Abstract
Purpose
Among the medications approved for Alzheimer’s disease (AD), rivastigmine is the only one available as transdermal patch. The aim of this study was to evaluate compliance and caregivers’ preference with oral and transdermal (rivastigmine) monotherapy in patients with mild-to-moderate AD from Taiwan.
Methods
Real-world Evaluation of Compliance And Preference in Alzheimer’s disease treatment (RECAP) in Taiwan was a prospective, noninterventional, observational study with a 24-week (±8 weeks) observational period for each participant. Eligible patients were grouped into one of the two treatment cohorts based on the baseline AD therapy: oral (donepezil, galantamine, rivastigmine, or memantine) or transdermal (rivastigmine patch). The primary end points were caregiver preference and caregiver assessment of patients’ compliance to the current medication (oral or transdermal medication) at Week 24 (end of the study). Safety was assessed by recording any adverse events.
Results
A total of 301 patients (age: 77.6±7.19 years) were enrolled from nine centers in Taiwan, of whom 138 (45.8%) patients were in the transdermal monotherapy cohort. Caregivers of patients who were exposed to both forms of therapies demonstrated a higher preference for transdermal rivastigmine monotherapy than the oral monotherapy (82.4% [n=61] versus 17.6% [n=13], P<0.0001); for patients treated with only one therapy, the caregivers’ preference was significantly in favor of the treatment to which the patient was exposed (both P<0.0001). In both cohorts, patients showed good compliance, with an overall score of 8.65±1.38 on an 11-point scale. Of 301 enrolled patients, 102 (33.9%) reported at least one adverse event during the study (51 patients each in the two cohorts).
Conclusion
With the higher caregiver preference and a good patient compliance, the trans-dermal rivastigmine patch is a suitable treatment choice for patients with mild-to-moderate AD, especially for patients intolerant to oral therapies.
Introduction
Alzheimer’s disease (AD), a progressive neurodegenerative condition, is the most common cause of dementia that accounted for 35.6 million (dementia) cases worldwide in 2010.Citation1 The prevalence of AD steadily increases with the aging population, and the risk for developing AD is higher in women than in men.Citation2,Citation3 Taiwan is among the countries with the highest prevalence of aged populations ≥65 years (12.0% in the year 2014).Citation4 AD imposes severe social and economic burden to patients as well as their family members, caregivers of patients, and the society; moreover, the costs involved in caring for patients with AD further increase with the aging population adding to the burden.Citation5
As the disease progresses, patients with AD require increasing levels of care. Family members play an important role as caregivers for individuals with AD and administer most of the treatments.Citation6,Citation7 The choice of treatments among the available options is influenced by caregiver preference, and their preference in choosing a therapy for the patient, which is easy to administer and use, would improve patient compliance to medication.Citation7
Currently, approved treatment options for AD in Taiwan are rivastigmine, donepezil, and galantamine (cholinesterase inhibitors), as well as memantine (N-methyl-D-aspartate receptor antagonist).Citation8,Citation9 Of these, rivastigmine is the only medication also available as transdermal patch. The once-daily transdermal formulation provides smooth and continuous delivery over 24 hours with fewer side effects compared with the twice-daily oral (rivastigmine) formulation.Citation8,Citation10 The transdermal rivastigmine (patch) has been associated with higher satisfaction and preference than the oral formulation as indicated by caregivers of patients with AD in a preference substudy of the Investigation of transDermal Exelon in ALzheimer’s disease (IDEAL) trial.Citation11,Citation12 However, there is a lack of real-world clinical setting data, and no such study is available in patients from Taiwan. The Real-world Evaluation of Compliance And Preference in Alzheimer’s disease treatment (RECAP) study is a noninterventional, observational study that evaluated caregiver preference, compliance, safety, and effectiveness in patients with AD treated with either oral or transdermal monotherapy.Citation13 The study was conducted in Taiwan, India, Egypt, South Korea, Lebanon, and Singapore.
In this study, we have reported the real-world evaluation of compliance and caregiver preference with oral (donepezil, galantamine, rivastigmine, or memantine) and transdermal (rivastigmine) monotherapy in patients with mild-to-moderate AD from Taiwan only.
