?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Despite clearly improved clinical outcomes for prophylaxis compared to on-demand therapy, on average only 56% of patients diagnosed with severe hemophilia receive prophylactic factor replacement therapy in the US. Prophylaxis rates generally drop as patients transition from childhood to adulthood, partly due to patients becoming less adherent when they reach adulthood. Assessment of patient preferences is important because these are likely to translate into increased treatment satisfaction and adherence. In this study, we assessed preferences and willingness to pay (WTP) for on-demand, prophylaxis, and longer acting prophylaxis therapies in a sample of US hemophilia patients.
Methods
Adult US hemophilia patients and caregivers (N=79) completed a discrete-choice survey that presented a series of trade-off questions, each including a pair of hypothetical treatment profiles. Using a mixed logit model for analysis, we compared the relative importance of five treatment characteristics: 1) out-of-pocket treatment costs (paid by patients), 2) factor dose adjustment, 3) treatment side effects, 4) availability of premixed factor, and 5) treatment effectiveness and dosing frequency. Based on these attribute estimates, we calculated patients’ WTP.
Results
Out-of-pocket treatment costs (P<0.001), side effects (P<0.001), and treatment effectiveness and dosing frequency (P<0.001) were found to be statistically significant in the model. Patients were willing to pay US $410 (95% confidence interval: $164–$656) out of pocket per month for thrice-weekly prophylaxis therapy compared to on-demand therapy and $360 (95% confidence interval: $145–$575) for a switch from thrice-weekly to once-weekly prophylaxis therapy.
Conclusion
Improvements in treatment effectiveness and dosing frequency, treatment side effects, and out-of-pocket costs per month were the greatest determinants of hemophilia treatment choice and WTP. The positive preferences and WTP for longer acting prophylactic therapies suggest that the uptake is likely to increase adherence, improving treatment outcomes. These preferences should also inform the Food and Drug Administration’s assessment of new longer acting hemophilia therapies.
Introduction
In the US, ~20,000 people are estimated to have hemophilia according to the Center for Disease Control and Prevention.Citation1 Hemophilia is an X-linked congenital bleeding disorder caused by a mutation in the genes coding for clotting factors VIII (hemophilia A) or IX (hemophilia B). Without properly functioning factors VIII and IX, patients are vulnerable to serious bleeding episodes and associated degenerative joint disease as well as other potential morbidities.Citation2 There are two approaches for the treatment of hemophilia: 1) on-demand therapy provided on an as-needed basis when bleeding occurs and 2) prophylactic (preventative) factor replacement therapy (given three to four times per week for hemophilia A or typically two times per week for hemophilia B) to prevent anticipated bleeding episodes and joint damage.Citation3 Prophylactic factor replacement therapy not only reduces the number of annual bleeds but also reduces the likelihood of permanent and disabling joint damage. For example, in a landmark 2007 study of hemophilia A, 93% of patients receiving prophylactic therapy showed no signs of joint damage compared to only 55% of those using on-demand therapy.Citation4
Despite clearly improved clinical outcomes for prophylaxis compared to on-demand therapy, only 56% of patients diagnosed with severe hemophilia receive prophylactic therapy in the US, as reported by the Universal Data Collection (UDC) system of the Center for Disease Control and Prevention that has health data on >80% of the patients treated at Hemophilia Treatment Centers (HTCs) in the US.Citation5 Rates of prophylaxis also vary widely with age. A significant proportion of patients choose to reduce or discontinue prophylaxis during their transition from childhood to adolescence and adulthood.Citation6–Citation9 Approximately one-third of the adults who receive prophylaxis as children choose to switch to on-demand treatment.Citation9 A recent survey on practice patterns in the US for hemophilia A and hemophilia B (71 of the 126 US hemophilia treatment centers participated in the survey) reported that only 33% of the adults with hemophilia over the age of 25 years were