Abstract
Objective
To conduct qualitative and quantitative assessment of yeast-like fungi in the feces of children and adolescents with type 1 diabetes mellitus (T1DM) with respect to their metabolic control and duration of the disease.
Materials and methods
The studied materials included samples of fresh feces collected from 53 children and adolescents with T1DM. Control group included 30 age- and sex-matched healthy individuals. Medical history was taken and physical examination was conducted in the two study arms. Prevalence of the yeast-like fungi in the feces was determined as well as their amounts, species diversity, drug susceptibility, and enzymatic activity.
Results
The yeast-like fungi were found in the samples of feces from 75.4% of T1DM patients and 70% controls. In the group of T1DM patients, no correlation was found between age (Rs=0.253, P=0.068), duration of diabetes (Rs=−0.038, P=0.787), or body mass index (Rs=0.150, P=0.432) and the amount of the yeast-like fungi isolated in the feces. Moreover, no correlation was seen between the amount of the yeast-like fungi and glycated hemoglobin (Rs=0.0324, P=0.823), systolic blood pressure (Rs=0.102, P=0.483), or diastolic blood pressure (Rs=0.271, P=0.345).
Conclusion
Our research has shown that children and adolescents with T1DM show higher species diversity of the yeast-like fungi, with Candida albicans being significantly less prevalent versus control subjects. Moreover, fungal species in patients with T1DM turn out to be more resistant to antifungal treatment.
Introduction
The overall balance in the composition of gut microbiota has a great impact on the proper functioning of the human immune system.Citation1–Citation3 According to the most recent research, the composition of the gut microflora can be an important factor that contributes to the development of autoimmune diseases, including diabetes.Citation1,Citation3 Some researchers suggest that the composition of microbiota can considerably differ between diabetic patients and healthy subjects.Citation4–Citation6 Moreover, the most recent research suggests that the differences in composition of the bacterial community in diabetic patients may influence the growth of the yeast-like fungi in the human alimentary tract.Citation1 There have been many reports published on the composition of the bacterial microflora in diabetic patients whereas studies investigating the occurrence of fungi in the gastrointestinal tract of diabetic patients are scarce. Especially, there are very few data on this issue in children and adolescents with type 1 diabetes mellitus (T1DM). The human gastrointestinal tract is a site where the yeast-like fungi live in small amounts as saprofites and may require only a small trigger to exert their harmful effects.Citation7–Citation10 However, in a healthy human body with properly maintained immune functions, fungal infections are not likely to develop. Nevertheless, when a factor occurs that results in increasing the number of fungal microorganisms and initiates their penetration deep into the body tissues, symptomatic candidiasis may develop. The fungal colonization itself of the digestive tract does not mean fungal infection. Factors contributing to the development of candidiasis include the amount of yeasts attacking the mucous membrane and fungal virulence, which is dependent on the fungal potential to proliferate, adhere, and produce enzymes and toxic metabolites.Citation11–Citation13 Symptomatic candidiasis most often develops in endogenous pathways as a result of a variety of factors. Fungal colonization may be a consequence of factors predisposing to fungal proliferation and growth, may precede the disease, or may precede local symptomatic infection.Citation14 Furthermore, the most recent studies have shown that increased quantity of the yeast-like fungi found in the digestive tract of diabetic versus nondiabetic patients is not likely to be associated with the pathogenesis of diabetes but is rather its consequence.Citation3,Citation15 The increased amount of fungal microorganisms may not only favor the development of symptomatic fungal disease but may also adversely affect the course of diabetes. As in the available literature only a few reports have been identified on the fungal colonization of the gastrointestinal tract in children, especially diabetic children, the aim of this research was to conduct qualitative and quantitative evaluation of the yeast-like fungi found in the feces of children and adolescents with T1DM with respect to their metabolic control and duration of diabetes.