Methods
Study design
The RECAP study in Taiwan was a 24-week, prospective, noninterventional, observational study conducted across nine centers between July 27, 2011 and March 19, 2013 (). Data were collected at the entry (baseline), Week 12±4 weeks (optional), and at the end of the study (Week 24±8 weeks). All individual patients who were prescribed a capsule or a transdermal patch therapy by their treating physician for AD were invited to participate in the study. Eligible patients were grouped into one of the two treatment cohorts based on the baseline AD therapy: oral (rivastigmine, donepezil, galantamine, or memantine) or transdermal (rivastigmine patch).
The RECAP study was conducted according to the definition of noninterventional trials.Citation14 In accordance with this definition, the medication was prescribed as per the marketing authorization; the assignment of the patient to the therapy was decided within current practice and the medical indication and was clearly separated from the decision to include the patient in the study. No diagnostic or monitoring procedures additional to the standard care and routine practice were performed. Patient treatment, visits, and assessments were independent of this study, and they were at the discretion of the treating physician following local standard medical practice and local prescribing information (PI; local approved product label).
The study was approved by the Joint Institutional Review Board, Taiwan and also by the Institutional Review Board of each participating sites and was conducted according to the Guidelines for Good Pharmacoepidemiology PracticeCitation15 as well as ethical principles of the Declaration of Helsinki. The reporting of this study followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.Citation16 Written informed consent was obtained from all patients or through their caregivers before any data were collected. Patients were free to withdraw their consent at any time during the study.
Patients’ eligibility criteria
Inclusion criteria were male and female outpatients (aged ≥50 years) diagnosed with mild-to-moderate AD, patients prescribed oral (cholinesterase inhibitors or memantine) or transdermal monotherapy (rivastigmine) in adherence with the local PI, and patients having a caregiver willing and able to answer the Caregiver Medication Questionnaire (CMQ). The questions in the CMQ were derived from the AD Caregiver Preference Questionnaire that was developed for use in the IDEAL study.Citation12,Citation17,Citation18 Patients were excluded according to the contraindications mentioned in the local PI of the treatment used.
Study end points and assessments
The primary end points were to assess “caregiver preference” for oral medication or transdermal medication and caregiver assessment of “patient compliance” as evaluated using the CMQ at Week 24. The CMQ comprised the following questions to be answered by the caregiver: questions on patient compliance and satisfaction with treatment, general preference for oral or transdermal medication, and top three reasons for medication preference. Caregivers of patients with exposure to only one form of medication for the treatment of AD compared the current medication available during the study with a hypothetical situation in which their patient could have received the alternative form of therapy. The patient compliance to the treatment was rated on an 11-point scale (from 0= “patient never took the medication as prescribed” to a value of 10= “patient always took the medication as prescribed”).
Secondary end points assessed at Week 24 included concomitant use of psychotropic medication (yes/no) and the number of psychotropic medications used (1, 2, 3, or >3) per patient, physicians’ preference for treatment (including top three reasons for preference) as evaluated using the short questionnaire, and the total daily dosage reached (percentage of patients on which dosage). Questions were asked to each prescribing physician at the end of the patient treatment period of the study. A short physician preference assessment questionnaire was used to assess physicians’ preference for treatment. Safety assessment included the incidence of adverse events (AEs) and serious AEs during the study.
AEs were coded using the Medical Dictionary for Regulatory Activities (Version 16.0). The mean Mini-Mental State Examination (MMSE) scores at the baseline, Week 12, and Week 24 were also recorded.
In this study, a subanalysis was performed to address the following: 1) proportion of patients who were exposed to both oral and transdermal medications and switched from oral to transdermal patch because of intolerability to oral medication and 2) the difference in the amount of the use of concomitant medication at baseline between oral and transdermal monotherapy cohorts.
Statistical methods
Two analysis sets were defined in this study: 1) full analysis set (FAS) consisted of all patients who provided informed consent and received at least one dose of the medication under observation during this study and 2) effectiveness set excluded patients without any post-baseline effectiveness assessment or data collected after the time of treatment switch. The effectiveness set was used to conduct effectiveness analysis, and all other analyses were performed on the FAS.
