 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
Osteoporosis is a chronic disease and an important health and social burden due to its worldwide prevalence. Literature and clinical experience report incomplete adherence to the therapy. This retrospective observational study aimed at assessing the adherence to first-line antiosteoporosis drugs (AODs; reimbursed by the National Health System, according to the Italian Medicine Agency recommendation number 79), alendronate or risedronate, with or without calcium and/or vitamin D supplements, in a real, Italian clinical setting.
Patients and methods
Analyses were carried out on data present in the ARNO Observatory, a population-based patient-centric Italian database. From a population of 5,808,832 inhabitants with available data, a cohort of 3.3 million of patients aged ≥40 years was selected. New users of first-line AODs as monotherapy (accrual period, 2007–2009) were followed up over 3 years to assess adherence at 6, 12, and 36 months to AODs and to supplements and related determinants.
Results
Approximately 40,000 new users were identified: mostly women, aged on average (standard deviation) 71±10 years. Alendronate was the most prescribed (38.2% of patients), followed by risedronate (34.9%) and alendronate with colecalciferol as a fixed-dose combination (25.8%). Adherence at the 6-month follow-up was 54%, and this constantly and significantly decreased after 1 year to 46%, and after 3 years to 33% (P<0.01). Adherence to the fixed-dose combination was higher than to plain alendronate throughout the follow-up period. Similarly, adherence to supplements constantly decreased with the duration of treatment. Women and patients aged >50 years were more likely to adhere to treatment regimen (P<0.001). The use of drugs for peptic ulcer and gastroesophageal reflux disease and of corticosteroids for systemic use were significantly associated with high adherence at different times. Polytherapy (>5 drugs), cardiovascular, and neurological therapies were significantly associated with low adherence throughout the follow-up period.
Conclusion
In a huge clinical practice sample, this study highlights suboptimal adherence to first-line AODs and to supplements and important determinants, such as concomitant therapies.
Introduction
Osteoporosis is a chronic disease and an important health and social burden due to its worldwide prevalence. The World Health Organization estimated that more than 75 million people in the United States, European Union, and Japan suffer from this disease.Citation1 In Italy, 23% of women over 40 years and 14% of men over the age of 60 suffer from osteoporosis, and 50% of women and 12.5% of men over the age of 50 experience a fragility fracture at least once in their lifetime.Citation2,Citation3 Moreover, as a consequence of the world population’s progressive aging, incidence is set to significantly increase, with severe social, health, and economic implications.Citation2,Citation4 Currently, many drugs are available to prevent and treat osteoporosis, but in the real clinical practice, therapeutic benefits are often compromised by low adherence.Citation5,Citation6 The adherence to pharmacological therapy includes concepts of compliance and persistence, which mean quality and length of the treatment, respectively.Citation7 Literature data and clinical experience report incomplete adherence during daily clinical practice. Indeed, 31% to more than 50% of patients discontinue oral therapy after 1 year of follow-up from the beginning of the therapy, and even the most careful and optimal choice of the treatment does not provide results if it is not properly taken.Citation5,Citation7,Citation8 The most important consequence of osteoporosis and poor adherence to antiosteoporotic drugs (AODs) is bone fractures, one of the main causes of reduced elderly self-sufficiency, increased long-term care, and mortality. This affects not only public health, but also socioeconomic factors.Citation4–Citation7 Between 2000 and 2008, elderly Italian citizens experienced more than half a million hip fractures, which have cost about €8.5 billion.Citation2 The underlying causes of low adherence are multifactorial and relate to patient, physician, and therapeutic choice.Citation9 Some of them have been widely defined in literature, but further studies are needed to get through to prevent consequences.Citation5–Citation7,Citation9,Citation10 The aim of this retrospective observational study was to assess the adherence to first-line antiosteoporosis therapy (alendronate or risedronate, with or without calcium and vitamin D supplements) in an Italian setting. Second, we wanted to identify determinants of adherence through demographic and clinical features.
