Abstract
Hereditary angioedema (HAE) is a rare genetic disease characterized by episodic subcutaneous or submucosal swelling. The primary cause for the most common form of HAE is a deficiency in functional C1 esterase inhibitor (C1-INH). The swelling caused by HAE can be painful, disfiguring, and life-threatening. It reduces daily function and compromises the quality of life of affected individuals and their caregivers. Among different treatment strategies, replacement with C1-INH concentrates is employed for on-demand treatment of acute attacks and long-term prophylaxis. Three human plasma-derived C1-INH preparations are approved for HAE treatment in the US, the European Union, or both regions: Cinryze®, Berinert®, and Cetor®; however, only Cinryze is approved for long-term prophylaxis. Postmarketing studies have shown that home therapy (self-administered or administered by a caregiver) is a convenient and safe option preferred by many HAE patients. In this review, we summarize the role of self-administered plasma-derived C1-INH concentrate therapy with Cinryze at home in the prophylaxis of HAE.
Introduction
Hereditary angioedema (HAE) due to C1 esterase inhibitor (C1-INH) deficiency is a rare autosomal dominant disorder characterized by episodic swelling typically involving the skin, abdomen, and larynx.Citation1,Citation2 Studies suggest that HAE affects up to one in 50,000 people worldwide, regardless of race or ethnicity.Citation3–Citation5 The majority of HAE cases are due to the deficiency of functional C1-INH. HAE due to C1-INH deficiency is further divided into HAE types I and II, and the presentations of these subtypes are clinically indistinguishable. There are ~300 identified mutations of the C1-INH gene (SERPING1) located at 11q12–q13.1 related to HAE.Citation6
HAE type I accounts for 85% of cases. Patients with this form of HAE have low-level production of antigenic C1-INH. These patients have normal antigenic levels but abnormal C1-INH function.Citation7,Citation8 Under physiological conditions, C1-INH regulates the activities of four interlinked proteolytic enzyme cascades, namely, the complement, contact (kallikrein–kinin), fibrinolytic, and coagulation pathways (). In particular, C1-INH is the primary inhibitor of the kallikrein–kinin system via inactivation of activated factor XII (factor XIIa) and kallikrein. The excessive production of bradykinin via an overactive kallikrein–kinin system accounts for episodic swelling in patients with HAE.Citation4,Citation9,Citation10
Figure 1 Pathways inhibited by C1-INH.
Abbreviations: C1-INH, C1 esterase inhibitor; FXI, factor XI; FXIa, activated factor XI; FXII, factor XII; FXIIa, activated factor XII; HMWK, high molecular weight kininogen; MASP, mannose-binding lectin-associated serine protease; tPA/uPA, tissue/urokinase plasminogen activator.
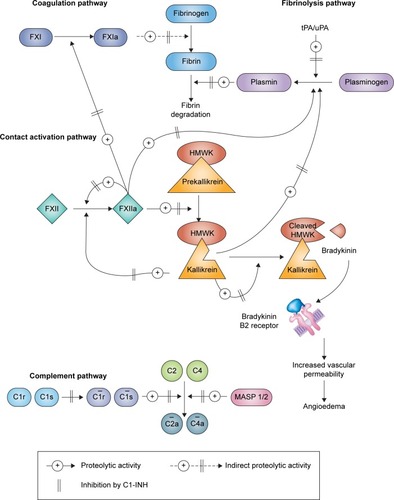
For most patients with HAE, their clinical presentation is often unpredictable. The mean time at which the initial symptoms of HAE appear is ~11 years of age. HAE attacks often become more frequent and severe during adolescence and adulthood.Citation1,Citation4,Citation12 The pattern of HAE attacks may vary tremendously among patients and throughout a patient’s life. Many attacks may involve multiple organ systems. Patients can experience symptoms weekly, while others have attacks less than once a year.Citation12–Citation15
Without treatment, most episodes of HAE last for 2–5 days.Citation16 Laryngeal attacks, which occur in up to 50% of HAE patients, are potentially life-threatening;Citation12,Citation17–Citation19 without medical intervention, the mortality rate due to laryngeal edema is up to 40%.Citation18 HAE-associated abdominal attacks have a high symptom burden because local mucosal swelling gives rise to severe abdominal pain, nausea, and vomiting, often necessitating hospitalization. Furthermore, unnecessary exploratory surgery is sometimes performed in undiagnosed patients.Citation20