The study was planned to include ~300 patients in Taiwan, and compiled into the global RECAP study for the final analyses. The details of the statistical power associated with sample size were described elsewhere.Citation13 Summary statistics for continuous variables were presented as number of observations (n), arithmetic mean, and standard deviation (SD), and categorical variables were presented as absolute and relative frequencies. In addition, two-sided 95% confidence intervals (CIs) as well as P-values were presented for the caregiver preferences for the oral or transdermal patch. The differences in the assessment of compliance between the two cohorts were tested using the Student’s t-test. The two-sided significance level was set at P<0.05.
The SAS statistical package Version 9.2 was used to perform all statistical analyses.
Results
Study population
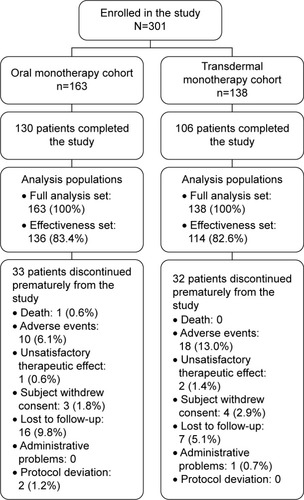
A total of 302 patients from Taiwan were enrolled, but only 301 patients were analyzed. One patient was excluded from the FAS as the patient was not prescribed any medication due to administrative reasons. Of the 301 enrolled patients, 163 (54.2%) were in the oral monotherapy cohort and 138 (45.8%) patients were in the transdermal monotherapy cohort. The majority of patients (n=236; 78.4%) completed the study: 130 in the oral monotherapy cohort and 106 in the transdermal monotherapy cohort. The remaining 65 patients (21.6%) discontinued the study prematurely, and the primary reasons for discontinuation were AEs (n=28; 9.3%) and patient lost to follow-up (n=23; 7.6%) followed by the withdrawal of informed consent (n=7; 2.3%).
The baseline and demographic characteristics were comparable between the two treatment cohorts (). The mean age (SD) was 77.6 (7.19) years, the mean (SD) duration of AD was 1.0 (1.54) year, and a total of 31 patients (10.3%) had a family history of AD. Female sex is associated with increased risks of AD in part because women live longer; similar to a previous study,Citation3 we observed, in our study, that the majority of 63.8% patients were women. The majority of patients (n=287; 95.3%) were living with caregivers or other individuals. Depression (n=78; 25.9%), insomnia (n=63; 20.9%), sleep disorder (n=48; 15.9%), anxiety (n=46; 15.3%), delusion (n=41; 13.6%), agitation (n=17; 5.6%), and neurosis (n=2; 0.7%) were the psychiatric disorders reported at baseline. There were 127 (42.2%) patients who received prior treatment for AD, and among them, the most common reason for changing treatment upon entering the study was “previous treatment was not well tolerated” (n=46; 15.3%) and “optimal dose on previous treatment was not reached” (n=30; 10.0%).
Table 1 Patient demographics and baseline characteristics
Primary assessments
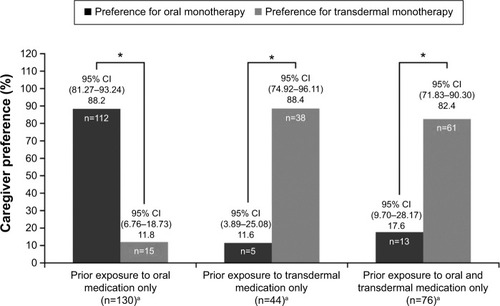
For patients with exposure only to oral or transdermal monotherapy, the caregivers’ preference was significantly in favor of the treatment to which the patient was exposed at Week 24 (both P-values <0.0001); however, caregivers of patients exposed to both forms of therapies demonstrated higher preference for transdermal than oral monotherapy at Week 24 (P<0.0001; ). Patients in both cohorts showed good compliance, with the overall mean (SD) score of 8.65 (1.38). At Week 24, the mean (SD) scores for compliance were 8.77 (1.33) (95% CI: 8.54–9.00) and 8.50 (1.43) (95% CI: 8.23–8.77) in the oral and transdermal monotherapy cohorts, respectively. The patient compliance in two treatment cohorts was not significantly different (P=0.1238).
Figure 2 Caregivers’ preference for the oral or transdermal medication at Week 24, by their patient’s prior exposure.
