Abstract
Introduction
The human immunodeficiency virus (HIV) stills remains a serious public health problem. HIV acquisition has several negative health consequences, such as a cognitive deterioration or health-related quality of life (HRQoL) impairment. Although these negative consequences could be directly related to a significant increase in self-stigma in this population, few previous studies have analysed the possible associations between these variables. This is especially the case in specific groups of people living with HIV, such as men who have sex with men who could be at a greater risk of stigmatisation. The main aim of the present study was to evaluate the association between cognitive functioning, HRQoL and self-stigma in a group of men with HIV who have sex with men.
Methods
The present study was conducted in the Infectious Diseases Unit of the General University Hospital of Alicante (Spain). The final sample was composed of 70 participants who passed the inclusion and exclusion criteria. All were men with HIV who had sex with men and the sample’s mean age was 45 years. Each participant completed questionnaires on HRQoL and HIV self-stigma. Moreover, they completed an online cognitive assessment through the previously validated platform for cognitive evaluation CogniFit, Inc.
Results
The obtained results showed a significant association between memory functioning impairment, lower levels of HRQoL and higher HIV self-stigma scores. Hence, HRQoL, in the mental summary domain, was shown to be a significant mediator in the relationship between low memory performance and higher HIV self-stigma.
Discussion
Neurocognitive impairment could decrease HRQoL in men with HIV who have sex with men, and hence, reinforce the idea widespread in society that having HIV holds serious consequences. This fact, together with the reduced cognitive abilities to fight against their own self-stigma could represent plausible explanations of the obtained results. In this sense, intervention strategies, oriented towards reducing cognitive impairment, such as those based on cognitive training, and other psychological interventions to promote HRQoL could be effective approaches to prevent the negative effects of HIV self-stigma in this population.
Introduction
The Human Immunodeficiency Virus (HIV) is a global public health problem. There were an estimated 1.7 million new infections in 2018,Citation1 reaching a total of 38 million infected individuals worldwide in 2019.Citation2 The figures in Spain are similar. Indeed, according to the annual report on HIV epidemiology issued by Spain’s Ministry of Health, a total of 3244 new HIV diagnoses were reported over 2018, of which 85.3% were men.Citation2 With the widespread application of Antiretroviral Therapy (ART), HIV has gradually become a chronic disease, infected people’s life expectancy being similar to that of the general population.Citation3,Citation4 However, stigma and discrimination has evolved unevenly in society compared to scientific progress. The psychological impact of receiving an HIV diagnosis is still a heavy burden and the experience is complex and diverse at the inter-individual level.Citation2,Citation4 Furthermore, due to related health problems in HIV-infected populations, levels of morbidity are higher, especially in terms of HIV-associated neurocognitive impairment. In this way, recent studies indicate that the prevalence of HIV-associated neurocognitive impairment ranges from 23.5% to 87.2%.Citation5–Citation7 More specifically, memory is the most commonly affected cognitive ability in people with HIV (27.4%).Citation8 One consequence of cognitive impairment, especially in men with HIV who have sex with men (MSM), is related to stigma. Stigma is a process through which individuals are ‘disqualified from full social acceptance due to an undesirable “mark” or “label”. This label can be a physical, health-related, or a behavioural attribute that is deemed “deeply discrediting”.Citation9 Crawford found that the degree of stigma associated with HIV infection is greater than that of other medical conditions such as hepatitis, drug abuse, diabetes, and cancer.Citation10 This stigma has major social and psychological implications that are rooted in perceptions of the implications of HIV in society.Citation11 Misconceptions about HIV transmission routes, perceived contagiousness, and overestimations regarding the risks of casual contact can evoke a certain stigmatisation of People Living With HIV (PLWHIV).Citation12–Citation14 HIV stigma carries serious consequences. It has been related to a decrease in HIV screening tests, poorer preventive behaviours, lesser visits to the doctor, poorer treatment engagement and retention, reduced disclosure of HIV status, and a lower quality of life for PLWHIV, generally.Citation15–Citation18 PLWHIV suffer from multiple stigmas. PLWHIV experiment HIV stigma and also multiple forms of social and structural discrimination, including racial discrimination, homophobia, powerlessness, social isolation, and self-stigma.Citation19,Citation20 However, the stigma could be internalised by individuals, resulting in what has been referred to as self-stigma. Self-stigma is defined as the maladaptive process in which a person accepts social prejudices and integrates societal beliefs as part of their own self-concept.Citation21 Self-stigma occurs when people who belong to a socially discredited group, such as PLWHIV, or people who use illicit drugs, endorse and internalise feelings of shame and worthlessness due to their socially devalued identity, or “spoiled identity”.Citation21 This self-stigma can be understood as a process of identity transformation, which leads to the loss of previously held beliefs about oneself and the acceptance of lowered expectations for oneself.Citation22
