Abstract
Daily life stress markedly affects the response toward stressful stimuli. DNA methy-lation is one of the factors that regulate this response, and is a normal mechanism of somatic cell growth, but its regulatory gene variations may cause alterations in the stress response. The aim of the present study was to investigate genotypic variants of the DNA methyltransferase 3A (DNMT3A) gene in 129 healthy subjects and evaluate its association with daily life stress. Blood samples were collected, and genomic DNA was isolated. DNA was amplified using specific tetra primers for DNMT3A (C/T) rs11683424 and visualized following 2% agarose gel electrophoresis. The association of DNMT3A genetic variants with daily life stress was analyzed using the Kessler Psychological Distress Scale (K10). We observed that the distribution of subjects with genotype CC (wild type), CT (heteromutant), and TT (homomutant) was 13.95%, 81.4%, and 4.65%, respectively. Genetic variations significantly affected the daily life stress condition (p=0.04) in Indonesian healthy subjects, but most of the subjects with the CT phenotype were classified in a stress condition.
Introduction
The response to stressful stimuli can be regulated by environmental experiences (early and later), genetic liability, and epigenetic mechanisms.Citation1,Citation2 Epigenetic mechanisms regulate the expression of several genes that are involved in experience-dependent plasticity, which is a structural and functional change in the brain.Citation3 Histone modification, non-protein coding RNA, and especially DNA methylation are epigenetic mechanisms that affect heritable gene expression or phenotype without causing a transformation in the DNA sequence or genotype.Citation4–Citation7
DNA methylation is required during normal somatic cell growth, and alterations in DNA methylation following stressful conditions have been shown in several stress-related genes.Citation8,Citation9 A key family of enzymes that plays a role in the addition of a methyl group to cytosine at the position 5 within cytosine–guanine dinucleotides (CpG) in the DNA sequence is the DNA methyltransferases (DNMTs: DNMT1, DNMT3A, and DNMT3B).Citation9–Citation11 The function of DNMT1 is to modulate the methylation process during DNA replication, whereas DNMT3A and DNMT3B are involved in de novo methylation.Citation9,Citation11 Inhibition of the DNMT enzymes may cause defects in cell growth, cell death, and genome instability, which shows that DNMT enzymes play a pivotal role in basic cell functions.Citation9,Citation12–Citation14
Modification in DNA methylation affects the binding of transcription factors and RNA polymerase into the DNA. Gene expression, thus, will be influenced by the location of DNA methylation and recruitment of cofactors – usually associated with decreased transcription and increased gene expression.Citation1 Alteration in DNA methylation has been shown in several stress-related conditions. Low expression of hippocampal glucocorticoid receptor (GR) with hypermethylation of the GR gene was shown in subjects exposed to prenatal stressor, postnatal stressor, and childhood maltreatment.Citation15–Citation19
DNMT3A has 23 exons, two isoforms (DNMT3A1 and DNMT3A2), and is regulated by an additional unique promoter.Citation20,Citation21 Among three single-nucleotide polymorphisms (SNPs) of DNMT3A (rs11683424, rs1465764, and rs1465825), rs11683424 has been shown to be involved in emotional responses to daily life stress. The variability of DNMT3A genes mainly exist in the intronic regions, including DNMT3A rs11683424, which is correlated with the alternative splicing of the DNMT3A gene.Citation3,Citation22
Currently, studies about the correlation between DNA methylation and mental health are limited, especially in the Indonesian population. In the present study, we examined the association between epigenetic gene variants, such as SNP rs11683424 of DNMT3A (C/T), with daily life stress. A questionnaire tool determined the level of daily life stress. The Kessler Psychological Distress Scale (K10) is a 10-question tool that is used to screen for nonspecific psychological distress and is usually used in general purpose health surveys; it is highly precise and is effective at identifying mental illnesses. Furthermore, the K10 questionnaire is an easy and quick tool that only takes 2–3 minutes for self-administration or interviewer-administration.Citation23 Results of this study may provide a method for preliminary screening that may enable the targeted prevention and early intervention of mental health in Indonesian citizens.