Abbreviation: CIs, confidence intervals.
Secondary assessments
Overall, 76 (46.6%) patients in the oral monotherapy cohort and 72 (52.2%) patients in the transdermal monotherapy cohort took concomitant psychotropic medications during the observational period. There were 54 (17.9%), 59 (19.6%), 27 (9.0%), and 8 (2.7%) patients in the total study population who took one, two, three, and more than three psychotropic medications, respectively. The use of concomitant psychotropic medication and the number of different psychotropic medications per patient were comparable between the two treatment cohorts ().
Of the nine participating physicians, five indicated preference for oral monotherapy and four preferred transdermal monotherapy at Week 24. The most important reason (preference rank 1) for oral medication preference was “easier to comply and better acceptance by the patient” as indicated by five (100%) physicians. The most important reason for medication preference, however, varied among the physicians preferring the transdermal monotherapy. The most important reason (preference rank 1) for transdermal medication preference was “more convenient and fits better into daily life” as indicated by two (50%) physicians followed by “easier to use” and “easier to comply/better acceptance by the patient” as indicated by one physician each for one reason.
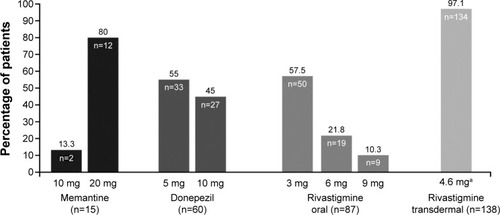
The most common medications used by patients in the oral monotherapy cohort (n=163) at the end of the study were rivastigmine (oral) at 3 mg for 50 patients and donepezil at 5 mg and 10 mg for 33 and 27 patients, respectively, whereas in the transdermal monotherapy cohort most of the patients (n=134) were on the 4.6 mg rivastigmine (patch; ). The data on change in the MMSE score from the baseline at Week 24 were obtained from 195 of the 250 patients in the effectiveness set. The mean (SD) MMSE scores at Week 24 (199/250 patients) were 18.6 (4.76) and 17.8 (5.91) in the oral and transdermal monotherapy cohorts, respectively. At Week 24, there was no change in the MMSE score (195/250 patients) as shown by the median change of zero.
Figure 3 Drug regimens among patients on cholinesterase inhibitors and memantine at Week 24.
Results of the subanalysis showed that a very small number of patients (n=4; 4.3%) switched from oral to patch formulation due to intolerability at baseline. At baseline, 22.7% patients in the oral monotherapy cohort received at least one concomitant medication compared with 18.1% in the transdermal monotherapy cohort ().
Safety
During the study, a total of 102 (33.9%) patients reported at least one AE, and the proportion of patients who reported AEs was comparable between the two cohorts (oral: n=51 [31.3%]; transdermal: n=51 [37.0%]). The most frequent AEs in the oral monotherapy cohort were nausea (n=9; 5.5%), dizziness (n=8; 4.9%), decreased appetite (n=7; 4.3%), vomiting (n=7; 4.3%), and insomnia (n=5; 3.1%), whereas in the transdermal monotherapy cohort they were pruritus (n=11; 8.0%), dizziness (n=7; 5.1%), and rash (n=7; 5.1%).
Of the 301 enrolled patients, 27 (9.0%) discontinued prematurely from the study because of AEs (oral: 11 [6.7%]; transdermal: 16 [11.6%]). The most common AEs leading to discontinuation were skin and subcutaneous tissue disorders (oral: 0; transdermal: 14 [10.1%]), gastrointestinal disorders (oral: 8 [4.9%]; transdermal: 0 [0.0%]), and metabolism and nutrition disorders (oral: 3 [1.8%]; transdermal: 0). There was one death reported in the oral monotherapy cohort and was not considered to be related to the study medication as assessed by the investigator.
Discussion
RECAP in Taiwan was the first study regarding compliance and caregiver satisfaction with transdermal rivastigmine in a real-world clinical setting. The results of the current study showed higher caregivers’ preference (82.4%) to rivastigmine transdermal patch for patients exposed to both forms of medication, and the findings were consistent with previous studies.Citation12,Citation19 The current study, however, did not show a clear preference of caregivers for either oral or transdermal treatment, which was due to the prior exposure to particular form of therapy influencing the caregivers’ preference.