HIV self-stigma is particularly dangerous and has many terrible consequences. Indeed, it influences affective, cognitive, and mental health outcomes, as well as healthcare behaviours, which ultimately affects the physical health outcomes of PLWHIV.Citation23–Citation25 More specifically, the HIV self-stigma keeps people from visiting HIV consultants and other health services,Citation26–Citation28 hindering adherence to ART. Thus, PLWHIV with a high level of self-stigma progress more quickly from HIV to AIDS,Citation29 self-stigma, therefore, being attributed to the high risk of morbidity and mortality related to HIV.Citation26,Citation27 At a psychological level, high HIV self-stigma is associated with avoidance, rejection, abandonment, social exclusion,Citation30,Citation31 an increase in the risk of suicide,Citation32,Citation33 and greater levels of psychological distress.Citation34 As such, it has an impact on mental health and the quality of life of PLWHIV.Citation32,Citation35 When experiencing HIV self-stigma, men with HIV are not only affected by feelings of shame and guilt, they also internalise a level of negative self-image and have a greater tendency to withdraw from social interactions.Citation36 The high level of self-stigma around contracting HIV and these severe consequences could be explained by the act that leads to contracting the disease, because it is regarded as controllable. This makes it more likely that a sense of responsibility for acquiring the illness is inferred, bringing about further emotional reactions such as pity or blame.Citation37,Citation38 HIV self-stigma is a very extensive field of study.
Regarding HIV self-stigma prevalence, a recent studyCitation39 estimated that nearly two-thirds of MSM with HIV had a moderate to high level of HIV self-stigma, and eventually internalised and endorsed the stigmatising thoughts and feelings associated with their identity as people living with HIV. In this regard, cognitive impairment could negatively affect HIV self-stigma, as the person’s ability to reason is reduced. Previous studies have highlighted that metacognitive abilities are related to the ability to deflect self-stigma in people with schizophrenia.Citation40 This suggests that neurocognitive deficits, including memory, limit a person’s ability to reject social stereotypes.Citation39,Citation41 In addition, people with HIV are vulnerable to HIV self-stigma, which translates into a greater level of agreement with negative beliefs associated with their identity.Citation39 However, studies analysing the relationship between cognitive abilities and HIV self-stigma in this group are scarce. In this regard, unlike HIV-related discrimination, HIV self-stigma remains strikingly understudied, with no evidence of well-established programmes that address the issue.Citation33,Citation42–Citation45 It is particularly urgent to start to work in this line, not only to decrease the serious consequences and the high impact on the quality of life of PLWHIV, but also to compensate the shortage of interventions targeting HIV-related self-stigma.Citation42,Citation46,Citation47 To understand how to manage HIV self-stigma, studies on the factors affecting self-stigma and how they operate are necessary.
Moreover, the deterioration of cognitive functions, such as memory, not only affects self-stigma but also health-related quality of life (HRQoL). The latter is recognised as a subjective and multidimensional well-being variable that involves an individual’s physical, mental, emotional, and social functioning, and is essential for the management of chronic diseases such as HIV.Citation48,Citation49 According to a recent review, few studies have hitherto analysed the relationship between HRQoL and neurocognitive impairment in MSM with HIV.Citation48 Nevertheless, some previous studies have found that the presence of neurocognitive impairment is significantly associated with lower HRQoL scores in HIV-positive MSM.Citation49–Citation51 As a result, HRQoL has become an important health outcome for people living with HIV.Citation49,Citation52 A recent study found that cognitive performance indirectly affects HRQoL through its impact on cognitive difficulties and the ability of men with HIV to participate in meaningful activities such as leisure, household management, and sports.Citation53
Several studies have also shown that HRQoL is related to self-stigma in clinical samples.Citation54–Citation57 In this sense, low levels of HRQoL were significantly associated with cognitive deficits and negative emotions, high levels of self-stigma and low levels of self-esteem among people diagnosed with depression,Citation54 as well as impaired psychological well-being, self-esteem, and autonomy in people with schizophrenia.Citation58,Citation59 Self-stigma and quality of life may have a bidirectional relationship and focusing on some specific areas of quality of life (such as autonomy through self-empowerment approaches) may also improve the effectiveness of programmes that reduce the impact of self-stigma on the quality of life of subjects with schizophrenia.Citation54 While this association has been addressed in different clinical populations, studies examining this relationship in MSM with HIV are scarce.