Material and methods
Data source
Analyses for the retrospective observational study were carried out on data present in the ARNO Observatory,Citation11 a population-based patient-centric Italian database. Since 1987, ARNO Observatory routinely collects and integrates National Health Service (NHS) administrative data for each single patient (ie, patient demographics, outpatient drug-filled prescriptions, inpatient hospital discharges, and imaging and laboratory tests prescriptions) with high-quality and complete information. It provides local health units (LHUs) with a comprehensive database for epidemiological and economic planning for decision-making. Today, ARNO manages a network of nearly 10,000 general practitioners from 32 LHUs in Italy. It covers a population of over 11 million of patients, and it is a valid instrument to monitor patients care pathways and real-world data. Pharmaceutical data consist of the dispensed drug name, ATC (Anatomical Therapeutic Chemical classification group), dose, number of packages, and dispensing date.Citation12 Demographic information about patients was made anonymous, according to Italian law regarding the protection of privacy. Ethical approval for conducting this retrospective observational study was unnecessary because it was based on the collection of anonymous administrative data (ARNO Observatory database) and it was conducted for institutional purposes.
Cohort selection and follow-up
The population with available data from 2006 to 2012 consisted of 5,808,832 inhabitants. Starting from this population, a cohort of 3.3 million patients aged 40 years and more (57% of the overall population) was selected.Citation8,Citation13,Citation14 “Patients treated with first-line AODs” were new users of AODs as a monotherapy over the 3-year follow-up period (prescribed according to Italian Medicines Agency – AIFA – recommendation number 79, an instrument for regulating NHS reimbursements of some drugs), combined or not to calcium and/or vitamin D supplements.Citation15 They were identified by at least one filled prescription of AODs (). During the accrual period (2007–2009), from the initial cohort, we selected a subsample of naïve patients who had not had an AODs prescription during the 12 months before the index date (first prescription of AODs), but had been treated with AODs (AIFA recommendation number 79) as monotherapy during the 3-year follow-up. This providing free drug prescriptions analysis has not considered the private purchase that amounts approximately to 13% of the overall pharmaceutical expenditure.Citation16 The percentage of private purchase of bisphosphonates is about 18% (of the total expenditure for this chemical subgroup). It is slightly higher particularly for alendronic acid than for other bisphosphonates, probably due to its accessible cost. Moreover, about 7% of the expenditure for calcium and vitamin D is due to private purchase.Citation14,Citation16
Table 1 Specific drugs used to identify and analyze the cohort of patients with osteoporosis
Definition of indicators and statistical analysis
The use of administrative databases was ascertained several times as a reliable source of data for “prescription continuity”. However, we used the term “adherence” instead of it, because even if we cannot obtain information on the real medication use, but only on the filled prescription, from these databases, we assumed that prescription continuity could act as a trustworthy indicator of treatment adherence. According to international definition of “prescription adherence”, this was assessed as the ratio between the number of days of medication supplied within the refill interval and the number of days in refill interval (MPR – medical possession ratio), with a 20% tolerance permitted (≥300 days), during the 1-year follow-up:Citation17–Citation19
This means that a patient was considered adherent if treated with 300 unit doses or more during the 365 days of follow-up. In case of “combination therapy”, at least 600 unit doses should be prescribed to patients. Adherence was assessed:
at different times: sixth month, first year, and third year of follow-up;
to first-line AODs as monotherapy (drug): alendronic acid, alendronic acid + colecalciferol, and risedronic acid; and
to different calcium and/or vitamin D supplements, focusing on different colecalciferol formulations prescribed to patients treated with bisphosphonates (drops, vials, and monodose bottles).
Adherence to colecalciferol formulations was assessed by using international units (IU): patients were considered adherent if taking at least 300,000 IU/yr of colecalciferol (in accordance with the package leaflet and guidelines). For every AOD/supplement treatment, standard authorized dosage was considered, ie, alendronic acid 70 mg once a week or risedronic acid 35 mg once a week. A McNemar test, a special case of Cochran’s Q-test, was used to compare adherence by matched-pair analysis: 6-month adherence to 1-year adherence, 6-month adherence to 3-year adherence, and 1-year adherence to 3-year adherence. All statistical analyses were conducted using R software version 3.1 (R Foundation for Statistical Computing, Vienna, Austria), and 5% of significance level was used. Factors potentially associated with high adherence, often called “determinants” in literature, were also assessed: age, sex, concomitant therapies, polytherapy, and previous fractures. In particular, we analyzed the systemic use of corticosteroids and other drugs used to treat the most common (≥1/10) adverse events to first-line AODs, grouped by the anatomical site they affect: gastrointestinal disease (such as nausea and hypercholesterolemia), cardiovascular diseases (such as blood pressure abnormalities and peripheral edema), musculoskeletal diseases (such as pain and spasms), and nervous disorders (such as dizziness and migraine).Citation6,Citation7,Citation9 Logistic regression was applied to detect those variables that could be considered related to treatment adherence. Logistic regression coefficients were used to estimate odds ratios for each of the independent variables included in the final model.