on continuous prophylactic factor replacement therapy. The same survey reported that patients’ adherence to treatment is lowest in the 18–24 years age group (64% adherence) followed by 25–44 years age group (65% adherence).Citation10 This is partly because patients become more independent and less adherent as they reach adulthoodCitation6,Citation11 and partly due to a reduction in the perceived benefits associated with prophylaxis in adulthood.Citation6,Citation12 The most frequent patient-reported barriers to adherence to prophylactic therapy are the reduction of symptoms, forgetfulness, lack of time, and inconvenience associated with frequent injections.Citation6,Citation11,Citation13 In addition, financial constraint can be a significant barrier to prophylactic therapy; the cost of factor concentrate inhibits the widespread use and uptake of prophylactic therapy.Citation6,Citation11,Citation13 In one retro spective study, clotting factor consumption accounted for an average of 72% of total hemophilia treatment costs.Citation14 In terms of treatment strategy, the volume of factor concentrate required for prophylactic therapy is typically two to three times greater than that required for on-demand therapyCitation4,Citation15 and therefore entails much higher costs for Medicaid and third-party payers. In a randomized controlled trial comparing the clinical outcomes of on-demand versus prophylactic factor replacement treatment, Manco-Johnson et alCitation4 noted that given an average cost of US $1 per unit of recombinant factor VIII, the cost of prophylactic therapy could reach as high as $300,000 per year for a 50 kg child.
In 2012, the US Food and Drug Administration (FDA) was congressionally mandated to incorporate patient perspective in the regulatory processes through the patient-focused drug development initiative. Hemophilia was one of 20 diseases selected by the FDA for this drug development program that attempts to incorporate patient preferences.Citation16 Health care providers, third-party payers, and policy makers should also consider patient preferences because studies from other disease areas suggest that treatment preferences are likely to translate directly into increased treatment satisfaction and adherence. In turn, this will lead to greater treatment effectiveness and ultimately a reduced patient and societal burden of disease.Citation17–Citation21 Patient preferences for different treatment options depend not only on clinical efficacy but also on other treatment characteristics such as the frequency and convenience of drug administration and the likelihood, severity, and importance of side effects.
The UDC system also reports that 39% of the hemophilia patients have some sort of government-funded insurance, while 51% of the patients have commercial insurance.Citation5 Although most of the cost related to factor concentrates is reimbursed via insurance in the US for these hemophilia patients, a “willingness-to-pay” (WTP) study provides an opportunity to quantify the value of these treatment characteristics simultaneously and in the familiar units of money. Here, we conducted a discrete-choice experiment (DCE) to evaluate patient preferences and WTP for relevant treatment characteristics associated with on-demand, prophylactic, and longer acting prophylactic hemophilia therapies.
Methods
Discrete-choice experiment
DCEs have been used to assess preferences for a variety of health interventions such as outcomes of life-saving technology with liver transplant applications, osteoporosis and osteoarthritis drug treatment, chronic hepatitis B treatment outcomes, non-small-cell lung cancer treatment outcomes, screening tests for Down syndrome, asthma-related treatment risks, and irritable bowel syndrome treatment outcomes.Citation22–Citation29 The theoretical background and rationale supporting DCEs have been discussed in detail by Johnson et al in a report that summarizes good research practices for constructing DCEs.Citation30 In a DCE, respondents must choose their most preferred treatment alternative from a set of treatment profiles, assuming that these are the only treatment options available. DCEs therefore allow one to compare the relative importance of various treatment attributes. This study was approved by the Children’s Hospital Los Angeles Institutional Review Board, and informed consent of the participants was waived due to the anonymous nature of the survey.