The triggers for a particular attack in a patient with HAE are not always clear, but several factors have been linked to the onset of HAE attacks. The common triggers may include trauma, surgical and dental procedures, stress, infection, hormonal changes, and treatment with estrogens or angiotensin-converting enzyme inhibitors.Citation21–Citation23 Due to the unpredictable nature of the disease, many patients with HAE are constantly living in fear of another severe attack, disfigured face, impaired functions of hands and feet, agonizing abdominal pain, and possible airway compromise. In addition, they worry about their children inheriting the disease.Citation15,Citation24 Consequently, HAE exerts a profound humanistic burden, with effects including physical and emotional trauma, educational or professional underachievement, financial hardship, and poor quality of life.Citation15,Citation24–Citation28
HAE treatments have been developed for short-term or long-term prophylaxis and for treating acute attacks.Citation29–Citation31 There are a few human plasma-derived C1-INH concentrates that are currently approved by the US Food and Drug Administration (FDA) or European Medicines Agency (EMA) for HAE treatment (). In line with the current recommendations, all HAE attacks should be treated.Citation29–Citation31 Moreover, long-term prophylaxis should be considered when the patient has severe and frequent attacks that cannot be adequately controlled by on-demand therapies or when rapid access to treatment of an attack is unavailable.Citation29–Citation31
Table 1 Approval status of intravenously delivered human plasma-derived C1-INH concentrates for treatment of or prophylaxis against acute HAE attacks
Prophylactic treatment with C1-INH concentrates
To minimize the effect of the disease on patients, effective prophylaxis against HAE attacks is most desirable. The effectiveness of androgens such as danazol is limited by adverse events (AEs). Oral antifibrinolytics such as tranexamic acid have relatively poor efficacy for this rare indication.Citation29–Citation31 Prophylactic administration of C1-INH concentrates replenishes plasma C1-INH activity and thus addresses the fundamental cause of HAE attacks. There are three highly purified human plasma-derived C1-INH concentrate preparations that are commercially available for HAE treatment (), but only Cinryze® (Shire ViroPharma Incorporated, Lexington, MA, USA), a nanofiltered human C1-INH concentrate, is recommended as a first-line therapy for routine long-term prophylaxis (in adolescent and adult patients). Berinert® (CSL Behring, King of Prussia, PA, USA) is approved for the short-term prevention (in the EU), and Cetor® (Sanquin, Amsterdam, the Netherlands) has no approval for prophylactic use, although it has the same qualitative and quantitative composition in terms of the active substance as Cinryze.Citation37 Cinryze is also the only approved C1-INH concentrate for the treatment, preprocedure prevention, and long-term prevention of HAE attacks (in adolescents and adults) albeit only in the EU; in the US, its approved indication is limited to long-term prophylaxis.Citation38
Ruconest® (Pharming Group NV, Leiden, the Netherlands),Citation39,Citation40 which is a recombinant C1-INH produced in transgenic rabbits, ecallantide (Kalbitor®; Dyax Corporation, Burlington, MA, USA),Citation41 a kallikrein inhibitor, and icatibant (Firazyr®; Shire, Lexington, MA, USA),Citation42,Citation43 a bradykinin B2 receptor antagonist, are approved treatments for acute HAE attacks in the US, the EU, or both. Because they are not plasma-derived C1-INH concentrates, these drugs are not discussed any further in this review.
The objective of this review is to summarize the role of self-administered plasma-derived C1-INH concentrate therapy at home for the prevention of HAE.