Subjects and methods
The study involved 53 adolescent patients (19 girls and 34 boys) with T1DM. All patients were under intensive insulin therapy (0.9±0.2 IU of insulin per day/kg of body weight). Diabetes was diagnosed according to the Polish Diabetes Association guidelines, which correspond with the guidelines of the World Health Organization.Citation16–Citation17 Glycated hemoglobin (HbA1c) was measured with an immunoturbidometric method using a Unimate 3 set (Hoffmann-La Roche AG, Basel, Switzerland). Blood pressure was measured using a 24-hour ambulatory blood pressure monitoring (ABPM) method.Citation18 Various sizes of the cuff were used according to age, weight, and arm circumference of the studied subjects. All the ABPM results which had less than 80% of technically correct measurements were excluded from the study. Threshold values defining the range of normal blood pressure values, prehypertension, and hypertension state were according to the centile tables which took into consideration sex, age, and height centile. Arterial hypertension was diagnosed when mean ABPM values were above the 95th centile for the corresponding age, sex, and height on at least three separate measurements.Citation18
The control group consisted of 30 healthy children and adolescents, age- and body mass index (BMI)-matched (16 girls and 14 boys). Patients with T1DM and their matched controls were examined by a pediatrician on the day of collection of the fecal samples. Medical history was taken and physical examination was performed and did not reveal any gastrointestinal complaints in either study group. Moreover, the study participants had not been receiving antibiotics for up to 3 months prior their participation in the study. Children with symptoms of infection or systemic somatic illness other than diabetes mellitus were excluded from the study. Written informed consent was obtained from all children and adolescents participating in the study, or from their parent or guardian. The study was approved by The Ethics Committee of The Medical University of Gdańsk (no NKBBN/125/2014) and the investigation was carried out in accordance with the principles of the Declaration of Helsinki as revised in 1996.
Yeast-like fungal cultures
The study materials were samples of fresh feces collected from the study participants. The feces were collected in sterile containers and provided to the laboratory on the day of collection. On the same day, the samples were used to establish cultures. In order to isolate and enumerate yeast-like fungal colonies in 1 g feces, quantitative cultures on Sabouraud Dextrose Agar were used. Fecal suspensions in normal saline in serial dilutions 1:10, 1:100, 1:1,000, and 1:10,000 were prepared. Five growth lines were drawn on the Sabouraud medium plate. On the first line, 10 μg feces was equally spread. Then, 10 μg of each fecal dilution was plated and equally spread on each subsequent line, respectively. The cultures were incubated for 72 hours at 37°C. Then, the number of fungal colonies that had grown on the plate were counted. According to the plate dilution, the number was converted into colony forming units in 1 g feces. Growth units were established as 103, 104, 105, and 106 CFU.
In order to identify whether the tested samples contained more than one Candida species, the feces was additionally plated on the CHROMagar medium (Graso, Starogard Gdański, Poland). The cultivated fungi were identified according to their morphology and biochemical properties, and tested with commercial API 20 C AUX assays (BioMerieux, Paris, France) as instructed by the manufacturer.
Yeast-like fungal drug susceptibility testing
Drug susceptibility of the isolated strains was tested with FUNGITEST® kit (BIO-RAD, Marnes-la-Coquette, France) designed to test the growth of yeast-like fungi in the presence of six drugs at two concentration levels each – 5-fluorocitosine (2 and 32 μg/mL), amphotericin B (2 and 8 μg/mL), miconazole (0.5 and 8 μg/mL), ketoconazole (0.5 and 4 μg/mL), itraconazole (0.5 and 4 μg/mL), and fluconazole (8 and 64 μg/mL). The test was conducted according to the manufacturer’s instructions.
Enzymatic activity testing
Enzymatic activity of the isolated strains was investigated with the API ZYM system (BioMerieux) according to the manufacturer’s instructions. The API ZYM test contains substrates to detect activities of 19 hydrolases.
Statistical analyses
Statistical analyses were performed with the RKWard Data Analysis Tool Version 0.6.1 using the KDE Development Platform 4.13.3. Quantitative variables were expressed as arithmetic mean and standard deviations. Qualitative variables were presented as numbers and percentages. The Shapiro–Wilk W test was used to verify whether the quantitative variable came from normally distributed population. To investigate differences for the two data sets, Snedecor’s F-test for homogeneity of variances was performed. When inhomogeneity was found, Wilcoxon–Mann–Whitney U-test was used to confirm equality of mean. In case of homogeneity, Student’s t-test was used for two means. For larger data set, the analysis of variance was conducted with Bartlett’s test (more advanced form of Snedecor’s F-test for a bigger number of samples). When inhomogeneity of variance was detected, differences between the study groups were tested with Kruskal–Wallis test. For groups with homogeneous variance, analysis of variance test was applied. Moreover, chi-square test for independence was used for qualitative variables. To assess the strength and direction of the relationship between two variables, a correlation analysis was performed with the calculation of Pearson’s and Spearman’s correlation coefficients. Probability value P, that is, the level of significance, was established as 0.05.