Based on all the above, the main objective of the present study was to understand the relationship between neurocognitive functioning, HRQoL and HIV self-stigma in MSM with HIV. Moreover, the present study aimed to identify the possible mediation effect of HRQoL in the relation between cognitive functioning and HIV self-stigma. Based on previous research, we hypothesised that a poorer cognitive functioning is associated with a deterioration of HRQoL and higher levels of self-stigma.Citation53,Citation57,Citation60,Citation61 Furthermore, although no previous studies have specifically tested the mediation effect of HRQoL in the relationship between cognitive functioning and HIV self-stigma, we expected to find a significant mediation role of HRQoL based on the results of previous studies.
Materials and Methods
Study Design and Participants
The study’s design was cross-sectional and took place at the Infectious Diseases Unit of the General University Hospital of Alicante (Spain). Inclusion criteria were: (1) having an HIV infection diagnosis; (2) being aged ≥ 18 years; (3) being a man who has sex with men; (4) receiving antiretroviral treatment: (5) and signing an informed consent for the whole study. Exclusion criteria included: the presence of comorbidities identified in medical records (dementia or other central nervous system diseases, mental health condition(s) diagnosis, viral chronic hepatitis, active cancer or infection, diabetes mellitus, high blood pressure, cardiovascular disease, hypothyroidism, malnutrition, and other severe health conditions) and mental or physical impairments that could hinder the participant’s ability to complete or understand the study questionnaires. All patients in usual care between February 2020 and December 2020 who met the inclusion criteria were invited to participate in the study. The patient cohort followed by the Infectious Diseases Unit of the General University Hospital of Alicante (HGUA) includes 900 patients with HIV. Of these, 630 patients met the study’s inclusion criteria and not its exclusion criteria. After performing the sample calculation using G*Power,Citation62 the resulting required sample was 62 MSM with HIV. To prevent the expected loss of patients, we decided to include 72 HSH PHIV. Ultimately, 2 subjects failed to answer the questionnaires adequately, so the final sample was composed of 70 HIV-positive MSM.
The study was conducted in two phases. During the first phase, participants were informed about the study’s characteristics, and were notified that the confidentiality and anonymity of all data provided would be preserved throughout the project. To ensure data confidentiality and anonymity, codes were assigned to identify the participants. Once the participants had signed the informed consent document, they completed questionnaires related to sociodemographic data, HRQoL, and Internalised HIV Stigma. The patients who enrolled in the first part were invited to continue in the study. The second phase consisted of an online multidomain cognitive test named “Online General Cognitive Assessment Battery (CogniFit, Inc.)”. Both the first and the second part of the study were conducted at the hospital unit with the support of specialised researchers.
No participants received any remuneration for their participation in the study. The study was conducted following the guidelines of the Declaration of Helsinki and the European Union Good Clinical Practice standards. The study was also approved (26 February 2020) by the Ethics Committee of the General University Hospital of Alicante (PI2019/083).
Variables
Sociodemographic and clinical data. Sociodemographic variables were evaluated through an ad hoc questionnaire developed for this study. It asked about age, educational level, nationality, employment status and income level. Clinical data were extracted from each participant’s medical records, including the following variables: time since HIV diagnosis, current CD4+ lymphocytes, Nadir CD4+ lymphocyte and viral load.
Internalised HIV Stigma Scale (HIV-ISS).Citation63 This questionnaire is a Spanish self-report instrument that evaluates the level of internalised stigma related to HIV over the most recent month. This scale is comprised of 10 items with a 5-point Likert-type response format (1=never or nearly never, 2=seldom, 3=sometimes, 4=frequently, 5=always or almost all the time). The total score of the HIV-ISS is obtained by adding the 10 item scores (range: 10–50). A higher score in the questionnaire indicates a higher level of perceived internalised HIV stigma.