Materials and methods
Subjects
This study was an experimental study including 129 healthy subjects in Bandung city, Indonesia, who were >18 years old, without a history of mental disorders, not under any antipsychotic or antidepressant drugs, and not undergoing any psychological therapy. Participant information was anonymized during the analyses. All procedures conducted in studies involving human participants were approved (No. 727/UN6.C2.1.2/KEPK/PN/2014), and in accordance with, the ethical standards of the institutional and/or national research committee (Medical Research Ethics Committee of the Medical Faculty at Universitas Padjadjaran) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Genotyping of DNMT3A rs11683424
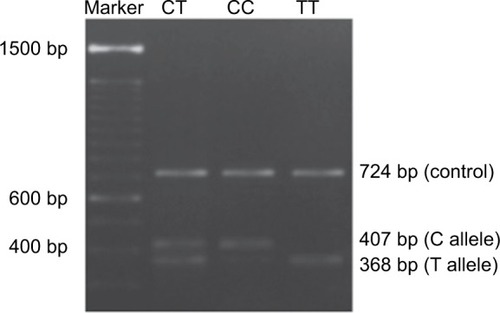
A variant of the DNMT3A gene was identified as rs11683424 (C/T) and described in The National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov). Samples (whole blood) were collected from all subjects and stored at −80°C until used in the assay. Genomic DNA was isolated using the PureLink™ Genomic DNA Mini Kit (Thermo Fisher Scientific, Waltham, MA, USA), and PCR was undertaken using 1.1× PCR SuperMix (Thermo Fisher Scientific) with specific tetra primers to identify SNPs in the DNMT3A gene (rs11683424). The tetra primers used in this analysis are as follows: primer forward 1 (F1) 5′-CTGTGCCTACTCCAAACATCATCATT-3′; primer forward 2 (F2) 5′-AGTTCAACACCCTTTCCCTGGT-3′; reverse primer 1 (R1) 5′-CAAAAATAACATCACCCTTGAAGGAG-3′; and reverse primer 2 (R2) 5′ CTCCTCTGACTTTACAACCCTGC-3′ (Sigma-Aldrich Co., St Louis, MO, USA). The PCR fragment was visualized by agarose gel electrophoresis (2%) followed by ultraviolet light at 312 nm. Three bands were visualized: the control (724 bp), C (407 bp), and T (368 bp) alleles. The genotyping of DNMT3A gene was repeated three times for each samples.
Kessler Psychological Distress Scale
All participants filled out the Kessler Psychological Distress Scale (K10) questionnaire to determine nonspecific psychological distress. Frequencies in the K10 questionnaire were described on a 5-point Likert scale with the responses: 1) all, 2) most, 3) some, 4) little, and 5) none of the time. These responses were scored from 4 to 0 and summed for a possible score range of 0–40. Scores <20 were classified as not stressed, 20–24 as mild stress, 25–29 as moderate stress, and ≥30 as severe stress.
Statistical analysis
Analysis of allele frequencies in each locus were calculated by chi-square test using the Hardy–Weinberg equilibrium (df = 1). Correlations between the DNMT3A genetic variants and stress conditions or among the DNMT3A genetic variants, stress condition, and gender were determined by chi-square (χ2) bivariate analysis (binary and multinomial logistic regression) with odds ratios (ORs) and 95% CI. A p<0.05 was considered as statistically significant.
Results
Genotyping of DNMT3A rs11683424 in healthy subjects
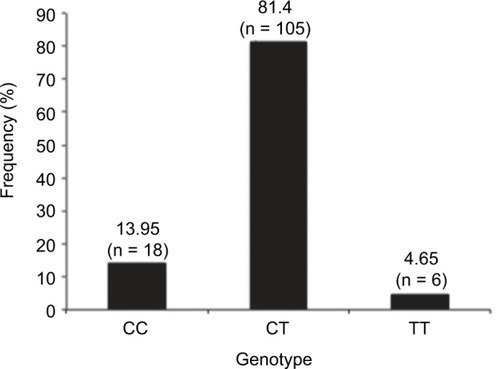
Profiling of the DNMT3A gene (rs11683424) was undertaken using specific tetra primers. The PCR result was visualized on a 2% agarose gel: the CC genotype was shown at 407 bp, CT at 368 bp and 407 bp, and TT at 368 bp; the control band was present in all genotypes (). The distribution of DNMT3A rs11683424 in healthy subjects was as follows: 18 subjects were wild-type CC homozygotes (13.95%); 105 subjects were CT heterozygotes (81.4%); and six subjects were TT homozygotes (4.65%; ). The Hardy–Weinberg equilibrium showed that the frequencies of the genotypes were in a disequilibrium state with p<0.05 ().