Results
We identified 40,003 new users of first-line AODs, according to AIFA recommendation number 79 and as monotherapy, during the accrual period that lasted from 2007 to 2009: 88.25% women and 11.75% men, mean age (standard deviation) 71±10 years. Characteristics of prescriptions are described in . ARNO Observatory network data, according to epidemiological literature data, show that the age group 70–79 years was the most treated by first-line AODs (36.8%).Citation1 Of 40,003 patients treated, women received the highest number of prescriptions, and alendronic acid was the most commonly prescribed treatment (38.2% patients), followed by risedronic acid (34.9%) and alendronic acid with colecalciferol as a fixed combination (25.8%). Prevalence in the use of supplements in patients taking bisphosphonates, not in fixed combination with vitamin D, was assessed for every single follow-up period. At 6 months, calcium was prescribed to 3.7% of patients, vitamin D to 17.7%, and calcium with vitamin D to 28.6%; at 12 months, 4.4% received calcium, 21.2% vitamin D, and 31.3% calcium with vitamin D; and at 36 months, 6.3% used calcium, 34.8% vitamin D, and 38% calcium with vitamin D.
Table 2 Demographic characteristics of patients with first-line AODs prescriptions
Adherence to antiosteoporosis therapy
Adherence was assessed at 6, 12, and 36 months from the index date on the total naïve patients cohort (N=40,003). Adherence at the 6-month follow-up was 54% (), which constantly and significantly decreased after 1 year to 46%, and after 3 years, to 33% (**P<0.01). Adherence was first evaluated to AODs as a monotherapy (). It constantly decreased during the 3-year follow-up period for all AODs. Moreover, patients were significantly more adherent to the fixed-dose combination (FDC) than to the plain alendronic acid throughout the follow-up (P<0.001 at 6 months and at 1 year, P<0.01 at 3 years). Then, as patients with osteoporosis using AODs should receive supplemental calcium and vitamin D if dietary intake is inadequate, we assessed the adherence to these supplements (). The use of calcium supplements in Ital-ian patients appears generally low and at high risk of low adherence. Colecalciferol prescriptions were analyzed in subsets of patients taking bisphosphonates where FDC was excluded (N=29,518). “Drops” (bottle of 10 ML containing 10,000 UI/ML per os) resulted in the most prescribed pharmaceutical form of colecalciferol, followed by “Vials” vials of 1 ML containing 300,000 UI intramuscular/per os administered every 3/6/12 months. We assessed adherence to “drops”, because it is a chronic treatment, while “vials” is mostly an induction therapy. Only 51% of patients taking plain bisphosphonates were adherent to the recommended dose of vitamin D in the first year of follow-up, decreasing to 23% in the third year.
Figure 1 Adherence to AODs by overall population.
Abbreviation: AODs, antiosteoporosis drugs.
