreasing the hepatic vascular resistance (alpha1-adrenergic blocker effect). As such, carvedilol has the potential to achieve greater reduction of portal pressure than the propranolol.Citation10,Citation25,Citation26 However, some earlier observationsCitation15,Citation16,Citation27 demonstrated that carvedilol significantly decreased the MAP compared to baseline values and/or compared to propranolol-treated patients, leading to concerns over the use of this drug for long-term administration in patients with portal hypertension. The present meta-analysis of seven studies was conducted to compare the treatment effect on portal hypertension between carvedilol and propranolol and to evaluate the %MAP reductions caused by the two drugs.
Across all included trials, patients recruited in the two groups were comparable without pronounced differences regarding the number of patients, mean age, sex, etiology of cirrhosis, Child-Pugh score, and history of previous variceal bleeding.
The current meta-analysis reflects that in the available RCTs focusing on carvedilol and propranolol for the prevention of variceal bleeding in patients with portal hypertension, carvedilol reduces HVPG significantly more than propranolol, regardless of acute or long-term drug administration. The pooled MD of %HVPG reduction achieved by two drugs was −8.62 (CI −11.76, −5.48), favoring carvedilol. No heterogeneity was found. When attention was paid to the subgroups, the figures for acute and long-term drug administration were −10.05 (CI −14.24, −5.86) and −6.80 (CI −11.53, −2.07), respectively. The results were consistent with the principal assessment.
With regard to the number of hemodynamic responders, an Austria research conducted by Reiberger et alCitation28 claimed that carvedilol demonstrated great efficacy in the proportion of hemodynamic responders, which was also the case in the current review. In our meta-analysis, the number of patients achieving a reduction in HVPG to ≥20% or to ≤12 mmHg was reported in six out of the seven studies (carvedilol: 89/149 vs propranolol: 55/147). The pooled RR of hemodynamic nonresponders reached statistical significance with carvedilol at 0.64 (CI 0.51–0.81; fixed-effect model), denoting superiority of carvedilol in hemodynamic response. The subgroup results, 0.63 (CI 0.47–0.85) and 0.67 (CI 0.47–0.97) for acute and long-term drug administration, respectively, were assessed in accordance with the overall evaluation. However, the results were inconsistent with a previous meta-analysis studyCitation29 published in early 2014.
As for the %MAP reduction, significant heterogeneity (P<0.1, I2=92%) existed between the two treatment groups, hampering evaluation of a pooled effect. No significant difference could be observed in the %MAP reduction between two drugs across all trials as was shown by the poor overlapping CI boundaries in our study, indicating that carvedilol and propranolol had similar effects in MAP reduction.
Interestingly, when the source of the heterogeneity was explored, we found that if one studyCitation15 with unclear risk was excluded in the acute assessment, the heterogeneity would be eliminated (P>0.1, I2=0%), suggesting that carvedilol decreased MAP in a more pronounced fashion than propranolol when administered acutely. However, the remaining three studies reviewed were all high-risk trials. Therefore, this result might conceivably be considered as a type I error.
Nevertheless, this difference was clinically meaningful. One patient with symptomatic hypotensionCitation17 over 90 minutes (recovered over the next 2 days with conservative therapy) was reported in the carvedilol group and two (necessitating cessation of treatment) in the propranolol group. A total of 15 patients developed orthostatic hypotension out of 77 patients treated by carvedilol, compared to nine out of 73 treated by propranolol (P=0.27>0.05, Fisher’s exact test) in threeCitation16,Citation19 out of the seven studies.
A final attention was paid to bias. We may have not included all trials comparing carvedilol and propranolol, particularly, those with negative results had been performed but not published. On the other hand, the small number of available studies and their small sample sizes precluded the achievement of a convincing estimation. Furthermore, from the results of the methodological quality assessment, high-risk factors existed in three included studiesCitation18 with oneCitation15 incorporating unclear risk, making selection bias, performance bias, and reporting bias possible ().
Conclusion
In conclusion, this meta-analysis of the existing controlled trials suggests that carvedilol reduces HVPG significantly more than propranolol, irrespective of acute or long-term drug administration. The number of hemodynamic responders is significantly higher with carvedilol treatment, and this is supported by a recent studyCitation28 confirming that carvedilol leads to a significantly greater reduction in HVPG than propranolol and is a rescue treatment for hemodynamic nonresponders to propranolol. However, conclusion that carvedilol reduces MAP more than propranolol cannot be drawn from the data in our meta-analysis. The finding that the acute administration of carvedilol decreased MAP to a greater extent than propranolol did should be viewed with caution. On the basis of the present study, carvedilol is shown to be more effective than propranolol regarding the effect of portal hypertension in patients with cirrhosis. Further comparative trials of carvedilol and propranolol are encouraged to identify the effect of the MAP.
The current meta-analysis suggests that carvedilol might be recommended for the reduction of portal hypertension in patients.Citation28 However, the MAP should be monitored.
Disclosure
The authors report no conflicts of interest in this work.