Table 1 Hardy–Weinberg equilibrium for the observed and expected genotype frequencies for DNMT3A rs11683424
Stress levels in healthy subjects by the Kessler Psychological Distress Scale
The K10 questionnaires were validated with p<0.05. Analyses of the stress level in 129 healthy subjects by the K10 questionnaire showed 33 subjects with no stress (25.58%), 48 subjects with mild stress (37.21%), 33 subjects with moderate stress (25.58%), and 15 subjects with severe stress (11.63%; ).
Table 2 The Kessler Psychological Distress Scale (K10) of healthy subjects: distribution correlated with the variations of genotype
Correlation of stress level with the DNMT3A genotype variant
The rs11683424 variant of DNMT3A showed multiple distributions among stress levels (K10 analysis), but the majority of subjects carried the heterozygous (CT) genotype (). In each stress group, the CT genotype was present and accounted for 87.88%, 79.17%, 87.9%, and 60% of the no, mild, moderate, and severe stress groups, respectively. Using bivariate analysis, the genotype variant significantly affected the stress level (p=0.04). Within the subjects with CT genotype (), if we compared between no stress (n=29) and all stress conditions taken together (mild, moderate, and severe; n=76), the incidence of stress was observed at a higher extent (72.38%). There were no significant correlations among DNMT3A genetic variants, stress condition, and gender. Analysis using binary logistic regression for no stress and all stress conditions (mild, moderate, and severe were grouped together as stress) resulted in a p-value of 0.88, whereas multinomial logistic regression for no, mild, moderate, and severe stress resulted in a p-value of 0.93 ().
Figure 3 The Kessler Psychological Distress Scale (K10) of healthy subjects: distribution correlated with the variation of genotype (bar graphs).
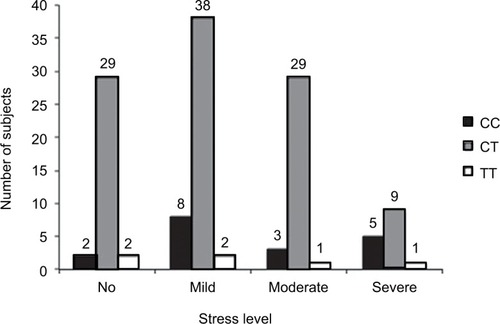
Table 3 The rs11683424 variant of DNMT3A and gender
Discussion
This is the first study to study the correlations between DNMT3A variants and the daily life stress response in healthy Indonesian subjects. Our results showed that most of the stress-condition subjects (mild, moderate, and severe), as determined with the K10 questionnaire, were T-allele carriers (CT or TT) of DNMT3A rs11683424. Our results were different with the previous study in the Netherlands, which showed that the T-allele carriers of rs11683424 were less affected by daily stress events and also that the T genotype seemed to be buffering the emotional changes.Citation2
All subjects were in a healthy condition with no psychological disorders or use of antipsychotic therapy. The K10 questionnaire result showed that 74.42% of the respondents were in stress conditions (mild, moderate, or severe) in which only daily life stress caused their psychological condition. Although the analysis result showed that the DNMT3A genotype variant (rs11683424) significantly correlated with psychological distress (p=0.04), a specific genotype (CC, CT, or TT) was not significantly associated with distress.
There might be certain gene regulatory pathways that mediate this nonspecific psychological distress. Epigenetic alterations involved in gene regulation initiated by environmental stress occur in early infancy and regulate the hypothalamic–pituitary–adrenal (HPA) pathways.Citation1 DNA methylation of the cytosine residue is one of the epigenetic mechanisms that cause gene silencing and gene activation.Citation24–Citation26 Unpleasant experiences will modify DNA methylation in different stress-related genes, which may affect emotional reactivity.Citation8
Levels of emotional reactivity to daily life stress are individual-dependent, where higher levels of emotional reactivity indicate symptoms of psychological disorder.Citation27,Citation28 Studies on the DNMT3A gene in the regulation of daily life stress are still limited. Other studies on the polymorphisms of DNMT3A rs11683424 have shown a significant correlation with the emotional response to daily life stress as analyzed by the Experience Sampling Methodology (ESM).Citation2 Furthermore, variations in the DNMT3A genotype induces several diseases, such as myelodysplastic syndrome (MDS)Citation29 and acute myeloid leukemia (AML), in southeast Asian populations.Citation30,Citation31 The expression of DNMT3A SNPs was increased in hepatocellular carcinoma, but it was not significantly correlated as a predictive marker.Citation32 Another variant of DNMT3A polymorphism at rs2289195 was observed to be associated at the genotypic level with schizophrenia in India, but was not significantly associated with onset of schizophrenia. DNMT3B rs2424932, rs1569686, and DNMT3L rs2070565 were observed to be significantly associated with early onset and familial early onset of schizophrenia in males.Citation33
Other stress-related disorders that are affected are more prevalent in females. The RNA expression of DNMT3A in female mice after subchronic variable stress (SCVS) induction was highly stimulated.Citation34 Epigenetic mechanisms involved in the methylation of the estrogen receptor and histone acetylation in the brain, which underline sex differences in behaviors, are vulnerable to repeated social defeat stress and mental disorders.Citation35–Citation43 Our results showed that there was no correlation between the rs11683424 polymorphism of the DNMT3A gene and psychological stress. Although the CT genotype was mainly observed in females, it was distributed in all types of stress as shown by K10 analysis. Therefore, the gene variants were not significantly correlated with stress in females.