Clinical studies
Plasma-derived C1-INH concentrates have performed well as preventative measures against HAE attacks in controlled studies, as well as in observational and descriptive studies.Citation44,Citation45
Cinryze
The preprocedural administration of Cinryze before dental, surgical, or interventional diagnostic procedures was found to prevent edematous episodes; in a retrospective analysis of data from two acute treatment trials, 89 of 91 procedures did not trigger a subsequent HAE attack.Citation46
The long-term protective efficacy of Cinryze (open label) was evaluated in a placebo-controlled crossover study of patients with a history of at least two attacks per month. These patients were randomly assigned 1,000 units of open-label nanofiltered C1-INH concentrate for the prevention of acute HAE attacks or a placebo, administered twice weekly.Citation45 Cinryze had a significant benefit over placebo treatment, as evidenced by a reduction in the number of attacks per 12-week period (6.26 with C1-INH concentrate vs 12.73 with placebo; P<0.001). The severity and duration of attacks, the need for open-label rescue therapy, and the total number of days with symptoms of swelling were also reduced.Citation45 This study also revealed that patients with HAE had significantly better health-related quality of life following 12 weeks of routine prophylaxis with Cinryze. This preventative treatment was compared with acute treatment of attacks without long-term prevention (patients receiving a placebo).Citation47 In an open-label multicenter extension phase of this study, treatment with Cinryze for up to 2.6 years also exerted durable prophylaxis in most patients with HAE, with a 93.7% reduction in the median number of attacks per month (3.00–0.19).Citation48
A further study found that escalating the dose of Cinryze up to 2,500 U every 3 or 4 days is well tolerated and may be required in patients who are not responsive to the approved dose of 1,000 U administered at the same frequency.Citation49
Berinert
In a retrospective study based on clinical record review, preprocedural Berinert administration was associated with a lower incidence of facial swelling or laryngeal edema after tooth extraction, compared with patients who did not receive prophylaxis. In a large-scale observational study with long-term follow-up, short-term prophylaxis with Berinert for various dental and nondental surgical procedures reduced the number of patients who experienced postprocedural attacks significantly more than tranexamic acid and danazol.Citation50
The data regarding the use of Berinert as a long-term prophylactic measure are limited. However, non-placebo-controlled studies showed that this preparation reduced the severity and number of HAE attacks.Citation51–Citation53
Real-world and home use
Administration of plasma-derived C1-INH in a health care facility may be hindered by accessibility and convenience factors, and this is particularly challenging for those who require treatment twice per week. This may affect the patient’s time needed to receive treatment and patients’ adherence to the regimen. Home infusion provides an easy and convenient modality of C1-INH delivery and use, and a significant proportion of patients prefer self-administered plasma-derived C1-INH at home ().
Table 2 Summary of real-world observational data for home-based HAE therapy with human plasma-derived C1-INH
Home administration with plasma-derived C1-INH concentrates results in reduced frequency of attacks compared with previous treatment such as danazol or tranexamic acid, fewer days spent in a hospital, and fewer days missed from school or work.Citation51,Citation54,Citation55 Overall, self-administration of intravenous C1-INH concentrate is associated with enhancing the quality of life of patients with HAE.Citation51,Citation56
Safety and tolerability
Long-term prophylaxis with Cinryze was well tolerated in a placebo-controlled crossover study and its open-label extension phase.Citation45,Citation48 The most common AEs observed were headache, nausea, rash, and vomiting. A recent systematic review of the literature concluded that Berinert also has a comparable side effect profile.Citation44
Hypersensitivity reactions and serious arterial and venous thromboembolic events have been reported at the recommended dose of BerinertCitation32 and the prescribed dose of Cinryze.Citation38
The transmission of blood-borne diseases (viruses or prions) is an inherent risk for all human plasma-derived products, including Cinryze and Berinert (). In a report of ~260 patients who received Cinryze in clinical studies, a total of ~14,000 doses were administered; none of these patients became positive for parvovirus B19, hepatitis B, hepatitis C, or HIV.Citation38 A review of the literature for Berinert also ascertained that it was not associated with transmission of viruses.Citation44
Table 3 Treatment-related AEs associated with commercially available human plasma-derived C1-INH concentrates for HAE treatment
Overall, numerous studies have demonstrated that home administration of HAE medications is safe, with a low occurrence rate of AEs.Citation51,Citation54,Citation56–Citation59
The role of home self-infusion in HAE management
Current clinical guidelines recommend home-based treatment where feasible and that all patients have access to medication supply at all times.Citation30,Citation60–Citation63 Therefore, a large proportion of patients on long-term prophylaxis using Cinryze choose the home therapy option. The result is significantly reduced HAE-related hospitalizations, androgen-derivative usage, and greater patient satisfaction.Citation57,Citation64
Cinryze, initially approved by the FDA in 2008, was approved for self-administration (in patients who receive sufficient training) by the FDA in June 2009Citation65,Citation66 and by the EMA in June 2011.Citation67 In June 2010, in a study performed in the US, 243 of 516 patients (47%, 5–84 years of age) administered Cinryze at home, with the remainder of patients receiving treatment in the physician’s office or at an infusion center. The proportion of patients receiving treatment in a home setting (ie, 20% of the total study population) who self-administered the drug was 42%, and 16% and 23% of patients were administered the drug by a family member or a home health care worker, respectively.Citation68 In 2012, following the implementation of an infusion training program in December 2010, the proportion of patients receiving Cinryze at home (rather than at a physician’s office or infusion center) increased to 76% and the percentage who self-administered increased from 20% to 44%.Citation69 In 2010, 30- to 64-year-olds were the largest age group in which 50% self-administered, but the patient group who were reported to be self-administering Cinryze excluded children (0–12 years of age) or patients >65 years of age.Citation68,Citation69 In 2012, only one child and ten older patients were found to be self-administering.Citation69 The percentage of patients who received C1-INH at home in 2012 was found to be similar across all age groups (≤12 to ≥65 years of age). A total of 57.9% of patients receiving home infusions of Cinryze did so by self-administration.Citation69