Results
Clinical characteristics of the study participants
Clinical characteristics of the studied patients with T1DM as well as the control healthy subjects are presented in . The study involved 53 children and adolescents with T1DM aged 10.9±3.9 years, and 30 healthy children and adolescents, age range 10.3±4.9 years. The group of patients with T1DM showed significantly higher HbA1c levels versus control subjects, P=0.0000. No significant differences were seen in age (P=0.588), sex (P=0.187), BMI (P=0.635), or systolic and diastolic blood pressure in T1DM children versus healthy control subjects.
Table 1 Clinical characteristics of patients with T1DM and healthy control subjects
No yeast-like fungi were grown in fecal samples of 13 T1DM children and nine control subjects. The culture was considered negative. In fecal samples of 40 T1DM patients and 21 control subjects, yeast-like fungal growth was detected in the amount of 103–106 CFU. However, the differences were not statistically significant (P=0.777) ().
Table 2 Quantitative evaluation of yeast-like fungal colony growth in fecal samples of T1DM children and control subjects
Qualitative evaluation of yeast species in fecal samples of T1DM children and control subjects
Out of T1DM patients whose 40 fecal samples were yeast-like fungus positive, 33 (82.5%) cultures contained one species of the yeast-like fungi and in seven T1DM (17.5%) patients, two yeast-like fungal species were grown in one culture. In the control group of 30 nondiabetic children, fungus-positive cultures were seen in 21 (70%) children. The differences were not statistically significant (P=0.106) (). In all of the fungus-positive samples, single-species cultures were obtained. In total, 47 strains of the yeast-like fungi were grown in the fungus-positive fecal samples of T1DM children (). The isolated strains belonged to nine species. Candida spp. accounted for 83% of the isolated strains and the remaining 17% were strains of the other yeast-like fungi. Among the isolated strains, Candida albicans was the main species and made up 62% of all of the grown strains. The other Candida species that were grown included C. krusei – 9% of all of the isolated strains; C. famata – 6%; and C. parapsilosis, C. lusitaniae, and C. guilermondii – 2% of all of the isolated strains each. The remaining yeast-like fungal species isolated were Rhodotorula spp. (9%), Geotrichum spp. (6%), and Saccharomyces spp. (2%). In the materials from control group, 21 yeast-like fungal strains were detected (). The isolated strains belonged to four species. Among the isolated strains, the main species was C. albicans which accounted for 85% of all of the yeast-like fungal strains grown. Moreover, C. famata and C. tropicalis were detected in this group accounting for 5% of all strains. The last fungal species detected was Rhodotorula spp., not being a member of the Candida genus, which accounted for 5% of isolates ().
Figure 1 The amount of the yeast-like fungal species in type 1 diabetes mellitus children (A) and the control subjects (B).
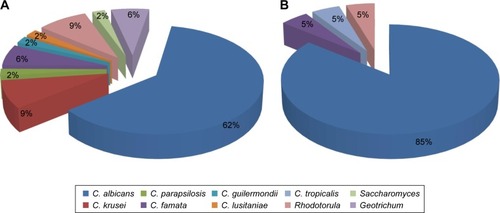
Table 3 Frequency of single and multiple species in cultures from the stool samples in children with T1DM and control subjects
Correlation between the amount of the yeast-like fungi and clinical parameters in T1DM children
The aim of the next part of the study was to investigate the relationship between the amount of the yeast-like fungi and clinical parameters in T1DM children versus control group. Therefore, Spearman’s rank correlation coefficient was calculated and the results are presented in .
Figure 2 Relationship between the amount of the yeast-like fungi and clinical parameters in the study groups.