A similar study was done in six SNPs (rs3736963, rs2767565, rs752016, rs1327175, rs2478813, and rs716461) of plexin A2 (PLXA2) in schizophrenic subjects, and showed that the rs2478813 variant was significantly correlated with anxiety, depression, neuroticism, and psychological distress (K10).Citation44 Validation of the K10 questionnaire representing nonspecific psychological distress in Aboriginal people was done. Higher levels of distress were observed in females with lower education and lower household income as well as in subjects who reported poor mental health (mood disorder, anxiety disorder, and suicidal ideation).Citation45 The K10 questionnaire is a method that is used to screen for nonspecific psychological distress as well as symptoms of depression and anxiety within 30 days. Moreover, this method was positively correlated with the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV).Citation23,Citation46 Analysis using the K10 questionnaire is suitable for analyzing psychological distress in Indonesia, especially with regard to the many community problems that are present in a developing country. However, further clinical analysis should be included, such as blood pressure, salivary alpha amylase (SAA) and levels of cortisol, and catecholamines in blood, saliva, and urine to confirm the correlation between genetic variations with K10 questionnaire analysis.
Some studies showed that SAA and salivary cortisol level are increased in stressed condition, especially during puberty.Citation47–Citation50 The cortisol level in the saliva can be used as a parameter of stress condition, but the level of salivary cortisol in the morning was observed to be at a higher level than in the evening. However, physical activities, psychological condition, and type of circadian rhythm also influenced the level of salivary cortisol.Citation51–Citation53 Moreover, salivary alpha-amylase can be used as a biological marker for the sympathetic nervous system (SNS) to describe physiological and psychosocial stress.Citation54–Citation57 Differing from cortisol, levels of SAA decline an hour after awakening, increase during the rest of the day, and peak in the late afternoon or evening (diurnal profile).Citation58,Citation59
This study is the first report of a correlation between genotype variations with psychological distress in one particular city in Indonesia, Bandung city, as first screening and preliminary research. However, the present study provides data for the Indonesian genotype variation of DNMT3A. Although DNMT3A (rs11683424) genotypic variants were significantly correlated with psychological distress, it was not affected by gender. However, more samples are needed to describe data that represents all areas in Indonesia. For further investigation, patients with psychological disorder should be included as a comparison with healthy subjects and to confirm the involvement of DNMT3A variations in daily life stress susceptibility. Confirmation of DNA methylation to confirm DNMT3A gene variations should also be included using bisulfite methylation sequencing or methylation-specific PCR for further analysis.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank our team: Henry Ng, Anzari Muhammad, Indah A Sagita, Casuarina Rusmawati, and Carissa P Purabaya for great team collaboration.
Disclosure
The authors report no conflicts of interest in this work.