Identification of treatment characteristics
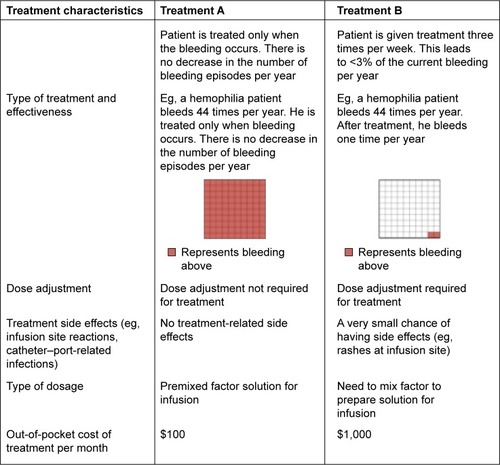
A comprehensive literature review was conducted to identify important treatment characteristics (also known as attributes) in hemophilia treatment. With the help of the hematologist coauthor, the five most relevant treatment characteristics and their possible variations (ranges; ) were used to create hypothetical treatment profiles. We included: 1) treatment effectiveness and frequency of dosing, 2) treatment side effects such as rashes and reactions at the infusion site or catheter–port infections, 3) a need for dose adjustments, 4) availability of premixed factor solution for infusion, and 5) out-of-pocket treatment costs. Choices designed included a variety of levels designed to reflect the entire relevant range of each attribute (). These selected treatment characteristics were shown to five hemophilia patients who confirmed that they were of interest and so this list was finalized for the rest of the study.
Table 1 Treatment characteristics (attributes) and ranges (levels) used to create hypothetical treatment profiles
Design of experiment
The five treatment characteristics with all included levels produced 128 possible hypothetical treatment profiles. Since it was not possible to evaluate each of the 128 treatment profiles within the study, a fractional factorial design was used.Citation31 The orthoplan procedure of SPSS statistical software, Version 22 (IBM Corporation, Armonk, NY, USA) was used to generate a main-effects design that reduced the number of required treatment profiles to 25. These 25 treatment profiles were randomized and paired to form choice tasks. For the DCE survey, each respondent answered demographic questions followed by ten treatment-choice questions. An example choice question is illustrated in .
Survey respondents and setting
For the pilot study, the survey was administered in the waiting room of the Children’s Hospital Los Angeles. A total of five patients (≥18 years old) or the parents/caregivers of patients (<18 years old) participated in the pilot survey. As is standard for this type of research, parents and caregivers of patients younger than 18 years acted as proxy respondents for the patients.
As the number of hemophilia patients is very small and thus potential respondents may have been hard to reach, we elected to conduct the survey during a major national convention for patients held by the National Hemophilia Foundation in Anaheim, CA, in 2013. As such, the surveys were self-administered on iPads provided to hemophilia patients (≥18 years old) or the parents/caregivers of patients (<18 years old). In all, 79 patients and caregivers completed the survey. This sample size was deemed sufficient to estimate the main effects in our statistical model by Louviere’s sample size estimation method.Citation31
Data analysis
Descriptive statistics were performed on demographic responses. All analyses were performed using Stata, Version 13.0 (StataCorp LP, College Station, TX, USA, 1997). To investigate preferences for hemophilia therapies, a theoretical framework based on a mixed logit model was adopted. Mixed logit models allow attribute coefficients to vary across respondents, accounting for preference heterogeneity and improving the realism of model assumptions. Mixed logit models also adjust the standard errors of utility estimates to account for repeated choices by the same individual. Our mixed logit model for estimation was (EquationEquation 1(1) ):
(1) where U is the utility derived from choosing a given treatment, V is the observed utility that can be calculated as the sum of parameters β1 through β8, estimates of the strength of preference for each attribute (on-demand therapy, twice per week prophylaxis, once per week prophylaxis, once biweekly prophylaxis, dose adjustment not required, treatment side effects, need to mix factor, and out-of-pocket costs), and ε represents an unobservable error term. The attribute levels of thrice-weekly prophylaxis, dose adjustment required, no treatment-related complications, and availability of premixed factor solution were treated as default reference levels for analyses. For example, in EquationEquation 1
(1) , β1 gives the change in utility for on-demand therapy rather than thrice-weekly prophylactic therapy.
