Abstract
Background
Non-adherence to inhaled corticosteroids (ICSs) is a major risk factor for poor asthma control in children. However, little is known about the effect of adherence to ICS on the incidence of asthma exacerbations. The objective of this study was to examine the effect of poor adherence to ICS on the risk of exacerbations in children with asthma.
Methods
In this nested case–control study using data from the Dutch PHARMO Record Linkage System, children aged 5–12 years who had an asthma exacerbation needing oral corticosteroids or hospital admission were matched to patients without exacerbations. Refill adherence was calculated as medication possession ratio from ICS-dispensing records. Data were analyzed using a multivariable multiplicative intensity regression model.
Results
A total of 646 children were included, of whom 36 had one or more asthma exacerbations. The medication possession ratio was 67.9% (standard deviation [SD] 30.2%) in children with an exacerbation versus 54.2% (SD 35.6%) in the control group. In children using long-acting beta-agonist, good adherence to ICS was associated with a higher risk of asthma exacerbations: relative risk 4.34 (95% confidence interval: 1.20–15.64).
Conclusion
In children with persistent asthma needing long-acting beta-agonist, good adherence to ICS was associated with an increased risk of asthma exacerbations. Possible explanations include better motivation for adherence to ICS in children with more severe asthma, and reduced susceptibility to the consequences of non-adherence to ICS due to overprescription of ICS to children who are in clinical remission. Further study into the background of the complex interaction between asthma and medication adherence is needed.
Introduction
Asthma is the most common chronic disease seen in children in the Western world, with an estimated prevalence of 5%–10%.Citation1,Citation2 Inhaled corticosteroids (ICSs) have an important place in asthma therapy and is prescribed when asthmatic symptoms cannot be sufficiently controlled by short-acting beta-agonist (SABA) alone. The aim of ICS treatment is reaching and maintaining good asthma control, which is characterized by a low frequency and severity of asthma symptoms, no limitation of physical activities, and a limited need for reliever/rescue treatment with SABA.Citation2 In clinical trials, ICS has proved to be effective, reducing exacerbations by 55% compared to placebo or SABA alone.Citation3 However, more than half of the childhood population (6–16 years) with doctor-diagnosed asthma has insufficient control according to the Global Initiative for Asthma (GINA) recommendations.Citation4 As a result of poor asthma control, asthma exacerbations needing hospitalization occur with an estimated incidence of one to two per 1,000 child-years (data from the USA).Citation5,Citation6
Risk factors for poor asthma control include poor socioeconomic status and ethnic minority affiliation,Citation7–Citation9 young age,Citation6 parental smoking,Citation10 negative parental perceptions about medication,Citation11 and exposure to allergens, pollutants, and viral infections.Citation2 A critical factor for maintaining good asthma control seems adherence to ICS treatment, which ranges from 40% to 70% in children.Citation12–Citation14 In a study among 102 children, adherence to ICS was 17% higher in patients with controlled asthma than in those with uncontrolled asthma (P<0.001).Citation15 A similar result was reported in a recent study in 81 Dutch children which showed a trend of higher levels of asthma control with higher levels of adherence to ICS (P=0.028).Citation16
Although the effect of adherence to ICS on asthma control is generally positive, conflicting evidence exists on the occurrence of episodes of very poorly controlled asthma: asthma exacerbations, needing a short course of oral corticosteroids, or hospital admission in children. A recent systematic review reported that high levels of adherence to ICS were associated with a reduced risk of severe asthma exacerbations in children,Citation17 but increasing evidence exists that the relation between adherence to ICS and the occurrence of exacerbations is less straight forward than we used to think. Several studies reported a reverse association between adherence and risk of severe asthma exacerbations.Citation18–Citation21 Rust et al,Citation22 for example, found that 1.9% of children with refill adherence to ICS <50% had a hospital admission for asthma versus 3.2% of children with refill rate >50% (P<0.01). In another study, patients reduced their prescribed controller medication without negative consequences,Citation23 whereas other patients continued to have poor outcomes despite good adherence.Citation24 Apart from the heterogeneity of the study results, all studies failed to address an essential methodological issue, being the temporal relation between (non-)adherence to ICS use and the asthma exacerbations. The former should precede the latter; otherwise, a causal relationship between both variables is not plausible.