References
- GudsnukKChampagneFAEpigenetic influence of stress and the social environmentILAR J2012533–427928823744967
- PishvaEDrukkerMViechtbauerWEpigenetic genes and emotional reactivity to daily life events: a multi-step gene-environment interaction studyPLoS One201496e10093524967710
- WeaverICCervoniNChampagneFAEpigenetic programming by maternal behaviorNat Neurosci20047884785415220929
- FeinbergAPEpigenetics at the epicenter of modern medicineJAMA2008299111345135018349095
- MeaneyMJEpigenetics and the biological definition of gene x environment interactionsChild Dev2010811417920331654
- MooreLDLeTFanGDNA methylation and its basic functionNeuropsychopharmacology2013381233822781841
- WeichenhanDPlassCThe evolving epigenomeHuman Mol Gen201322R1R1R6
- YangXEwaldERHuoYGlucocorticoid-induced loss of DNA methylation in non-neuronal cells and potential involvement of DNMT1 in epigenetic regulation of Fkbp5Biochem Biophys Res Commun2012420357057522445894
- TsumuraAHayakawaTKumakiYMaintenance of self-renewal ability of mouse embryonic stem cells in the absence of DNA methyltransferases Dnmt1, Dnmt3a and Dnmt3bGenes Cells200611780581416824199
- Turek-PlewaJJagodzińskiPPThe role of mammalian DNA methyltransferases in the regulation of gene expressionCell Mol Biol Lett200510463164716341272
- OkanoMBellDWHaberDALiEDNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian developmentCell199999324725710555141
- XuGLBestorTHBourc’hisDChromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase geneNature1999402675818719110647011
- Jackson-GrusbyLBeardCPossematoRLoss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulationNat Genet2001271313911137995
- HataKKusumiMYokomineTLiESasakiHMeiotic and epigenetic aberrations in Dnmt3L-deficient male germ cellsMol Reprod Dev200673111612216211598
- OberlanderTFWeinbergJPapsdorfMGrunauRMisriSDevlinAMPrenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responsesEpigenetics2008329710618536531
- MurgatroydCPatchevAVWuYDynamic DNA methylation programs persistent adverse effects of early-life stressNat Neurosci200912121559156619898468
- KemberRLDempsterELLeeTHSchalkwykLCMillJFernandesCMaternal separation is associated with strain-specific responses to stress and epigenetic alterations to Nr3c1, Avp, and Nr4a1 in mouseBrain Behav20122445546722950049
- MelasPAWeiYWongCCGenetic and epigenetic associations of MAOA and NR3C1 with depression and childhood adversitiesInt J Neuropsychopharmacol20131671513152823449091
- McGowanPOSasakiAD’AlessioACEpigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuseNat Neurosci200912334234819234457
- ChenTUedaYXieSLiEA novel Dnmt3a isoform produced from an alternative promoter localizes to euchromatin and its expression correlates with active de novo methylationJ Biol Chem200227741387463875412138111
- WeisenbergerDJVelicescuMPreciado-LopezMAIdentification and characterization of alternatively spliced variants of DNA methyltransferase 3a in mammalian cellsGene20022981919912406579
- FlicekPAmodeMRBarrellDEnsembl 2014Nucleic Acids Res201442Database issueD749D75524316576
- KesslerRCAndrewsGColpeLJShort screening scales to monitor population prevalences and trends in non-specific psychological distressPsychol Med200232695997612214795
- WeissmanJNaiduSBjornssonHTAbnormalities of the DNA methylation mark and its machinery: an emerging cause of neurologic dysfunctionSemin Neurol201434324925725192503
- ChahrourMJungSYShawCMeCP2, a key contributor to neurological disease, activates and represses transcriptionScience200832058801224122918511691
- GuoJUSuYShinJHDistribution, recognition and regulation of non-CpG methylation in the adult mammalian brainNat Neurosci201417221522224362762
- Myin-GermeysIvan OsJSchwartzJEStoneAADelespaulPAEmotional reactivity to daily life stress in psychosisArch Gen Psychiatry200158121137114411735842
- Myin-GermeysIPeetersFHavermansREmotional reactivity to daily life stress in psychosis and affective disorder: an experience sampling studyActa Psychiatr Scand2003107212413112534438
- ShahrabiSKhosraviAShahjahaniMRahimFSakiNGenetics and epigenetics of myelodysplastic syndromes and response to drug therapy: new insightsOncol Rev201610231128058097
- TanMNgIKSChenZClinical implications of DNMT3A mutations in a Southeast Asian cohort of acute myeloid leukaemia patientsJ Clin Pathol201770866967628100593
- LinPHLiHYFanSCA targeted next-generation sequencing in the molecular risk stratification of adult acute myeloid leukemia: implications for clinical practiceCancer Med20176234936028070990