All attribute variables were considered random factors except for out-of-pocket costs, which were considered fixed in our model. P-values <0.05 (two-tailed tests) and non-overlapping 95% confidence intervals (CIs) were considered statistically significant differences for parameter estimates. Mixed logit models employ simulation-based estimation techniques with the number of Halton draws indicating the number of unique times that the mixed logit simulation was run. In our case, the mixed logit simulation was run 500 times to generate a robust output. It is important to run these simulations enough times so that the model converges and model estimates stabilize (ie, simulation-induced variance is minimized).
WTP for any given attribute was calculated in a straightforward manner based on the ratio of parameter estimate of the considered attribute to the negative of the parameter estimate of cost attribute using the rationale derived from Lancaster’s theory of demand.Citation32
Results
presents the demographic details of the 79 patients and parents or caregivers of patients who completed the survey. The mean age of the respondents was 40 years (range: 18–69). More than half of the respondents were non-Hispanic white, married, working full time or part time, and had completed at least 2 years of college.
Table 2 Survey respondent demographics
Patient preferences
As shown in , patients and caregivers considered treatment effectiveness and dosing frequency, out-of-pocket treatment costs, and side effects to be important while making treatment-related decisions. The parameter estimates for these characteristics were found to be statistically significant in the mixed logit estimation models (P<0.05). However, a comparison of preferences for prophylaxis dosing frequencies revealed that improvement in dosing frequency from three to two times per week was not found to be statistically significant in the model. By convention, the coefficients of the levels for each of the attributes are interpreted as deviations from the reference level (as described previously).
Table 3 Patient preferences for hemophilia therapies – mixed logit model analysis
We also analyzed the data to see if a difference in annual household income affects patient preferences. Because the sample size was small, we subset the respondents into only two categories: those with annual household income <$50,000 and those with annual household income ≥$50,000. We found that “treatment-related side effects” were not statistically significant in the subset of patients with annual household income <$50,000. Also, for this category, the patients only considered improvement in therapy from on-demand to once-weekly prophylaxis and to once biweekly prophylaxis to be statistically significant. For the subgroup of patients with annual household income ≥$50,000, the results were similar to the main analysis.
Willingness to pay
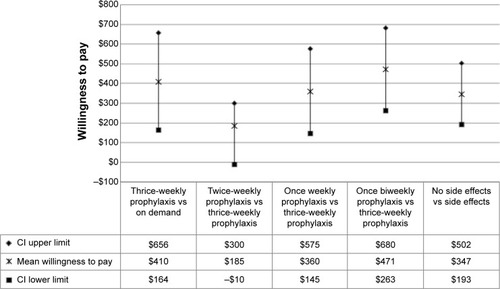
WTP was calculated only for those attributes found to be significant. For our patient survey, WTP was $410 out of pocket per month (95% CI: $164–$656) for thrice-weekly prophylaxis therapy compared to on-demand therapy (). WTP was $185 out of pocket per month (95% CI: −$10 to $300) for a switch from thrice-weekly to twice-weekly prophylaxis dosing, $360 (95% CI: $145–$575) for a switch from thrice-weekly to once-weekly prophylaxis dosing, and $471 (95% CI: $263–$680) for a switch from thrice-weekly to biweekly prophylaxis dosing. WTP was $347 out of pocket per month (95% CI: $193–$502) for a hemophilia therapy with no treatment side effects.
Figure 2 Willingness to pay (WTP) for significant treatment characteristics.
Abbreviation: CI, confidence interval.
Discussion
Hemophilia patients, physicians, and health care providers are faced with increasingly complex decisions regarding when and what treatments to initiate and continue as options expand and new, longer acting hemophilia therapies are introduced. The FDA’s patient-focused drug development initiative for hemophilia aims to incorporate patient and caregiver perspectives into the regulatory processes via direct testimonials.Citation16 Further incorporation of direct empirical evidence of patient and caregiver treatment preferences based on quantitative preference elicitation methods will allow the FDA to introduce formal evidence-based decision making into the regulatory process.