A European multicenter study of patients with HAE using Cinryze showed that the majority of doses (87%) used for routine or preprocedural prophylaxis were given at home and 67% were self-administered.Citation74
For Berinert, as of May 2013, a multicenter registry across the US and the EU found that >90% of intravenous infusions for prophylaxis were given by the patient or a caregiver at home,Citation75 compared with the 49% uptake rate reported by a German center 3 years earlier.Citation57
Relatively fewer pediatric patients with HAE are given long-term C1-INH prophylactic treatment.Citation76 In a UK survey of 111 children with HAE, one-third were receiving routine preventative medications, of whom only four were receiving C1-INH concentrate. Ten of 16 centers were able to offer training to administer C1-INH concentrate to children at home, but only two patients were participating in this process.Citation76
The model for delivering plasma-derived C1-INH concentrates in a home setting is based on other successful home therapy programs, such as those for managing immunodeficiency, hemophilia, and other chronic conditions.Citation57,Citation77–Citation79 The most notable benefit of C1-INH concentrate self-administration is flexibility and convenience, thus avoiding regular time-consuming visits to the clinic. The process empowers patients to take control of their disease to the extent that they can resume a normal, less restricted life, without the need to visit a doctor’s office for treatment. Although the concept of self-administration can be intimidating for both the patient and the physician, the provision of appropriate education and training, possibly with a few hours of counseling by their health care provider, enables most patients to feel comfortable with home therapy.Citation80,Citation81
However, it should be noted that, despite the fact that all patients with HAE requiring long-term prophylaxis using C1-INH concentrate are recommended to be considered for home therapy,Citation61,Citation62,Citation68 the eligibility of patients for this treatment is determined by several factors (). Patients may experience several challenges, such as acquiring the skill of infusion administration, and other potential barriers include managing nursing resources for patient training and the patient’s mental capacity.Citation83
Table 4 Patient eligibility criteria for home-based HAE treatment
For self-administration with C1-INH concentrate, venipuncture using a small (eg, 28G) butterfly needle infusion set is recommended;Citation82 however, safety concerns related to this self-administration procedure exist. Findings from an observational study of the use of Berinert indicated that all AEs (mild cases of redness at the injection site and vertigo) occurring after self-administration were due to either intravenous administration that was too rapid or administration at a suboptimum temperature (ie, <25°C).Citation51 Furthermore, indwelling venous ports are associated with complications such as infection and occlusion that can increase the frequency of attacks, and hence, they should be avoided whenever possible and only be considered where timely venous access is difficult.Citation82 In general, however, cannulation failure is uncommon with self-administration after sufficient training, and self-administration may support vein preservation more than that possible in a hospital setting.Citation54
Summary and future development
Intravenous administration of C1-INH concentrate is a safe and effective strategy for short-term (preprocedural) and long-term prophylaxis against HAE attacks. The majority of patients who receive Cinryze as a long-term prophylaxis measure can safely administer this product at home. Most notably, 50% of 30- to 64-year-old patients can perform self-infusion. The positive outcomes associated with Cinryze in the home setting may be even better with subcutaneous formulations of plasma-derived C1-INH concentrate, which are currently under clinical evaluation. Clinical trials are underway to establish whether subcutaneous delivery of plasma-derived C1-INH will provide a comparable efficacy benefit. A Phase III randomized, double-blind study for HAE types I and II was recently initiated (ClinicalTrials.gov identifier NCT02584959) to test a low-volume subcutaneous formulation of Cinryze,Citation85 following a Phase II trial with a different subcutaneous formulation (ClinicalTrials.gov identifier NCT01756157).Citation37 An open-label, dose-ranging, crossover Phase II trial (COMPACT) indicated that subcutaneous administration of plasma-derived C1-INH concentrate (CSL830; CSL Behring) was well tolerated, and there was a dose-dependent increase in physiologically relevant, functional C1-INH plasma levels.Citation86 A Phase III randomized, crossover, double-blind study to evaluate subcutaneously administered Berinert for the prophylactic treatment of HAE was recently conducted (ClinicalTrials.gov identifier NCT01912456).Citation87
Conclusion
Prophylactic treatment of HAE with EMA-approved and FDA-approved C1-INH concentrate can successfully control and prevent HAE attacks. The treatment can be given at home in the absence of a health care provider. Most patients can be trained for self-administration, which allows for more convenient dosing. The postmarketing safety and efficacy data indicate that self-infusion of Cinryze in a home setting is a safe and well-tolerated HAE treatment with few reported side effects. Further studies with subcutaneous administration of C1-INH (including current clinical trials for Cinryze and Berinert) may further increase the tolerability and acceptance of this important aspect of HAE treatment.