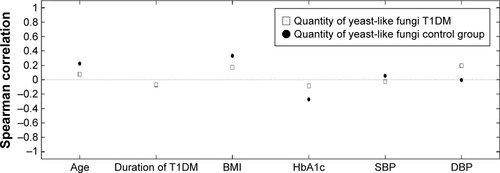
In the group of T1DM children, no statistically significant correlations were seen between age (Rs=0.253, P=0.0681), duration of diabetes (Rs=−0.0388, P=0.787), or BMI (Rs=0.150, P=0.432) and of the yeast-like fungi cultivated from the feces. Moreover, no correlations were seen between the yeast-like fungi and level of HbA1c (Rs=0.0324, P=0.823), systolic blood pressure (Rs=0.102, P=0.483), or diastolic blood pressure (Rs=0.271, P=0.345) ().
Quantitative evaluation of the yeast-like fungi in T1DM children according to their metabolic control and duration of diabetes
The next step involved an attempt to identify whether the level of metabolic control and duration of diabetes may affect the dynamics of the yeast-like fungi – at the range of 103 CFU/g to 106 CFU/g in the tested fecal samples from T1DM children. Duration of diabetes was stratified into <5 and >5 years. Metabolic control was assessed as HbA1c <7.5% and >7.5%.
In fecal samples collected from T1DM children, no correlation was detected between the yeast-like fungi in the range of 103 CFU/g to 106 CFU/g and HbA1c levels <7.5% and >7.5%.
In fecal samples collected from T1DM children, no correlation was detected between the yeast-like fungi in the range of 103 CFU/g to 106 CFU/g and duration of diabetes <5 and >5 years.
Investigation of drug susceptibility of C. albicans isolates from fecal samples in T1DM patients
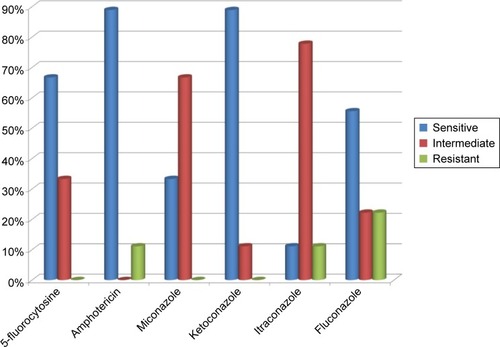
Susceptibility of the isolated strains of C. albicans to the following six antifungal drugs was tested: 5-fluorocytosine, amphotericin, miconazole, ketoconazole, itraconazole, and fluconazole. In the groups of T1DM children and control subjects, 100% of C. albicans strains were susceptible to 5-fluorocytozine and amphotericin. Only 28% of C. albicans strains were susceptible to itraconazole in T1DM patients and 54.5% in healthy control subjects ().
Figure 3 Drug susceptibility of Candida albicans strains isolated from fecal samples of type 1 diabetes mellitus children (A) and control subjects (B).
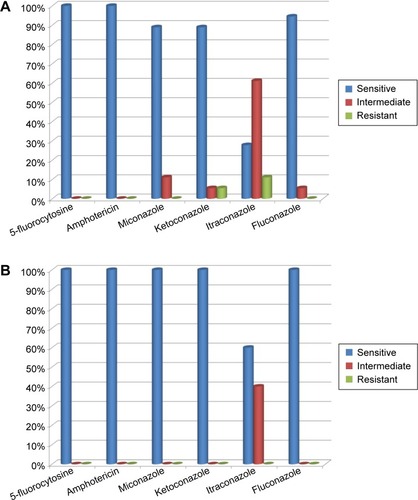
Candida non-albicans strains isolated from the tested samples of T1DM children appeared to be less susceptible to the tested drugs than C. albicans strains: only 11% were susceptible to itraconazole, 33% to miconazole, 56% to fluconazole, 67% to 5-fluorocytosine, and 89% to amphotericin and ketoconazole ().