This study was based on a straightforward and well-accepted discrete-choice preference methodology and provides useful information on how hemophilia patients view and value different aspects of hemophilia therapies. To our knowledge, it is the first systematic quantification of WTP (out-of-pocket costs) for improvements to various aspects of hemophilia therapy from the patients’ perspective. Our valuation study determined that improvements to treatment effectiveness and dosing frequency, treatment side effects, and out-of-pocket costs were the primary concerns regarding treatment preferences and WTP. Patients indicated strong preferences and WTP more for prophylactic therapy than on-demand therapy. This is not surprising since prophylactic therapy is associated with better clinical outcomes.Citation4 In monetary terms, this preference corresponded to a mean WTP of $410 more out of pocket per month for the patients (). Patients also preferred longer acting prophylactic therapies and were willing to pay more for weekly and biweekly prophylactic regimens compared to thrice-weekly treatments. It is interesting to note that although improvement in prophylaxis dosing frequency from thrice-weekly to twice-weekly was not statistically significant (), it was still trending toward significance in our survey.
One limitation of this study pertains to the fact that data were collected from patients and caregivers attending a national conference requiring travel and related expenses, meaning that patients of lower socioeconomic status were less likely to participate. This could have led to some bias, particularly in the WTP results. Second, although the study used rigorous discrete-choice methodology, data were obtained only from 79 patients (and caregivers). It was also not possible to take into account the effect of baseline characteristics such as effect of race, income, and other demographics in the mixed logit model analysis because of the low sample size. However, as hemophilia is a relatively rare disease, the type and size of the sample used in this study are reasonable and typical. Lastly, this study reflects only patient preferences. Future research should focus on a comparison of preferences in the general population and among health care providers. Research should also focus on understanding the preferences for newer treatment options such as gene therapy for hemophilia patients.
Conclusion
In conclusion, improvements in treatment effectiveness and dosing frequency, treatment side effects, and out-of-pocket costs per month (paid by patients) were the greatest determinants of hemophilia treatment choice and WTP. The positive preferences and WTP for longer acting prophylactic therapies observed in this study may translate into increased treatment satisfaction and adherence, improving treatment outcomes.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We thank the Hemostasis and Thrombosis Center at Children’s Hospital Los Angeles, CA, USA, for providing funding and logistical support for this study. We also thank the patients who graciously volunteered their time to complete the survey.
Disclosure
The authors report no conflicts of interest in this work. This manuscript is not under consideration for publication in any other journal.
References
- Centers for Disease Control and PreventionHemophilia data and statistics in the United States Available from: http://www.cdc.gov/ncbddd/hemophilia/data.htmlAccessed September 14, 2014
- CallaghanMKaufmanRCellular processing of factor VIII and factor IXLeeCABerntorpEEHootsKWTextbook of Hemophilia2nd edCambridge, UKWiley-Blackwell20101323
- CoppolaADi CapuaMDi MinnoMNTreatment of hemophilia: a review of current advances and ongoing issuesJ Blood Med2010118319522282697
- Manco-JohnsonMJAbshireTCShapiroADProphylaxis versus episodic treatment to prevent joint disease in boys with severe hemophiliaN Engl J Med2007357653554417687129
- Centers for Disease Control and PreventionReport on the universal data collection program2014 Available from: http://www.cdc.gov/ncbddd/blooddisorders/udc/documents/report-udcprogram_january2005-december-2009_jan-2014.pdfAccessed September 14, 2014
- FranchiniMMannucciMPProphylaxis for adults with hemophilia: towards a personalised approach?Blood Transfus201210212312422337279