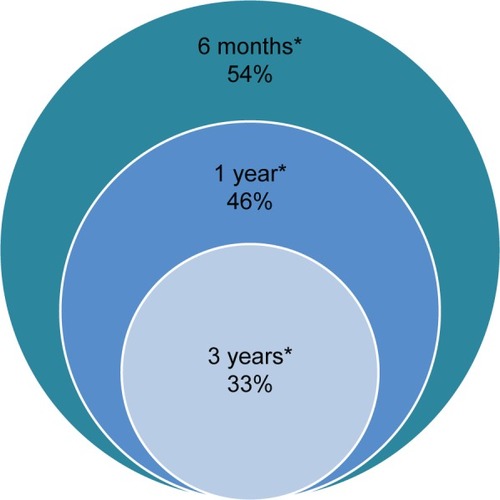
Table 3 Adherence to first-line AODs
Table 4 Adherence to calcium and/or vitamin D supplements of patients treated with alendronic or risedronic acid
Determinants of adherence to antiosteoporosis therapy
We carried out a multiple logistic regression analysis to identify factors potentially associated with adherence in the subcohorts of adherent patients: N=21,691 at 6 months, N=18,575 at 12 months, and N=13,372 at 36 months (). We found that patients aged >50 years and women were likely to be more adherent (P<0.001), and the more the age raises, the more adherent patients are. Concomitant peptic ulcer and gastroesophageal reflux disease treatments (among drugs for gastrointestinal diseases) and corticosteroid treatments can be related to high adherence up to 12 months from the beginning of the AODs therapy. However, both were likely to lose their potential association with high adherence from the 12th month of follow-up. Drugs for cardiovascular diseases (in order of decreasing number of prescriptions: lipid modifying agents, angiotensin converting enzyme inhibitors plain, β blockers, and selective calcium channel blockers with mainly vascular effects) and for nervous disorders (antidepressants, opioids, and antiepileptics) were associated with low adherence during the entire 3-year follow-up period (P<0.001). Finally, taking more than 5 drugs/d (polytherapy) can cause a significant reduction in adherence during follow-up period: P<0.05 at 6 months to P<0.001 at 1 and 3 years of follow-up.
Table 5 Determinants of adherence
Discussion
Osteoporosis is a “silent epidemic disease”, as it is often asymptomatic and implicates a low perception of the severity of the disease. Therefore, when fragility fractures occur as a first sign, the risk of poor outcomes and consequent disability is greater.Citation7,Citation20 A recent (2010) French observational study showed a large discrepancy between treatment compliance as evaluated by the investigator and as considered by the patient.Citation10 This is an important starting point in acquiring knowledge not only about how patients perceive osteoporosis and try to improve their quality of life but also about how general practitioners and public health can help them to do it. We decided to study a cohort of incident patients with osteoporosis who started first-line treatments, alendronic acid or risedronic, with or without calcium and/or vitamin D supplements. Assessment of the use of first-line medications to treat or prevent osteoporosis highlighted a suboptimal adherence in all LHUs of the ARNO Observatory network. Adherence significantly decreased (P<0.01) from 54% in the first six months from the index date to 46% in the second year (8% of patients that discontinued oral therapy after 1 year of follow-up), ranging to 33% in the third year, both for women and for men. As a whole, it appears borderline to ensure effectiveness and usefulness of the therapy.Citation8 Literature data confirm this constant reduction and are consistent with our findings on the use of oral bisphosphonates in our cohort.Citation7,Citation8 An easier administration regimen, weekly and monthly, certainly guarantees better adherence to bisphosphonates. Observational studies and clinical trials pointed out inverse correlation between adherence and dosage frequency.Citation7,Citation9,Citation21,Citation22 However, these regimens also do not ensure optimal effectiveness, as many observational studies have already reported.Citation6,Citation23 Insufficient calcium intake and lack of vitamin D are the most common causes of nonresponse to antiosteoporosis therapy.Citation13 In particular, incidence of hypovitaminosis D in Italy is extremely high, especially among the elderly.Citation13,Citation24 In this study, we found that adherence to calcium was very poor, and colecalciferol prescriptions showed a suboptimal intake too.Citation25 This observation supports the assertion that fixed combinations of AODs with vitamin D provide an opportunity. Interestingly, we found that by simplifying the association of alendronic acid and colecalciferol as FDC, the adherence appears to increase, compared with the administration of plain alendronic acid.