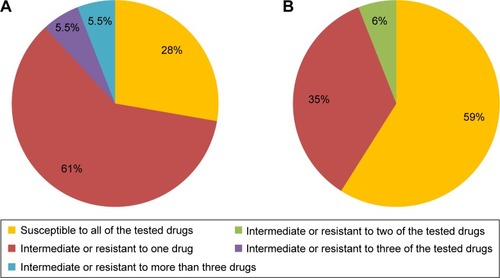
In the samples collected form T1DM children, 28% of C. albicans strains were susceptible to all of the tested drugs, 61% of the strains were intermediate or resistant to one drug, 5.5% of the strains were susceptible to three of the tested drugs with intermediate sensitivity or resistance to the remaining three drugs, and 5.5% of the strains were susceptible to as few as two of the tested drugs (). Out of the C. albicans strains isolated from healthy children, 59% were susceptible to all of the tested drugs, 35% were susceptible to five drugs, and 6% were not susceptible to two of the six tested drugs ().
Investigation of enzymatic activity of C. albicans isolates in T1DM children and healthy control subjects
Tests in the group of T1DM children have shown activities in 14 out of 19 hydrolytic enzymes of C. albicans strains isolated from fecal samples. In individual C. albicans strains, activities of nine to 12 enzymes were detected. No activity of lipase, α-chymotrypsin, β-glucosidase, β-glucuronidase, and α-fucosidase was detected. In the group of T1DM children, C. albicans strains showed the highest mean activities (mean score >4 on the scale of 0–5) of acid phosphatase (mean score of 4.3) and leucine arylamidase (4.8) (25–35 nmol of hydrolyzed substrate). High mean hydrolytic activity (>2 on the scale of 0–5) (10–20 nmol) was seen for C4 esterase and esterase lipase, valine arylamidase, and N-acetyl-beta-glucosaminidase. C. albicans strains isolated from fecal samples of healthy control subjects showed activities of ten out of 19 tested hydrolases. In individual strains of C. albicans, activities of eight to ten enzymes were detected. No enzymatic activity of C. albicans strains was detected for lipase, α-chymotrypsin, trypsin, α-galactosidase, β-galactosidase, β-glucuronidase, β-glucosidase, α-fucosidase, and α-mannosidase. The highest activity (mean score >3 on the scale of 0–5) was detected for acid phosphatase (4.2) and leucine arylamidase (4.4), followed by N-acetyl-β-glucosaminidase (3.1), esterase (2.1), and esterase lipase (2.2) and α-glucosidase (2.2).
Discussion
In our study, the yeast-like fungal growth in fecal samples was detected in 40 (75.4%) T1DM patients and 21 (70%) control subjects. Surprisingly, our analyses have shown high prevalence of yeast colonies occurring both in type 1 diabetic patients and healthy children. Some authors of the previous studies reported yeast-like fungal colonization in 91% of T1DM childrenCitation19 whereas in other reports the percentage of yeast-like fungus positive diabetic patients was lower and approximated 40%.Citation1,Citation20 Consistent with our results, also in children with malignant disease no significant differences in yeast-like fungal isolates were detected versus healthy children.Citation21 The authors suggested that fungal colonization may depend on the patient’s individual predisposing factors.Citation21
In our studies, in 17.5% of T1DM patients, two species of yeast-like fungi were detected whereas in healthy children only one species occurred. Moreover, although in the studied sample of T1DM children the dominant species was C. albicans, which accounted for 62% of all of the yeast-like fungal isolates, its prevalence was lower versus healthy children. The results of our study indicate increasing prevalence of C. non-albicans species. The decreasing prevalence of C. albicans in favor of other Candida species has already been reported by other authors in their most recent works.Citation19,Citation22–Citation25
The next step of our research was to investigate the relationship between the amount of the yeast-like fungi and clinical parameters in T1DM children and control subjects. In the group of T1DM children, no correlation was seen between the patient’s age, duration of diabetes, or BMI and the amount of the yeast-like fungi in the fecal samples. Moreover, no correlation was detected between the amount of the yeast-like fungi and systolic and diastolic blood pressure and the level of metabolic control. Consistent with our results, other authors in their recent report described no correlation between the amount of the yeast-like fungi and level of HbA1c.Citation15 However, the prevalence of Candida spp. was studied in fecal samples from adult patients with T1DM and type 2 diabetes mellitus (T2DM). Therefore, no correlation was detected between the level of fungal colonization and level of metabolic control either in T1DM children or T1DM and type 2 diabetes mellitus adults.Citation15 We believe that in patients with a short history of diabetes of <5 years and good metabolic control, the prevalence of the yeast-like fungi in the digestive tract is not significantly increased. In our study, the amount of the yeast-like fungi was not increased in T1DM children versus healthy control subjects. This could be related to the fact that the disease in T1DM children was of short duration and with good metabolic control but also to the fact that children had not complained of any gastrointestinal problems and had not been treated with any antibiotics for up to 3 months prior to their fecal sample collection. However, it seems interesting that in the samples collected from T1DM children, a higher diversity of fungal species was observed versus control subjects. In the studied group of T1DM children versus nondiabetic children, there were more C. albicans strains with lower susceptibility to the tested antifungal drugs. What is of concern is the relatively high number of strains showing low susceptibility or resistance to itraconazole and lower number of strains susceptible to fluconazole and ketoconazole in T1DM children. In the very few studies that are currently available in T1DM children, no increase in the number of C. albicans strains with lower susceptibility to the studied drugs was reported. Only 4% of C. albicans strains showed reduced susceptibility to fluconazole and 6% were resistant to ketoconazole.Citation26 However, increased prevalence of drug-resistant Candida strains was observed in children with malignant diseases.