To overcome this methodological issue, we designed a study into the temporal relation between adherence to ICS and the incidence of asthma exacerbations in children in a general real-life population of children with asthma. The aim of our study was to measure refill adherence to ICS in children with asthma aged 5–12 years, and to study its association with the frequency of asthma exacerbations needing a short course of oral corticosteroids or a hospital admission. Our hypothesis was that good refill adherence would be associated with a reduced risk of severe asthma exacerbations.
Methods
Setting
In this nested case–control study, a cohort of 150,000 patients was randomly selected from a subset of the PHARMO Record Linkage System (RLS). The PHARMO RLS contains medication-dispensing records from community pharmacies linked to hospital discharge records of more than two million inhabitants of the Netherlands. The computerized drug-dispensing histories contained detailed data about the dispensed medicines, dosing regimens, and type of prescriber. The hospital records included detailed information on primary and secondary diagnoses, procedures, and dates of hospital admission and discharge. All diagnoses were coded according to the International Classification of Diseases (ICD), 9th Revision, Clinical Modification.Citation25
The privacy regulation of the PHARMO institute was approved by the Dutch Data Protection Authority. According to the Dutch legislation, neither obtaining informed consent nor approval by a medical ethics committee is obligatory for database studies without direct patient involvement.Citation26 Hence, formal consent is not required for this retrospective, anonymized database study.
Study population
The study population included all children who were ≥5 and ≤12 years of age on the cohort entry date and had filled their first prescription of ICS between 1998 and 2008. Its dispensing date was considered the cohort entry date. The following types of ICS or combinations with beta-agonists were allowed: beclomethasone, budesonide, fluticasone, ciclesonide, salmeterol/fluticasone, and formoterol/budesonide. Patients were included if they did not use ICS in the 1 year preceding the cohort entry date. Patients had to be registered in the PHARMO database for at least 1 year before and 1 year after the cohort entry date. Patients taking ICS using a nebulizer were excluded from the cohort.
Outcome measures
Severe asthma exacerbations, requiring admission to hospital or a short course of oral corticosteroids, were used as primary outcome measure. The date of the exacerbation was called the “index date”. In the hospital discharge records, patients discharged with ICD-code 493 (asthma) or ICD-code 786.07 (wheezing) were counted as asthma-related admissions to hospital. Short courses of oral corticosteroid use were identified from drug-dispensing records as episodes of oral corticosteroid use (Anatomical Therapeutic Chemical code: H02AB) of not >15 days.Citation2 Incorrectly registered (eg, double) corticosteroid medication records, and records of oral corticosteroids not prescribed by a general practitioner, pediatrician, or pulmonologist were not considered.
Asthma exacerbations were not included: 1) if the cohort entry date was <3 months before the index date, as this observation period is too short to calculate a reliable measure for refill adherence; 2) if a previous event had occurred <12 months before the event date, as both events may not be independent; and 3) if there were less than two ICS prescriptions in the year prior to the index date, since no reliable calculation of refill adherence is then possible.
Determinants
The primary determinant in our study, that is, refill adherence to ICS, was calculated as the medication possession ratio (MPR). First, all ICS dispenses were converted into treatment episodes of consecutive use of ICS following the method of Catalan and Lelorier.Citation27 Switches from one to another type of ICS and changes in dose regimen were allowed. If possible, atypical ICS episodes caused by incorrect registration of medication records were corrected; otherwise, patients were excluded. The refill adherence was calculated as the ratio of the number of daily ICS dosages dispensed and the number of days in the episodeCitation28 for a period of 12 months preceding the index date.
The following co-variables were included: Sex and age were noted at cohort entry date. At index date, age, type of ICS, type of prescriber of ICS, type of inhaler, daily dose, dosing frequency of ICS, and time from cohort entry date to index date were obtained. Finally, we collected the number of dispenses of co-medications within 3 months and within 12 months before the index date.