- RichardsMAltisentCBatorovaAShould prophylaxis be used in adolescent and adult patients with severe hemophilia? An European survey of practice and outcome dataHaemophilia20071347347917880432
- WalshCEValentinoLAFactor VIII prophylaxis for adult patients with severe hemophilia A: results of a US survey of attitudes and practicesHaemophilia2009151014102119493018
- van DijkKFischerKvan der BomJGScheibelEIngerslevJvan den BergHMCan long-term prophylaxis for severe hemophilia be stopped in adulthood? Results from Denmark and the NetherlandsBr J Haematol200513010711215982352
- ZappaSMcDanielMMarandolaJAllenGTreatment trends for haemophilia A and haemophilia B in the United States: results from the 2010 practice patterns surveyHemophilia201218e140e153
- FischerKProphylaxis for adults with hemophilia: one size does not fit allBlood Transfus201210216917322575241
- ValentinoLAControversies regarding the prophylactic management of adults with severe hemophilia AHaemophilia200915suppl 251820041959
- MakrisMProphylaxis in hemophilia should be life-longBlood Transfus20121016516822337280
- GlobeDRCurtisRGKoerperMAHUGSSteeringCommittee. Utilization of care in haemophilia: a resource-based method for cost analysis from the Haemophilia Utilization Group Study (HUGS)Haemophilia200410suppl 1637014987251
- JohnsonKAZhouZYCosts of care in hemophilia and possible implications of health care reformHematology Am Soc Hematol Educ Program2011201141341822160067
- http://c-path.org/wp-content/uploads/2013/09/PRO_Consortium_FDA_PDUFA_V_PatientFocusedDD.pdfAccessed July 5, 2015
- ReginsterJYRabendaVNeuprezAAdherence, patient preference and dosing frequency: understanding the relationshipBone2006384 suppl 1S2S616520104
- HuynhTKOstergaardAEgsmoseCMadsenORPreferences of patients and health professionals for route and frequency of administration of biologic agents in the treatment of rheumatoid arthritisPatient Prefer Adherence20148939924470758
- MakitaKOkanoHFuruyaTSurvey of the utility of once-monthly bisphosphonate treatment for improvement of medication adherence in osteoporosis patients in JapanJ Bone Miner Metab2015331556024442791
- PoulosCHauberABGonzálezJMTurpcuAPatients’ willingness to trade off between the duration and frequency of rheumatoid arthritis treatmentsArthritis Care Res201466710081015
- González-RojasNGiménezEFernándezMÁPreferences for oral anticoagulant treatment in the medium and long term prevention of stroke in non valvular atrial fibrillationRev Neurol2012551111922718404
- RatcliffeJBuxtonMPatients’ preferences regarding the process and outcomes of life-saving technology. An application of conjoint analysis to liver transplantationInt J Technol Assess Health Care199915234035110507193
- de Bekker-GrobEWEssink-BotMLMeerdingWJPolsHAKoesBWSteyerbergEWPatients’ preferences for osteoporosis drug treatment: a discrete choice experimentOsteoporos Int20081971029103718193329
- ArdenNKHauberABMohamedAFHow do physicians weigh benefits and risks associated with treatments in patients with osteoarthritis in the United Kingdom?J Rheumatol20123951056106322422497
- MohamedAFJohnsonFRHauberABLescrauwaetBMastersonAPhysicians’ stated trade-off preferences for chronic hepatitis B treatment outcomes in Germany, France, Spain, Turkey, and ItalyEur J Gastroenterol Hepatol201224441942622330238
- BridgesJFMohamedAFFinnernHWWoehlAHauberABPatients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysisLung Cancer201277122423122369719
- LewisSMCullinaneFNBishopAJChittyLSMarteauTMHallidayJLA comparison of Australian and UK obstetricians’ and midwives’ preferences for screening tests for down syndromePrenat Diagn2006261606616378328
- McTaggart-CowanHMShiPFitzgeraldJMAn evaluation of patients’ willingness to trade symptom-free days for asthma-related treatment risks: a discrete choice experimentJ Asthma200845863063818951253
- JohnsonFRHauberABOzdemirSLyndLQuantifying women’s stated benefit – risk trade-off preferences for IBS treatment outcomesValue Health201013441842320230550
- JohnsonFRLancsarEMarshallDConstructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task ForceValue Health201316131323337210
- LouviereJHensherDSwaitJStated Choice Methods Analysis and ApplicationsCambridge, UKCambridge University Press2000261265
- LancasterKJA new approach to demand theoryJ Polit Econ1966741132157