References
- VillanuevaCPiquerasMAracilCA randomized controlled trial comparing ligation and sclerotherapy as emergency endoscopic treatment added to somatostatin in acute variceal bleedingJ Hepatol20064556056716904224
- AugustinSAltamiranoJGonzálezAEffectiveness of combined pharmacologic and ligation therapy in high-risk patients with acute esophageal variceal bleedingAm J Gastroenterol20111061787179521625271
- AbraldesJGVillanuevaCBañaresRCooperative Group for Portal Hypertension and Variceal BleedingHepatic venous pressure gradient and prognosis in patients with acute variceal bleeding treated with pharmacologic and endoscopic therapyJ Hepatol20084822923618093686
- BoschJAbraldesJGBerzigottiAGarcia-PaganJCThe clinical use of HVPG measurements in chronic liver diseaseNat Rev Gastroenterol Hepatology20096573582
- GroszmannRJGarcia-TsaoGBoschJPortal Hypertension Collaborative GroupBeta-blockers to prevent gastroesophageal varices in patients with cirrhosisN Engl J Med20053532254226116306522
- BoschJGarcia-PaganJCPrevention of variceal rebleedingLancet200336195295412648985
- D’AmicoGGarcia-PaganJCLucaABoschJHepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic reviewGastroenterology20061311611162417101332
- Garcia-TsaoGSanyalAJGraceNDCareyWPrevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosisHepatology20074692293817879356
- Garcia-TsaoGBoschJGroszmannRJPortal hypertension and variceal bleeding – unresolved issues. Summary of an American association for the study of liver diseases and European association for the study of the liver single-topic conferenceHepatology2008471764177218435460
- BoschJCarvedilol for portal hypertension in patients with cirrhosisHepatology2010512214221820513005
- TripathiDFergusonJWKocharNRandomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleedHepatology20095082583319610055
- LoGHChenWCWangHMYuHCRandomized, controlled trial of carvedilol versus nadolol plus isosorbide mononitrate for the prevention of variceal rebleedingJ Gastroenterol Hepatol2012271681168722849337
- ShahHAAzamZRaufJCarvedilol vs esophageal variceal band ligation in the primary prophylaxis of variceal hemorrhage: a multi-centre randomized controlled trialJ Hepatol20146075776424291366
- HobolthLBendtsenFHansenEFMollerSEffects of carvedilol and propranolol on circulatory regulation and oxygenation in cirrhosis: a randomised studyDig Liver Dis20144625125624290869
- BañaresRMoitinhoEPiquerasBCarvedilol, a new nonselective beta-blocker with intrinsic anti-alpha1-adrenergic activity, has a greater portal hypotensive effect than propranolol in patients with cirrhosisHepatology199930798310385642
- BañaresRMoitinhoEMatillaARandomized comparison of long-term carvedilol and propranolol administration in the treatment of portal hypertension in cirrhosisHepatology2002361367137312447861
- DeBKDasDSenSAcute and 7-day portal pressure response to carvedilol and propranolol in cirrhoticsJ Gastroenterol Hepatol20021718318911966949
- LinHCYangYYHouMCHuangYTLeeFYLeeSDAcute administration of carvedilol is more effective than propranolol plus isosorbide-5-mononitrate in the reduction of portal pressure in patients with viral cirrhosisAm J Gastroenterol2004991953195815447755
- HobolthLMollerSGronbaekHRoelsgaardKBendtsenFFeldager HansenECarvedilol or propranolol in portal hypertension? A randomized comparisonScand J Gastroenterol20124746747422401315
- RenRRThe comparison of recent curative effect on the reduction of hepatic vein pressure gradient in patients with cirrhosisShandong University2012
- QuCXThe preliminary research of the short-term reduction of hepatic vein pressure gradient to predict the long-term curative effect of beta-blockersShandong University2012
- GluudLLBias in clinical intervention researchAm J Epidemiol200616349350116443796
- KjaergardLLVillumsenJGluudCReported methodologic quality and discrepancies between large and small randomized trials in meta-analysesAnn Intern Med200113598298911730399
- Garcia-TsaoGCurrent management of the complications of cirrhosis and portal hypertension: variceal hemorrhage, ascites, and spontaneous bacterial peritonitisGastroenterology200112072674811179247
- TsochatzisEATriantosCKBurroughsAKGastrointestinal bleeding: carvedilol-the best beta-blocker for primary prophylaxis?Nat Rev Gastroenterol Hepatol2009669269419946301
- HemstreetBAEvaluation of carvedilol for the treatment of portal hypertensionPharmacotherapy2004249410414740791
- TripathiDTherapondosGLuiHFStanleyAJHayesPCHaemodynamic effects of acute and chronic administration of low-dose carvedilol, a vasodilating beta-blocker, in patients with cirrhosis and portal hypertensionAliment Pharmacol Ther20021637338011876689
- ReibergerTUlbrichGFerlitschAVienna Hepatic Hemodynamic LabCarvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non-response to propranololGut2013621634164123250049
- SinagraEPerriconeGD’AmicoMTineFD’AmicoGSystematic review with meta-analysis: the haemodynamic effects of carvedilol compared with propranolol for portal hypertension in cirrhosisAliment Pharmacol Ther20143955756824461301