In a study, Gualco et alCitation27 have observed a higher percentage of itraconazole-resistant C. albicans obtained in children versus adults. There is evidence in the literature that drug resistance of Candida spp. is increasing. For example, Bremenkamp et alCitation28 showed that 47.2% of Candida strains isolated from the oral cavity of T1DM patients were resistant to ketoconazole. Our studies have also shown an increased number of Candida spp. strains resistant to the studied drugs. Isolation of increasing numbers of strains with decreased drug susceptibility may significantly impact the conditions in which factors contributing to candidiasis occur and may cause difficulties in treatment.Citation29 The more the risk factors, such as impaired immunity or the use of antibiotics, occur in a patient and the higher the pathogenic potential of the isolated strain, the higher the risk of severe candidiasis-related problems.
Enzymatic activity is among the factors that strongly affects fungal virulence. Candida spp. can produce numerous enzymes that determine the nature and course of the infection. Enzymatic activity may contribute to disruption of the balance between the fungus and host. Lipases are considered important in the first phase of infection; proteases can damage epithelial cells and allow the fungi to invade deep into the tissues.Citation14 Enzymatic activity testing in the yeast-like fungi obtained from the oral cavity has shown higher protease activities in strains cultured from specimens collected from diabetic patients versus control subjects.Citation30 Higher enzymatic activity of strains obtained from T1DM children may contribute to their higher virulence and may confer more risks in conditions of impaired defense mechanisms in a patient.
In conclusion, the yeast-like fungi isolated from fecal samples of T1DM children showed more species diversity versus those isolated from control samples. Moreover, more C. albicans strains in this group showed lower susceptibility to the tested drugs and higher enzymatic activity. Mycological tests, including quantitative evaluation of fecal samples, should be considered as part of a diagnostic approach in T1DM children. When yeast-like fungi are isolated, their drug susceptibility should be determined as there are an increasing number of strains resistant to the commonly used antifungal drugs.
Acknowledgments
This work was supported by The State Committee for Scientific Research ST-120 (Medical University of Gdańsk).
Disclosure
The authors report no conflicts of interest in this work.