Acknowledgments
Medical writing support was provided by Shirley Teng, PhD, and Sally Hassan, PhD, of Excel Scientific Solutions and was funded by Shire.
Disclosure
Dr Li has served as a speaker for CSL Behring and Shire and received research grants from Shire/ViroPharma and consulting fees from CSL Behring, Salix, and Shire. The author reports no other conflicts of interest in this work.
References
- BorkKMengGStaubachPHardtJHereditary angioedema: new findings concerning symptoms, affected organs, and courseAm J Med2006119326727416490473
- WilliamsAHCraigTJPerioperative management for patients with hereditary angioedemaAllergy Rhinol (Providence)201561505525860171
- ZanichelliAArcoleoFBarcaMPA nationwide survey of hereditary angioedema due to C1 inhibitor deficiency in ItalyOrphanet J Rare Dis2015101125758562
- ZurawBLClinical practice. Hereditary angioedemaN Engl J Med2008359101027103618768946
- BernsteinJAUpdate on angioedema: evaluation, diagnosis, and treatmentAllergy Asthma Proc201132640841222221433
- HAE db [webpage on the Internet]C1 inhibitor gene mutation database Available from: http://hae.enzim.hu/stat.phpAccessed January 6, 2016
- RosenFSAlperCAPenskyJKlempererMRDonaldsonVHGenetically determined heterogeneity of the C1 esterase inhibitor in patients with hereditary angioneurotic edemaJ Clin Invest19715010214321494107267
- RosenFSPenskyJDonaldsonVCharachePHereditary angioneurotic edema: two genetic variantsScience1965148367295795814277836
- CugnoMNussbergerJCicardiMAgostoniABradykinin and the pathophysiology of angioedemaInt Immunopharmacol20033331131712639808
- NussbergerJCugnoMCicardiMAgostoniALocal bradykinin generation in hereditary angioedemaJ Allergy Clin Immunol199910461321132210589018
- BorkKPasteurized and nanofiltered, plasma-derived C1 esterase inhibitor concentrate for the treatment of hereditary angioedemaImmunotherapy20146553355124635050
- BorkKHardtJSchicketanzKHResselNClinical studies of sudden upper airway obstruction in patients with hereditary angioedema due to C1 esterase inhibitor deficiencyArch Intern Med2003163101229123512767961
- BorkKHereditary angioedema with normal C1 inhibitor activity including hereditary angioedema with coagulation factor XII gene mutationsImmunol Allergy Clin North Am200626470972417085286
- Aygören-PürsünEBygumABeusterienKSocioeconomic burden of hereditary angioedema: results from the hereditary angioedema burden of illness study in EuropeOrphanet J Rare Dis201499924996814
- CaballeroTAygören-PürsünEBygumAThe humanistic burden of hereditary angioedema: results from the Burden of Illness Study in EuropeAllergy Asthma Proc2014351475324268449
- GompelsMMLockRJAbinunMC1 inhibitor deficiency: consensus documentClin Exp Immunol2005139337939415730382
- FrankMMGelfandJAAtkinsonJPHereditary angioedema: the clinical syndrome and its managementAnn Intern Med19768455805931275365
- BorkKSiedleckiKBoschSSchopfREKreuzWAsphyxiation by laryngeal edema in patients with hereditary angioedemaMayo Clin Proc200075434935410761488
- CicardiMAgostoniAHereditary angioedemaN Engl J Med199633425166616678628365
- AliMABorumMLHereditary angioedema: what the gastroenterologist needs to knowClin Exp Gastroenterol2014743544525429234
- BorkKHardtJStaubach-RenzPWitzkeGRisk of laryngeal edema and facial swellings after tooth extraction in patients with hereditary angioedema with and without prophylaxis with C1 inhibitor concentrate: a retrospective studyOral Surg Oral Med Oral Pathol Oral Radiol Endod20111121586421601496
- ZotterZCsukaDSzaboEThe influence of trigger factors on hereditary angioedema due to C1-inhibitor deficiencyOrphanet J Rare Dis201494424678771
- MorcavalloPSLeonidaARossiGHereditary angioedema in oral surgery: overview of the clinical picture and report of a caseJ Oral Maxillofac Surg20106892307231120728035
- HuangSWResults of an on-line survey of patients with hereditary angioedemaAllergy Asthma Proc200425212713115176498
- GomideMAToledoEValleSOHereditary angioedema: quality of life in Brazilian patientsClinics (Sao Paulo)2013681818323420162