Matching cases and controls
Patients who had an asthma exacerbation (cases) were matched with control patients who at that moment had the same age (±1 year) and had no asthma exacerbation in the previous 12 months (incidence density sampling). Under the condition that there had not been a previous asthma exacerbation in the preceding 12 months, cases could also be analyzed as control patients, and control patients could be analyzed more than once at different moments in follow-up. For this reason, the results of this study were reported as number of “event moments” (with exacerbation) and matched “control moments” (without exacerbation) instead of “cases” and “control patients”. It is noted that control moments (without exacerbations) could originate both from patients with exacerbations and from patients without.
Data analysis
Using the approach of DupontCitation29 and software “PS Power and Sample Size Calculations”,Citation30 we determined the sample size required to detect a twofold, 2.5-fold, or threefold increase in risk of asthma exacerbation between ICS adherence ≥80% and <80% with 0.8 power at the 0.05 significance level. Assuming that each case is matched with minimal 30 controls, the probability of ICS adherence ≥80% among controls is 0.2, and the correlation coefficient for ICS adherence between matched cases and controls is 0.2; the required sample size is 88, 48, or 32 case patients with 30 matched control(s) per case.
A multiplicative intensity model was applied to assess the effect of refill adherence to ICS on the occurrence of asthma exacerbations, using statistical software “R” (version 2.15.2, Vienna, Austria) with library “survival”.Citation31 The multiplicative intensity model was introduced by Aalen in 1978Citation32 and is a generalization of Cox proportional hazards regression for multiple recurrent events per subject, time-dependent covariates, left truncated and left censored data, and calendar timescale.
Co-variables that showed (borderline) statistical significance (P<0.1) in the univariable analysis were investigated for confounding by adding them to the statistical model and leaving them in the model if the regression coefficient changed by >10%.
The following co-variables were investigated for effect modification of the association of adherence with asthma exacerbations: recent use of SABA or short-acting muscarinic antagonists, both as a measure of asthma control; recent use of long-acting beta-agonist (LABA), as a measure of asthma severity; and recent use of systemic antibiotics, since asthma exacerbations are often triggered by respiratory infections. These potential effect modifiers were investigated by adding the interaction term to the statistical model; if its regression coefficient was significantly >0.0 (P<0.05), the parameter was considered an effect modifier.
Results
A total of 934 children matched the inclusion criteria, and 646 children also met the requirements for correct calculation of refill adherence (). In this final study population, 365 (57%) children were male, and the mean age was 8.1 years (standard deviation [SD] 2.2) at cohort entry date and 9.6 years (SD 2.1) at index date.
Figure 1 Flowchart for patient selection.
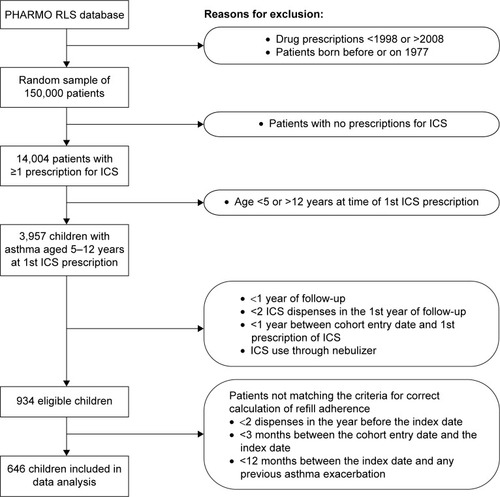
The frequency of recent use of SABA, LABA, combined ICS and LABA, nasal decongestants, and systemic antibiotics differed significantly between event moments (with an asthma exacerbation) and control moments (without exacerbations) (). The use of asthma medication not reported in was negligibly low in the 12 months preceding the events.
Table 1 Characteristics of analyzed patient moments
A total of 40 asthma exacerbations in 36 patients were included in the analysis: 32 short courses of oral corticosteroids and eight hospital admissions for asthma. The incidence density rate of asthma exacerbations needing hospital admission was 8.1/1,000 patient-years, and 43.8/1,000 patient-years for short courses of oral corticosteroids. Asthma exacerbations were matched to 1,596 control moments without an event with a mean of 42 control moments per stratum (range: 4–72).