Chronic illness in itself can be a factor potentially associated with low adherence, because the less the patient is motivated to a proper drug use, the less the condition to be treated is symptomatic. Other causes of a reduced adherence are inappropriate information and advice by the physician on possible risks and benefits. Moreover, there is evidence that gastrointestinal, musculoskeletal, neurological, and cardiovascular adverse events and the fear that they occur can affect adherence.Citation9 Finally, but no less important, feedback to the patient on laboratory tests and bone densitometry results, his or her physical and psychological state, his or her socioeconomic situation, comorbidities, and polytherapy are likely to be determinants of adherence.Citation4–Citation7,Citation10,Citation23 According to literature, this study showed that factors potentially associated with high adherence were: older age (>50 years), female sex, and some concomitant therapies (corticosteroids for systemic use, gastrointestinal diseases drugs). However, neurological and cardiovascular drugs and polytherapy continually reduced adherence throughout the follow-up (P<0.001). Moreover, our results interestingly showed that a factor associated with high adherence (use of corticosteroids) loses its potential of increasing it, while some other determinants of high (older age and female sex) and low (polytherapy) adherence were confirmed.Citation9
Strengths and limitations
This 3-year follow-up study assessed osteoporosis treatment and adherence in a huge cohort of men and postmenopausal, premenopausal, and menopausal women, aged 40 years and older. Unlike clinical trials, retrospective observational studies allow for an analysis of nonselected population in a real-life setting. The distribution of first-line AODs in ARNO population was consistent with findings from literature data.Citation7,Citation9 Another major strength of this study is the comparison of adherence at 6 months, 1 year, and 3 years. Our findings on poor medication adherence to bisphosphonates were also consistent with the number of studies in literature. We assessed and compared some important potential determinants of adherence at the three periods. Between them, we particularly aimed at pointing out the influence of major concomitant therapies on adherence (as already reported as determinants of low adherence by other studies) that identified the fear of common adverse reactions to adverse drug reaction too.Citation5,Citation9 Little information also exists on how corticosteroid concomitant therapy influences the persistence of AODs use.Citation6,Citation9 The use of fixed MPR is appropriate for chronic medical conditions.Citation18 Nevertheless, adherence with claims data provides evidence for receiving a drug, while none that it has been used.Citation26 Measurements errors due to inaccuracies in the identification of therapeutic and diagnostic codes might also have occurred.
Conclusion
Thanks to administrative databases, long considered a source of good quality data, analyses from the ARNO Observatory were able to provide a multitude of information about patients with osteoporosis. They were able to accurately describe demographic characteristics and treatment profiles in variously treated and differently adherent patients. This study highlights suboptimal adherence to first-line antiosteoporosis therapy and its major determinants in the real clinical practice. It is an example of how administrative databases can be used to monitor drug use and identify areas in which improvement is needed to increase compliance and persistence to treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
- GualanoMRSferrazzaACadedduCde WaureCLa TorreGRicciardiWEpidemiologia dell’osteoporosi post menopausale nel mondo e in Italia [Epidemiology of post-menopausal osteoporosis worldwide and in Italy]IJPH20118Suppl 2S3S22 Italian
- Ars.Toscana.it [homepage on the Internet]Firenze, ItalyPharmaco-epidemiology and Osteoporosis in Europe and in Italy Available from: https://www.ars.toscana.it/it/aree-dintervento/cure-e-assistenza/farmacoepidemiologia.htmlAccessed January 31, 2015
- Worldosteoporosisday.org [homepage on the Internet]Osteoporosis epidemiology worldwide Available from: www.worldosteoporosisday.org/Accessed February 1, 2016
- GhirardiADi BariMZambonAEffectiveness of oral bisphosphonates for primary prevention of osteoporotic fractures. Evidence from the AIFA-BEST observational studyEur J Clin Pharmacol20147091129113724951915