Previously, the IDEAL,Citation12 Alzheimer disease: eXamination of patiEnt comPliance and caregiver satisfacTion (AXEPT),Citation20 Effective Management of Alzheimer’s Disease By TReating pAtients and relieving Caregivers with Exelon Patch (EMBRACE),Citation19 ENTERPRISE,Citation21 global RECAP study,Citation13 and an observational clinical studyCitation22 have assessed the caregiver preference and/or satisfaction for the treatment of AD. IDEAL was the first pivotal trialCitation17,Citation23 to demonstrate the non-inferiority of the rivastigmine transdermal patch over oral capsules; the studyCitation12 also showed higher caregivers’ preference (72%) for the rivastigmine transdermal patch compared with oral capsules. Moreover, the results from the EMBRACE studyCitation19 showed 88.2% caregivers of patients with AD preferred rivastigmine transdermal patch over other oral medication.
The overall patient compliance in the current study was good in both the treatment cohorts, unlike other studies including AXEPTCitation20 and global RECAPCitation13 that showed greater patient compliance with rivastigmine transdermal patch over other oral medications as indicated by the caregivers of patients with AD. Results from the ENTERPRISE studyCitation21 also showed high compliance rates (60.5%) with transdermal rivastigmine patch compared with the oral formulation. A decrease in caregiver burden is associated with the use of rivastigmine patch, and patch contributes to improved patient compliance.Citation24
The MMSE score in the current study was comparable in both the treatment cohorts, and the median change of zero in the MMSE score indicates neither improvement nor deterioration, unlike other studies where improvement in the MMSE score was observed.Citation25 In the current study, there were no patients with serious AEs of skin and subcutaneous tissue disorder, yet the number of patients who discontinued due to AEs of skin and subcutaneous tissue disorder is high (14 patients, 10.1%), implying more education/information should be provided to optimize skin tolerability. Higher gastrointestinal AEs were noted in the oral monotherapy cohort compared with the transdermal monotherapy cohort (n=21 [12.9%] versus n=7 [5.1%], respectively), as observed in a previously reported study.Citation13
This study has limitations. First, it was a nonrandomized open-label study with inherent potential for bias. Second, only 30% of patients from whom the caregiver preference was available were exposed to both forms of therapy and were able to provide a comparative preference. The remainder had the preferences for the oral or the transdermal treatment clearly related to the prior exposure to one or other form of treatment. Third, there was a slight imbalance in patients assigned to the oral therapy (n=163; 54.2%) and those assigned to the transdermal therapy (n=138; 45.8%) and the imbalance in the use of the drug therapy for AD prior to enrollment in this study (46 [28.2%] patients to whom oral treatment was prescribed versus 81 [58.7%] to whom transdermal treatment was prescribed).
Conclusion
The RECAP study showed good patient compliance for both oral and transdermal treatments and higher caregiver preference with the rivastigmine transdermal monotherapy, suggesting rivastigmine transdermal monotherapy as a suitable treatment choice for patients with mild-to-moderate AD in Taiwan.
Author contributions
All authors were involved in data collection, analysis, and/or interpretation of the results and critical revision and approval of the article.
Acknowledgments
The authors thank K Ananda Krishna from Novartis Healthcare Pvt Ltd, Hyderabad, India, for medical writing assistance and incorporating subsequent revisions. This study was funded by Novartis Pharma AG, Basel, Switzerland. The study results were presented as a poster presentation at the First International Taiwanese Congress of Neurology and 2015 Annual Meeting of Taiwan Neurological Society, Taipei, Taiwan, May 7–10, 2015.
Disclosure
The authors report no conflicts of interest in this work.