The mean MPR for ICS was 67.9% (SD 30.2%) in the 12 months before the event moments versus 54.2% (SD 35.6%) for the control moments. The proportion of patient moments with MPR ≥80% was 35.0% (SD 48.3) for event moments and 20.2% (SD 40.1) for control moments ().
Table 2 Refill adherence to ICS in all children, in children with recent LABA use, and in children without recent LABA use
Recent LABA use, within 3 months before the index date, was identified as an effect modifier. Therefore, data were stratified, and two separate models were presented (). In the non-LABA stratum, the intensity ratio of asthma exacerbations was 1.07 (95% confidence interval [CI]: 0.39–2.92) for refill adherence to ICS ≥80% and 4.34 (95% CI: 1.20–15.64) in patients with recent LABA use, both adjusted for recent SABA use (within 3 months before the index date) as the only confounder.
Table 3 Association of refill adherence to ICS with the intensity of asthma exacerbations in children using LABA and in children not using LABA
Discussion
In children with persistent asthma needing the use of LABA, we found that good refill adherence to ICS was associated with an increased risk of asthma exacerbations. No association was found in children not using LABA. Therefore, we rejected our hypothesis that good refill adherence was associated with a reduced risk of severe asthma exacerbations.
These results contrast to earlier findings in which high adherence to ICS was associated with good improved asthma controlCitation15,Citation33 and with a reduced risk of asthma exacerbations.Citation17 Only a few earlier studies reported a reverse association.Citation18–Citation21 A possible explanation for the higher observed level of adherence in children with exacerbations is that the children with exacerbations had a lower level of asthma control to start with, which would have motivated them to take their ICS more adherently. Poorly controlled asthma would therefore be associated with higher adherence rates. In answer to the question why higher levels of adherence not necessarily lead to better asthma control and to less asthma exacerbations, Klok et al hypothesized that the minimum level of adherence needed for achieving asthma control is higher in patients with ongoing persistent asthma, than in patients with asthma in clinical remission.Citation16 Patients in the latter group, who are easily overtreated with ICS, would maintain asthma control at a lower level of adherence than the former.
In our study, recent LABA use, as a proxy for asthma severity, was identified as an effect modifier. Good adherence to ICS was only associated with a higher risk of asthma exacerbations in children with more severe asthma (needing the use of LABA). Apparently, the intake of ICS was less critical for maintaining asthma control in children with less severe asthma. As a result, these children were possibly less motivated for taking ICS adherently.
A strength of this study is our large patient sample (n=934) and long follow-up period (10 years). Also, contrary to earlier studies that used pharmacy data,Citation34,Citation35 we limited the refill-adherence calculation to the period immediately preceding the asthma exacerbation. Regarding the limited biological half-life of ICS, it is considered unlikely that non-adherence to ICS leads to the occurrence of an asthma exacerbation >12 months in the future. Our approach also ruled out the effect of ICS test doses and short episodes of ICS use after the occurrence of an exacerbation or pulmonary infection, which may otherwise bias adherence calculation. Another strength is that pharmacy records were combined with hospital discharge data, so that both asthma exacerbations treated with a short course of oral corticosteroids and those needing hospital admission could be included into the analysis.
A limitation of our study is that the use of pharmacy record data tends to overestimate the actual medication adherence. In one of the sparse studies evaluating the magnitude of this overestimation, a 9% difference was found between refill adherence and electronically measured dose count.Citation36 This overestimation seems too small to explain why we have found a higher adherence rate in children with an exacerbation. Another potential source of overestimation of adherence to ICS was our inclusion criterion that demanded a minimum of two ICS dispenses in the 12 months before the index date. This criterion was introduced to ensure valid MPR calculation (ie, limited discontinuation of ICS use). However, our sensitivity analysis with the inclusion criterion of at least one ICS dispensing in the preceding 12 months showed a similar association between adherence and exacerbations (data not reported). Like most patient databases, PHARMO RSL does not contain detailed data on asthma control. We have dealt with this issue by using recent SABA use as a proxy for asthma control, but this is only one out of three GINA indicators for asthma control.Citation2 It can also not be ruled out that asthma exacerbations needing a short course of oral corticosteroids were missed if patients used oral corticosteroids as chronic treatment. This is unlikely to change the study results, since the chronic use of systemic corticosteroids was rare (0.4%) in this study. In addition, asthma exacerbations that were treated with only a temporary increased ICS dose might have remained undetected in our study. These mild asthma exacerbations, however, were outside the scope of our study, since we focused on severe asthma exacerbations. A final limitation of our database study is that it required a highly developed Information and Communication Technology infrastructure for administering pharmacy records and hospital discharge data. This may be an obstacle for researchers trying to reproduce the results of our study in regions where this infrastructure is lacking.