- ZhaoCYanFWuHQiaoFQiuXFanHDNMT3A -448A>G polymorphism and the risk for hepatocellular carcinomaBiomed Rep20131466466824649006
- SaradalekshmiKRNeethaNVSathyanSNairIVNairCMBanerjeeMDNA methyl transferase (DNMT) gene polymorphisms could be a primary event in epigenetic susceptibility to schizophreniaPLoS One201495e9818224859147
- HodesGEPfauMLPurushothamanISex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stressJ Neurosci20153550163621637626674863
- KurianJROlesenKMAugerAPSex differences in epigenetic regulation of the estrogen receptor-alpha promoter within the developing preoptic areaEndocrinology201015152297230520237133
- SchwarzJMNugentBMMcCarthyMMDevelopmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life spanEndocrinology2010151104871488120702577
- MurrayEKHienAde VriesGJForgerNGEpigenetic control of sexual differentiation of the bed nucleus of the stria terminalisEndocrinology200915094241424719497973
- MatsudaKIMoriHNugentBMPfaffDWMcCarthyMMKawataMHistone deacetylation during brain development is essential for permanent masculinization of sexual behaviorEndocrinology201115272760276721586557
- KimDRBaleTLEppersonCNPrenatal programming of mental illness: current understanding of relationship and mechanismsCurr Psychiatry Rep2015172525617041
- TsankovaNMBertonORenthalWKumarANeveRLNestlerEJSustained hippocampal chromatin regulation in a mouse model of depression and antidepressant actionNat Neurosci20069451952516501568
- ElliottEEzra-NevoGRegevLNeufeld-CohenAChenAResilience to social stress coincides with functional DNA methylation of the Crf gene in adult miceNat Neurosci201013111351135320890295
- LaPlantQVialouVCovingtonHE3rdDnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbensNat Neurosci20101391137114320729844
- GoldenSAChristoffelDJHeshmatiMEpigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depressionNat Med201319333734423416703
- WrayNRJamesMRMahSPAnxiety and comorbid measures associated with PLXNA2Arch Gen Psychiatry200764331832617339520
- BougieEArimRGKohenDEFindlayLCValidation of the 10-item Kessler Psychological Distress Scale (K10) in the 2012 Aboriginal Peoples SurveyHealth Rep2016271310
- KesslerRCBarkerPRColpeLJScreening for serious mental illness in the general populationArch Gen Psychiatry200360218418912578436
- StrahlerJSkoludaNKappertMBNaterUMSimultaneous measurement of salivary cortisol and alpha-amylase: application and recommendationsNeurosci Biobehav Rev Epub2017831
- JiJNegriffSKimHSusmanEJA study of cortisol reactivity and recovery among young adolescents: heterogeneity and longitudinal stability and changeDev Psychobiol201658328330226517401
- StrahlerJMuellerARosenloecherFKirschbaumCRohlederNSalivary alpha-amylase stress reactivity across different age groupsPsychophysiology201047358759520070573
- AlmelaMHidalgoVVilladaCSalivary alpha-amylase response to acute psychosocial stress: the impact of ageBiol Psychol201187342142921664412
- BonatoMLa TorreASaresellaMMarventanoIMeratiGVitaleJASalivary cortisol concentration after high-intensity interval exercise: time of day and chronotype effectChronobiol Int201734669870728409690
- KudielkaBMFederenkoISHellhammerDHWüstSMorningness and eveningness: the free cortisol rise after awakening in “early birds” and “night owls”Biol Psychol200672214114616236420
- DickersonSSKemenyMEAcute stressors and cortisol responses: a theoretical integration and synthesis of laboratory researchPsychol Bull2004130335539115122924
- ChattertonRTJrVogelsongKMLuYCEllmanABHudgensGASalivary alpha-amylase as a measure of endogenous adrenergic activityClin Physiol19961644334488842578
- NaterUMLa MarcaRFlorinLStress-induced changes in human salivary alpha-amylase activity – associations with adrenergic activityPsychoneuroendocrinology2006311495816002223
- NaterUMRohlederNGaabJHuman salivary alpha-amylase reactivity in a psychosocial stress paradigmInt J Psychophysiol200555333334215708646
- SchumacherSKirschbaumCFydrichTStröhleAIs salivary alpha-amylase an indicator of autonomic nervous system dysregulations in mental disorders?–a review of preliminary findings and the interactions with cortisolPsychoneuroendocrinology201338672974323481259
- NaterUMRohlederNSchlotzWEhlertUKirschbaumCDeterminants of the diurnal course of salivary alpha-amylasePsychoneuroendocrinology200732439240117418498
- RohlederNNaterUMWolfJMEhlertUKirschbaumCPsychosocial stress-induced activation of salivary alpha-amylase: an indicator of sympathetic activity?Ann N Y Acad Sci2004103225826315677423