References
- PrinceMBryceRAlbaneseEWimoARibeiroWFerriCPThe global prevalence of dementia: a systematic review and metaanalysisAlzheimers Dement2013916375.e223305823
- ViñaJLloretAWhy women have more Alzheimer’s disease than men: gender and mitochondrial toxicity of amyloid-beta peptideJ Alzheimers Dis201020suppl 2S527S53320442496
- AndersenKLaunerLJDeweyMEGender differences in the incidence of AD and vascular dementia: the EURODEM Studies. EURODEM Incidence Research GroupNeurology19995391992199710599770
- Department of Statistics, Ministry of the Interior, Taiwan [webpage on the Internet]Statistical Yearbook of Interior 2.01 Available from: www.moi.gov.tw/stat/english/index.aspAccessed December 23, 2015
- BurnsAThe burden of Alzheimer’s diseaseInt J Neuropsychopharmacol200037313811343622
- HaberstrohJHampelHPantelJOptimal management of Alzheimer’s disease patients: clinical guidelines and family adviceNeuropsychiatr Dis Treat2010624325320520788
- SmallGDuboisBA review of compliance to treatment in Alzheimer’s disease: potential benefits of a transdermal patchCurr Med Res Opin200723112705271317892635
- CummingsJWinbladBA rivastigmine patch for the treatment of Alzheimer’s disease and Parkinson’s disease dementiaExpert Rev Neurother20077111457146317997695
- FuhJLWangSJDementia in Taiwan: past, present, and futureActa Neurol Taiwan200817315316118975520
- KurzAFarlowMLefèvreGPharmacokinetics of a novel transdermal rivastigmine patch for the treatment of Alzheimer’s disease: a reviewInt J Clin Pract200963579980519392927
- BlesaRBallardCOrgogozoJMLaneRThomasSKCaregiver preference for rivastigmine patches versus capsules for the treatment of Alzheimer diseaseNeurology2007694 suppl 1S23S2817646620
- WinbladBKawataAKBeusterienKMCaregiver preference for rivastigmine patch relative to capsules for treatment of probable Alzheimer’s diseaseInt J Geriatr Psychiatry200722548549117407176
- PaiMCArefHBassilNReal-world evaluation of compliance and preference in Alzheimer’s disease treatmentClin Interv Aging2015101779178826622172
- Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the member states relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human useMed Etika Bioet200291–2121916276663
- EpsteinMInternational Society of PharmacoepidemiologyGuidelines for good pharmacoepidemiology practices (GPP)Pharmacoepidemiol Drug Saf200514858959515918159
- von ElmEAltmanDGEggerMSTROBE InitiativeThe strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studiesLancet200737095961453145718064739
- WinbladBCummingsJAndreasenNA six-month double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease – rivastigmine patch versus capsuleInt J Geriatr Psychiatry200722545646717380489
- AbetzLRofailDMertzanisPAlzheimer’s disease treatment: assessing caregiver preferences for mode of treatment deliveryAdv Ther200926662764419495575
- GauthierSRobillardACohenSEMBRACE investigatorsReal-life effectiveness and tolerability of the rivastigmine transdermal patch in patients with mild-to-moderate Alzheimer’s disease: the EMBRACE studyCurr Med Res Opin2013298989100023647369
- BernabeiRRossiniPMDi CioccioLCompliance and caregiver satisfaction in Alzheimer’s disease: results from the AXEPT StudyDement Geriatr Cogn Dis Extra20122141843223139687
- Cruz JentoftAJHernándezBManejo terapéutico con rivastigmina en pacientes con enfermedad de Alzheimer de leve a moderadamente grave en condiciones de práctica clínica habitual. Estudio ENTERPRISE [Rivastigmine as treatment for patients with mild to moderately severe Alzheimer disease under normal clinical practice conditions. The ENTERPRISE study]Neurologia2014291110 Spanish23582372
- BoadaMArranzFJTransdermal is better than oral: observational research of the satisfaction of caregivers of patients with Alzheimer’s disease treated with rivastigmineDement Geriatr Cogn Disord2013351–2233323306147
- WinbladBGrossbergGFrölichLIDEAL: a 6-month, double-blind, placebo-controlled study of the first skin patch for Alzheimer diseaseNeurology2007694 suppl 1S14S2217646619
- AdlerGMuellerBArticusKThe transdermal formulation of rivastigmine improves caregiver burden and treatment adherence of patients with Alzheimer’s disease under daily practice conditionsInt J Clin Pract201468446547024588972
- BirksJSGrimley EvansJRivastigmine for Alzheimer’s diseaseCochrane Database Syst Rev20154CD00119125858345