- LumryWRCastaldoAJVernonMKBlausteinMBWilsonDAHornPTThe humanistic burden of hereditary angioedema: impact on health-related quality of life, productivity, and depressionAllergy Asthma Proc201031540741420929608
- WilsonDABorkKSheaEPRentzAMBlausteinMBPullmanWEEconomic costs associated with acute attacks and long-term management of hereditary angioedemaAnn Allergy Asthma Immunol2010104431432020408341
- BouilletLLaunayDFainOHereditary angioedema with C1 inhibitor deficiency: clinical presentation and quality of life of 193 French patientsAnn Allergy Asthma Immunol2013111429029424054366
- ZurawBLBanerjiABernsteinJAUS Hereditary Angioedema Association Medical Advisory Board 2013 recommendations for the management of hereditary angioedema due to C1 inhibitor deficiencyJ Allergy Clin Immunol Pract20131545846724565617
- BowenTCicardiMFarkasH2010 International consensus algorithm for the diagnosis, therapy and management of hereditary angioedemaAllergy Asthma Clin Immunol201062420667127
- BowenTHereditary angioedema: beyond international consensus – circa December 2010 – The Canadian Society of Allergy and Clinical Immunology Dr. David McCourtie LectureAllergy Asthma Clin Immunol20117121310025
- CSL Behring Ltd [webpage on the Internet]Full Prescribing Information. Berniert [C1 Esterase Inhibitor (Human)] Available from: http://labeling.cslbehring.com/PI/US/Berinert/EN/Berinert-Prescribing-Information.pdfAccessed January 20, 2016
- US Food Drug AdministrationOctober 9, 2009, Approval Letter - Berinert2009 http://www.fda.gov/BiologicsBloodVaccines/Blood-BloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/ucm186265.htmAccessed January 6, 2015
- European Medicines AgencyCinryze (C1 Inhibitor (Human))CHMP Assessment Report for Paediatric Use Studies Submitted According to Article 46 of the Regulation (EC) No 1901/20062013 http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/001207/WC500152686.pdfAccessed January 6, 2015
- US Food and Drug AdministrationOctober 10, 2008, Approval Letter - CINRYZE 2008 http://www.fda.gov/biologicsbloodvaccines/bloodbloodproducts/approvedproducts/licensedproductsblas/fractionatedplasmaproducts/ucm093602.htmAccessed January 6, 2016
- Sanquin Ltd. CetorPatient Information http://www.haenet.hu/doc/patient/Cetor%20Patient%20information.pdfAccessed January 6, 2016
- European Medicines Agency [webpage on the Internet]Assessment Report: Cinryze2011 http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001207/WC500108898.pdfAccessed January 6, 2015
- Shire Medical Information [webpage on the Internet]Cinryze® (C1 Esterase Inhibitor [Human]). Highlights of Prescribing Information Available from: http://pi.shirecontent.com/PI/PDFs/Cinryze_USA_ENG.pdfAccessed October 18, 2015
- Pharming Group NV [webpage on the Internet]Ruconest 2100 U Powder for Solution for Injection. Summary of Product Characteristics Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001223/WC500098542.pdfAccessed March 7, 2016
- Pharming Group NV [webpage on the Internet]Medical Information. Ruconest® (C1 Esterase Inhibitor [Recombinant]). Highlights of Prescribing Information Available from: http://shared.salix.com/shared/pi/ruconest-pi.pdfAccessed March 7, 2016
- Dyax Medical Information [webpage on the Internet]Kalbitor® (ecallantide). Highlights of Prescribing Information2015 Available from: https://www.kalbitor.com/hcp/rems/pdf/KalbitorFullPrescribingInformation.pdfAccessed March 7, 2016
- Shire Orphan Therapies GmbH [webpage on the Internet]Firazyr 30 mg Solution for Injection in Pre-Filled Syringe. Summary of Product Characteristics Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000899/WC500022966.pdfAccessed March 7, 2016
- Shire Medical Information [webpage on the Internet]Firazyr® (icatibant). Highlights of Prescribing Information Available from: http://pi.shirecontent.com/PI/PDFs/Firazyr_USA_ENG.pdfAccessed March 7, 2016
- BorkKSteffensenIMachnigTTreatment with C1-esterase inhibitor concentrate in type I or II hereditary angioedema: a systematic literature reviewAllergy Asthma Proc201334431232723710659
- ZurawBLBussePJWhiteMNanofiltered C1 inhibitor concentrate for treatment of hereditary angioedemaN Engl J Med2010363651352220818886