Based on the results of this study, clinicians treating children with asthma should be aware of the complex relation between adherence to ICS and asthma control. Patients having an asthma exacerbation often have good adherence to ICS, while other patients with poor adherence to ICS do not suffer any clinical consequences. The former phenomenon may involve patients who self-manage their ICS therapy according to, for example, their current asthma control or disease burden. We think that the latter is likely to be caused by overprescription of ICS, in case of which stepwise dose reductions or even discontinuation of ICS therapy may be required.
In future research, prospective studies using objective measures are needed to further assess the complex relation between asthma control and adherence to ICS, both in children who are in clinical remission and in children with unstable asthma. This would require longitudinal cohort studies in which objective adherence measures such as electronic medication monitoring are used, and in which important potential confounders, like asthma control, are taken into account. However, this would require costly electronic monitoring, long follow-up periods, and large study samples (considering the generally low incidence of severe asthma exacerbations). In order to avoid bias by overprescription of ICS or patient-initiated dose adjustments and interruptions, we suggest a study approach in which, prior to the study, ICS doses are titrated to the lowest levels on which asthma control is just maintained. This would enhance the clinical impact of non-adherence to ICS, providing a clearer view on the complex association of adherence to ICS with asthma control and the risk of asthma exacerbations.
Conclusion
In children with persistent asthma who also used LABA, adherence to ICS was associated with an increased risk of asthma exacerbations. No association was found in children not using LABA. Prospective studies into the complex relation between adherence to ICS and asthma control are needed.
Disclosure
Patricia MLA van den Bemt has received an unconditional research grant from the Dutch government (ZonMw) and from GlaxoSmithKline for a different, investigator-initiated study on medication adherence in children with asthma. Liset van Dijk has received unrestricted grants from Astra Zeneca, Bristol-Myers Squibb, and Pfizer for studies not related to this study. The other authors report no conflicts of interest in this work.
References
- MasoliMFabianDHoltSBeasleyRGlobal Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee reportAllergy200459546947815080825
- GINAGlobal strategy for asthma management and prevention. Global Initiative for Asthma (GINA)2015 Available from: http://www.ginasthma.org/Accessed December 29, 2015
- SinDDManJSharpeHGanWQManSFPPharmacological management to reduce exacerbations in adults with asthma – a systematic review and meta-analysisJAMA2004292336737615265853
- HammerSCRobroeksCMHHTvan RijCActual asthma control in a paediatric outpatient clinic population: do patients perceive their actual level of control?Pediatr Allergy Immunol200819762663318221469
- McConnochieKMRussoMJMcBrideJTSzilagyiPGBrooksAMRoghmannKJSocioeconomic variation in asthma hospitalization: excess utilization or greater need?Pediatrics19991036e7510353972
- JohnstonNWSearsMRAsthma exacerbations. 1: epidemiologyThorax200661872272816877691
- LieuTALozanoPFinkelsteinJARacial/ethnic variation in asthma status and management practices among children in managed medicaidPediatrics2002109585786511986447
- ThakurNMartinMCastellanosESocioeconomic status and asthma control in African American youth in SAGE IIJ Asthma201451772072824654704
- KopelLSPhipatanakulWGaffinJMSocial disadvantage and asthma control in childrenPaediatr Respir Rev201415325626224928775
- ZahranHSBaileyCMQinXMoormanJEAssessing asthma severity among children and adults with current asthmaJ Asthma201451661061724506700