- RossiniMDi MunnoOGattiDOptimising bisphosphonate treatment outcomes in postmenopausal osteoporosis: review and Italian experienceClin Exp Rheumatol201129472873521813068
- CasulaMCatapanoALPiccinelliRAssessment and potential determinants of compliance and persistence to antiosteoporosis therapy in ItalyAm J Manag Care2014205e138e14525326928
- SantiIZanoniCICettaFFattori determinanti l’aderenza alla terapia farmacologica per l’osteoporosi e possibili strategie per migliorarla [Determinants of adherence to osteoporosis treatment and strategies to improve it]Riv Psichiatr201058110116 Italian
- RossiniMRossiEDe RosaMCinconzeEAderenza alla terapia per l’osteoporosi in una coorte di pazienti dell’Osservatorio ARNO [Adherence to osteoporosis therapy in a cohort of ARNO Observatory]Arno Journal serial on the Internet2014 Available from: https://osservatorioarno.cineca.org/journal/Accessed March 4, 2016
- RossiniMBianchiGDi MunnoOTreatment of Osteoporosis in clinical Practice (TOP) Study GroupDeterminants of adherence to osteoporosis treatment in clinical practiceOsteoporos Int200617691492116538553
- HuasDDebiaisFBlotmanFCompliance and treatment satisfaction of post menopausal treated for osteoporosis. Compliance with osteoporosis treatmentBMC Women’s Health20101026 Available from: http://www.biomedcentral.com/1472-6874/10/26Accessed February 1, 201620727140
- CINECAARNO Observatory Available from: https://osservatorioarno.cineca.orgAccessed March 14, 2016
- WHO Collaborating Centre for Drug Statistics Methodology [homepage on the Internet]ATC/DDD methodology Available from: http://www.whocc.no/atc/structure_and_principles/Accessed February 1, 2016
- AdamiSGianniniSBianchiLVitamin D status and response to treatment in post-menopausal osteoporosisOsteoporos Int200920223924418551242
- AdamiSDe RosaMMartiniNPedriniARossiniMScroccaroGOsservatorio ARNO Patologie Osteoarticolari – Focus su Osteoporosi e Artrite Reumatoide [Osteoarticular disorders in ARNO Observatory]Bologna, ItalyCENTAURO Srl2012
- AgenziaFarmaco.gov.it [homepage on the Internet]Roma, ItalyAIFA Recommendation Available from: http://www.agenziafarmaco.gov.it/it/content/note-aifaAccessed February 1, 2016
- AIFARapporto Osmed 2013 [National Report of Medicines use in Italy. Year 2013] (English edition) Available from: http://www.agenziafarmaco.gov.it/it/content/national-report-medicines-use-italy-year-2013-english-editionAccessed February 1, 2016
- RaebelMASchmittdielJKarterAJKoniecznyJLSteinerFStandardizing terminology and definitions of medication adherence and persistence in research employing electronic databasesMed Care2013518 Suppl 3S11S2123774515
- ColomboGLRossiEDe RosaMBenedettoDGaddiAVAntidiabetic therapy in real practice: indicators for adherence and treatment costsPatient Prefer Adherence2012665366123055698
- PetersonAMNauDPCramerJAA checklist for medication compliance and persistence studies using retrospective databasesValue in Health200710131217261111
- SeemanEon the behalf of IOF Committee of Scientific AdvisorsOsteoporosis in men. The ‘silent epidemic’ strikes men too. IOF – Invest in your bones2004 Available from: http://www.iofbonehealth.org/osteoporosis-men-report-2004Accessed February 1, 2016
- ClaxtonAJCramerJPierceCA systematic review of the associations between dose regimens and medication complianceClin Ther20012381296131011558866
- BianchiMLDucaPVaiSImproving adherence to and persistence with oral therapy of osteoporosisOsteoporos Int2015261629163825619634
- ChiuCKuoMYuSSuBYChengTAdherence to osteoporosis regimens among men and analysis of risk factors of poor compliance: a 2-year analytical reviewBMC Musculoskelet Disord201314276 Available from: http://www.biomedcentral.com/1471-2474/14/276Accessed February 1, 201624060442
- VarennaMBinelliLCasariSZucchiFSinigagliaLEffects of dietary calcium intake on body weight and prevalence of osteoporosis in early postmenopausal womenAm J Clin Nutr200786363964417823428
- AdamiSBertoldoFBrandiMLLinee guida su prevenzione e trattamento dell’ipovitaminosi D con Colecalciferolo [Guidelines for the diagnosis, prevention and treatment of osteoporosis]Reumatismo2011633129147 Italian22257914
- KozmaCMDicksonMPhilipsALMeleticheDMMedication possession ratio: implications of using fixed and variable observation periods in assessing adherence with disease-modifying drugs in patients with multiple sclerosisPatient Prefer Adherence2013750951623807840