- GrantJAWhiteMVLiHHPreprocedural administration of nanofiltered C1 esterase inhibitor to prevent hereditary angioedema attacksAllergy Asthma Proc201233434835322856635
- LumryWRMillerDPNewcomerSFittsDDaynoJQuality of life in patients with hereditary angioedema receiving therapy for routine prevention of attacksAllergy Asthma Proc201435537137625295804
- ZurawBLKalfusISafety and efficacy of prophylactic nanofiltered C1-inhibitor in hereditary angioedemaAm J Med20121259938.e1938.e722800873
- BernsteinJAManningMELiHEscalating doses of C1 esterase inhibitor (CINRYZE) for prophylaxis in patients with hereditary angioedemaJ Allergy Clin Immunol Pract201421778424565773
- FarkasHZotterZCsukaDShort-term prophylaxis in hereditary angioedema due to deficiency of the C1-inhibitor – a long-term surveyAllergy201267121586159323025435
- KreuzWMartinez-SaguerIAygören-PürsünERusickeEHellerCKlingebielTC1-inhibitor concentrate for individual replacement therapy in patients with severe hereditary angioedema refractory to danazol prophylaxisTransfusion20094991987199519497056
- BorkKMengGStaubachPHardtJTreatment with C1 inhibitor concentrate in abdominal pain attacks of patients with hereditary angioedemaTransfusion200545111774178416271103
- BorkKStaubachPHardtJTreatment of skin swellings with C1-inhibitor concentrate in patients with hereditary angio-oedemaAllergy200863675175718445189
- LeviMChoiGPicavetCHackCESelf-administration of C1-inhibitor concentrate in patients with hereditary or acquired angioedema caused by C1-inhibitor deficiencyJ Allergy Clin Immunol2006117490490816630950
- KreuzWRusickeEMartinez-SaguerIAygören-PürsünEHellerCKlingebielTHome therapy with intravenous human C1-inhibitor in children and adolescents with hereditary angioedemaTransfusion201252110010721756262
- BygumAAndersenKEMikkelsenCSSelf-administration of intravenous C1-inhibitor therapy for hereditary angioedema and associated quality of life benefitsEur J Dermatol200919214715119264579
- Aygören-PürsünEMartinez-SaguerIRusickeEKlingebielTKreuzWOn demand treatment and home therapy of hereditary angioedema in Germany – the Frankfurt experienceAllergy Asthma Clin Immunol201062120667124
- LonghurstHJCarrSKhairKC1-inhibitor concentrate home therapy for hereditary angioedema: a viable, effective treatment optionClin Exp Immunol20071471111717177958
- TourangeauLMCastaldoAJDavisDKKoziolJChristiansenSCZurawBLSafety and efficacy of physician-supervised self-managed C1 inhibitor replacement therapyInt Arch Allergy Immunol2012157441742422123229
- CaballeroTBaezaMLCabañasRConsensus statement on the diagnosis, management, and treatment of angioedema mediated by bradykinin. Part I. Classification, epidemiology, pathophysiology, genetics, clinical symptoms, and diagnosisJ Investig Allergol Clin Immunol2011215333347 quiz follow 347
- CicardiMBorkKCaballeroTEvidence-based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an International Working GroupAllergy201267214715722126399
- CraigTAygören-PürsünEBorkKWAO guideline for the management of hereditary angioedemaWorld Allergy Organ J201251218219923282420
- WahnVAbererWEberlWHereditary angioedema (HAE) in children and adolescents – a consensus on therapeutic strategiesEur J Pediatr201217191339134822543566
- RiedlMGowerRGChrvalaCACurrent medical management of hereditary angioedema: results from a large survey of US physiciansAnn Allergy Asthma Immunol20111064316.e4322.e421457880
- US Food and Drug Administration [webpage on the Internet]Cinryze [C1 Inhibitor (Human)] Freeze Dried Powder US Prescribing Information2011 Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM129918.pdfAccessed January 20, 2015
- UK Medicines Information [webpage on the Internet]New Drugs Online Report for C1 Inhibitor (Human) Available from: http://www.ukmi.nhs.uk/applications/ndo/record_view_open.asp?newDrugID=4234Accessed January 6, 2016
- European Medicines Agency [webpage on the Internet]Cinryze 500 Units Powder and Solvent for Solutions for Injection: Summary of Product Characteristics2011 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001207/WC500108895.pdfAccessed January 6, 2016