References
- SoyucenEGulcanAAktuglu-ZeybekACOnalHKiykimEAydinADifferences in the gut microbiota of healthy children and those with type 1 diabetesPediatr Int201456333634324475780
- VaaralaOAtkinsonMANeuJThe “perfect storm” for type 1 diabetes – the complex interplay between intestinal microbiota, gut permeability, and mucosal immunityDiabetes2008572555256218820210
- DunneJLTriplettEWGeversDThe intestinal microbiome in type 1 diabetesClin Exp Immunol20141771303724628412
- BoernerBPSarvetnickNEType 1 diabetes: role of intestinal micro-biome in humans and miceAnn N Y Acad Sci2011124310311822211896
- VaaralaOGut microbiota and type 1 diabetesRev Diabet Stud2012925125923804264
- MurriMLeivaIGomez-ZumaqueroJMGut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control studyBMC Med2013114623433344
- BernhardtHKnokeMMycological aspects of gastrointestinal micro-floraScand J Gastroenterol Suppl19972221021069145460
- KumamotoCAInflammation and gastrointestinal Candida colonizationCurr Opin Microbiol201114438639121802979
- WhiteSJRosenbachALephartPSelf-regulation of Candida albicans population size during GI colonizationPLoS Pathog2007312e18418069889
- HuffnagleGBNoverrMCThe emerging world of the fungal micro-biomeTrends Microbiol201321733434123685069
- SzypowskaAPańkowskaESelected parameters of immunological system in children and adolescents with poorly controlled type-1 diabetes and fungal infectionsNowa Pediatria200424650
- SchulzeJSonnenbornUYeasts in the gut: From commensals to infectious agentsDtsch Arztebl Int200910651–5283784220062581
- KowalewskaBKawkoMZorenaKMyśliwiecMYeast-like fungi in the gastrointestinal tract in children and adolescents with diabetes type 1Pediatr Endocrinol Diabetes Metab201520417017726615584
- Batura-GabryelHBrajerBKuźnarBMłynarczykWCan hydrolytic activity of Candida strains isolated from COPD patients change with the basic disease progression Post Derm Alerg20033148155
- GosiewskiTSalamonDSzopaMSrokaAMaleckiMTBulandaMQuantitative evaluation of fungi of the genus Candida in the feces of adult patients with type 1 and 2 diabetes – a pilot studyGut Pathog201415;6143
- Position of the Polish Diabetes AssociationClinical Recommendations for treatment of patients with diabetesClin Diabetol20143SupplementA3A5
- WHO: DefinitionDiagnosis and Classification of Diabetes Mellitus and its Complications Report of a WHO Consultation Part 1 Diagnosis and Classification of Diabetes MellitusGenevaWHO2006
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and AdolescentsThe fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescentPediatrics200411455557615286277
- CisłoMWąsik-KuprianowiczABaranENoczyńskaAOccurrence of yeast and moulds in the digestive tract in children with diabetes type 1. Part II. Qualitative and quantitative fungi evaluation in the stool samplesMed Mycol2003103193198
- NowakowskaMJarosz-ChobotPBacterial and fungal flora in some clinical materials in children with diabetes type 1Pediatr Endocrinol Diabetes Metab2002828388 Polish [with English Abstract]
- GammelsrudKWSandvenPHøibyEASandvikLBrandtzaegPGaustadPColonization by Candida in children with cancer, children with cystic fibrosis, and healthy controlsClin Microbiol Infect201117121875188121745258
- MacuraAWitalisJFungi isolated from the stool in patients with gastrointestinal disorders in 2005–2009Przegl Epidemiol20106431331720731244
- KhatibRRiedererKMRamanathanJBaranJJrFaecal fungal flora in healthy volunteers and inpatientsMycoses200144515115611486452
- AgirbasliHOzcanSAGedikoğluGFecal fungal flora of pediatric healthy volunteers and immunosuppressed patientsMycopathologia2005159451552015983737
- TsaiMHWangSHHsuJFClinical and molecular characteristics of bloodstream infections caused by Candida albicans in children from 2003 to 2011Clin Microbiol Infect201521111018.e1e826148466
- NawrotUCisłoMNoczyńskaASusceptibility to selected antifungal agents of Yeats-like fungi isolated from gastrointestinal tract of diabetes children type 1Med Mycol20061313538
- GualcoLDebbiaEABandettiniRAntifungal resistance in Candida spp. isolated in Italy between 2002 and 2005 from children and adultsInt J Antimicrob Agents200729217918417175140
- BremenkampRMCarisARJorgeAOPrevalence and antifungal resistance profile of Candida spp. oral isolates from patients with type 1 and 2 diabetes mellitusArch Oral Biol201156654955521183157
- ArendrupMCCandida and candidaemia. Susceptibility and epidemiologyDan Med J20136011B469824192246
- RajendranRRobertsonDPHodgePJLappinDFRamageGHydrolytic enzyme production is associated with Candida albicans biofilm formation from patients with type 1 diabetesMycopathologia2010170422923520512529