- KlokTKapteinAADuivermanEJBrandPLHigh inhaled corticosteroids adherence in childhood asthma: the role of medication beliefsEur Respir J20124051149115522362847
- BenderBWamboldtFSO’ConnorSLMeasurement of children’s asthma medication adherence by self report, mother report, canister weight, and Doser CTAnn Allergy Asthma Immunol200085541642111101187
- VasbinderEDahhanNWolfBThe association of ethnicity with electronically measured adherence to inhaled corticosteroids in childrenEur J Clin Pharmacol201369368369022955894
- CelanoMGellerRJPhillipsKMZimanRTreatment adherence among low-income children with asthmaJ Pediatr Psychol19982363453499824922
- JentzschNSCamargosPSarinhoESBousquetJAdherence rate to beclomethasone dipropionate and the level of asthma controlRespir Med2012106333834322188844
- KlokTKapteinAADuivermanEJBrandPLIt’s the adherence, stupid (that determines asthma control in preschool children)!Eur Respir J201443378379123845718
- EngelkesMJanssensHMDe JongsteJCSturkenboomMCJMVerhammeKMCMedication adherence and the risk of severe asthma exacerbations: a systematic reviewEur Respir J201545239640725323234
- PriceDThomasMHaughneyJReal-life comparison of beclometasone dipropionate as an extrafine- or larger-particle formulation for asthmaRespir Med20131077987100023643486
- McMahonADLipworthBJDaveyPGMorrisADMacdonaldTMContinuity of prescribing with inhaled corticosteroids and control of asthmaPharmacoepidemiol Drug Saf20009429330319025832
- SmithKWarholakTArmstrongELeibMRehfeldRMaloneDEvaluation of risk factors and health outcomes among persons with asthmaJ Asthma200946323423719373629
- OsmanLMFriendJALeggeJSDouglasJGRequests for repeat medication prescriptions and frequency of acute episodes in asthma patientsJ Asthma199936544945710461934
- RustGZhangSReynoldsJInhaled corticosteroid adherence and emergency department utilization among Medicaid-enrolled children with asthmaJ Asthma201350776977523734973
- RiekertKAButzAMEgglestonPAHussKWinkelsteinMRandCSCaregiver-physician medication concordance and undertreatment of asthma among inner-city childrenPediatrics20031113e214e22012612274
- SmythARBarbatoABeydonNRespiratory medicines for children: current evidence, unlicensed use and research prioritiesEur Respir J201035224726519840958
- Breekveldt-PostmaNSErkensJAAalbersRde VenMJTVLammersJWJHeringsRMCExtent of uncontrolled disease and associated medical costs in severe asthma – a PHARMO studyCurr Med Res Opin200824497598318282372
- Breekveldt-PostmaNSKoerselmanJErkensJATreatment with inhaled corticosteroids in asthma is too often discontinuedPharmacoepidemiol Drug Saf200817441142218205251
- CatalanVSLelorierJPredictors of long-term persistence on statins in a subsidized clinical populationValue Health20003641742616464201
- AndradeSEKahlerKHFrechFChanKAMethods for evaluation of medication adherence and persistence using automated databasesPharmacoepidemiol Drug Saf200615856557416514590
- DupontWDPower calculations for matched case control studiesBiometrics1988444115711683233252
- DupontWDPlummerWDPower and sample size calculations: a review and computer programControlled Clinical Trials1990111161282161310
- AndersenPKBorganØGillRDKeidingNStatistical Models Based on Counting Processes, Springer Series in StatisticsNew YorkSpringer-Verlag1993
- AalenOONon-parametric inference for a family of counting processesAnn Statist19786701726
- KosterESRaaijmakersJAMVijverbergSJHMaitland-van der ZeeAHInhaled corticosteroid adherence in paediatric patients: the PACMAN cohort studyPharmacoepidemiol Drug Saf201120101064107221953846
- LasmarLCamargosPChampsNSAdherence rate to inhaled corticosteroids and their impact on asthma controlAllergy200964578478919183166
- MilgromHBenderBAckersonLBowryPSmithBRandCNoncompliance and treatment failure in children with asthmaJ Allergy Clin Immunol1996986 Pt 1105110578977504
- ChooPWRandCSInuiTSValidation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapyMed Care199937984685710493464