- LandmesserLTillotsonGMarianoDSite of care of nanofiltered C1 esterase inhibitor [human] (nf-C1INH) in patients with hereditary angioedema (HAE)American College of Clinical Pharmacy Annual Meeting30Austin, TexasPharmacotherapy2010456e Abstract 324
- GregoryCLandmesserLMCorriganLMarianoDFeasibility of home infusion and self-administration of nanofiltered C1 esterase inhibitor for routine prophylaxis in patients with hereditary angioedema and characterization of a training and support programJ Infus Nurs2014371293424384882
- CSL Behring LtdImportant Safety Information. Berinert [C1 Esterase Inhibitor (Human)] http://www.berinert.com/professional/important-safety-information.aspxAccessed January 6, 2016
- Shire PlcImportant safety informationCinryzeC1 Esterase Inhibitor (Human) http://www.cinryze.com/Accessed January 6, 2016
- Sanquin LtdSummary of Product Characteristics (Cetor 100 U/ml powder and solvent for solution for injection) http://www.sanquin.nl/repository/documenten/en/prod-en-dienst/plasmaproducten/139660/Summary_of_Product_Characteristics.pdfAccessed January 6, 2016
- Sanquin LtdPackage Leaflet Information for the User. Cetor 500 U Powder and Solvent for Solution for Injection http://www.sanquin.nl/repository/documenten/en/prod-en-dienst/plasmaproducten/139660/Package_Leaflet.pdfAccessed January 6, 2016
- Aygören-PürsünEMagerlMMartinez-SaguerICharacterising the safety and use of C1 inhibitor in routine clinical practice: interim results from a European Registry studyEuropean Academy of Allergy and Clinical Immunology CongressBarcelona, Spain2015
- BussePBygumAEdelmanJSafety of C1-esterase inhibitor in acute and prophylactic therapy of hereditary angioedema: findings from the ongoing international Berinert patient registryJ Allergy Clin Immunol Pract20153221321925609333
- ReadNLimETarziMDHildick-SmithPBurnsSFidlerKJPaediatric hereditary angioedema: a survey of UK service provision and patient experienceClin Exp Immunol2014178348348825113655
- DalyPBEvansJHKobayashiRHHome-based immunoglobulin infusion therapy: quality of life and patient health perceptionsAnn Allergy19916755045101958004
- GardulfANicolayUMathDChildren and adults with primary antibody deficiencies gain quality of life by subcutaneous IgG self-infusions at homeJ Allergy Clin Immunol2004114493694215480339
- RosendaalFRSmitCVarekampIModern haemophilia treatment: medical improvements and quality of lifeJ Intern Med199022866336402280241
- SymonsCRossiOMagerlMAndritschkeKPractical approach to self-administration of intravenous C1-INH concentrate: a nursing perspectiveInt Arch Allergy Immunol2013161suppl 1172023689240
- ShapiroRIntravenous self-administration of C1-INH concentrate for hereditary angioedema: a retrospective report of real-world experience in 13 patientsJ Angioedema20131228
- LonghurstHJFarkasHCraigTHAE international home therapy consensus documentAllergy Asthma Clin Immunol201062220667125
- BoysenHBBouilletLAygören-PürsünEChallenges of C1-inhibitor concentrate self-administrationInt Arch Allergy Immunol2013161suppl 1212523689241
- GowerRGLumryWRDavis-LortonMAJohnstonDTBussePJCurrent options for prophylactic treatment of hereditary angioedema in the United States: patient-based considerationsAllergy Asthma Proc201233323524022584192
- ClinicalTrials.gov [webpage on the Internet]A Study to Evaluate the Clinical Efficacy and Safety of Subcutaneously Administered C1-Esterase Inhibitor for the Prevention of Angioedema Attacks in Adolescents and Adults with Hereditary Angioedema2015 NCT02584959. Available from: www.ClinicalTrials.gov/ct2/show/NCT02584959Accessed January 6, 2016
- ZurawBLCicardiMLonghurstHJPhase II study results of a replacement therapy for hereditary angioedema with subcutaneous C1-inhibitor concentrateAllergy201570101319132826016741
- ClinicalTrials.gov [webpage on the Internet]A Study to Evaluate the Clinical Efficacy and Safety of Subcutaneously Administered C1-Esterase Inhibitor in the Prevention of Hereditary Angioedema2013 NCT01912456. Available from: www.ClinicalTrials.gov/ct2/show/NCT01912456Accessed January 6, 2016