Health-Related Quality of Life (HRQoL). The Spanish version of the Medical Outcomes Study-HIV Health Survey was employed to evaluate the HRQoL.Citation64 This questionnaire includes 11 subscales of HRQoL: General Health Perceptions (5 items), Pain (2 items), Physical Functioning (6 items), Role Functioning (2 items), Social Functioning (1 item), Mental Health (5 items), Energy/Fatigue (4 items), Cognitive Functioning (4 items), Health Distress (4 items), Quality of Life (1 item), and Health Transition (1 item). Moreover, it has two general indexes that are based on the calculation of the scores obtained in the subscales: Physical Health Summary (PHS) and Mental Health Summary (MHS). In the questionnaire, the questions refer to the 2 previous weeks, the scale points are 2, 3, 5, and 6, and the final score is given on a scale from 0 to 100. Higher scores correspond to greater health. A recent meta-analysis of this instrument highlights the high reliability of the HRQoL evaluation, the average α coefficient for the total score of MOS-HIV (Medical Outcome Study-HIV) being 0.91 and above 0.80 for all the subscales, except for role functioning.Citation65
Neurocognitive domains. The General Cognitive Assessment Battery (CogniFit, Inc.) is a software application used to measure cognitive performance (https://www.cognifit.com). This battery has been used previously in research on a wide range of populations and has demonstrated its efficacy.Citation66–Citation69 The battery is composed of a test that assesses the cognitive domains: reasoning, memory, attention, coordination, and perception. This cognitive function test lasts about 30 minutes. Following the instructions and normative data, the highest score in each domain is 800. Scoring over 400 indicates adequate performance, and lesser scores equate to low performance.
Data Analysis
Descriptive analyses of the sociodemographic and HIV-related clinical characteristics of the sample were carried out. Pearson’s correlations were applied to test the relationship between scores in the neurocognitive domains, HRQoL, and HIV self-stigma. To analyse the predictive value of HIV self-stigma based on the neurocognitive domains and HRQoL, a step-by-step hierarchical regression analysis was used. Participants were divided into two groups following the normative data obtained in the validation study of the General Cognitive Assessment Battery conducted by CogniFit, Inc. (scores below 400 indicated low performance and those above 400, high performance). For the mediation analyses, the PROCESS macro by Hayes was employed.Citation70 This macro is a path analysis modelling tool used to estimate direct and indirect effects in mediation models. It is an empirical bias-corrected bootstrapping procedure that serves to approximate confidence intervals from the repeated resampling of the observed data. A mediation effect is significant only when the 95% confidence interval does not include zero. In this case, it would be concluded that in 95% of the bootstrapped samples, the effect of memory on HIV self-stigma is mediated by the effect of HRQoL (PHS and MHS). In the present study, the data was resampled 10,000 times, as recommended by Hayes.Citation70 In small samples, bootstrapping has been demonstrated to be the most effective and powerful method to test indirect effects compared to other traditional methods, such as linear regression or the Sobel test. In all cases, p<0.05 was considered significant. Each statistical analysis was conducted using SPSS, Version 24.0 (Armonk, NY, USA).
Results
Sociodemographic and Clinical Variables of the Participants
Regarding sociodemographic dimensions, most participants had advanced education (72.9%) and were employed (78.6%). With respect to clinical variables, mean time since HIV diagnosis was less than 10 years, and over 95% of the men had an HIV-VL <50 cop. RaN/mL and high CD4+ cell counts. Participant characteristics are summarised in .
Table 1 Participants’ Sociodemographic and HIV-Related Clinical Variables
Relationships Between Neurocognitive Domains, HRQoL, and HIV Self-Stigma
As can be observed in , only memory was positively correlated with the summary domains in the HRQoL, both PHS (r=0.257, p<0.05) and MHS (r=0.291, p<0.05); and memory was negatively associated with HIV self-stigma (r=−0.276, p<0.05). Regarding HRQoL, both PHS (r=−0.428, p<0.01) and MHS (r=−0.596, p<0.01) were negatively related to HIV self-stigma. The full correlation patterns are summarised in .
Table 2 Pearson Correlation Coefficients Among Neurocognitive Domains, Physical, and Mental Health Summary of HRQoL and HIV Self-Stigma
Regression Analysis of Neurocognitive Domains and HRQoL Affecting HIV Self-Stigma
To examine how neurocognitive domains and HRQoL affect HIV self-stigma, hierarchical regression analyses were conducted. In the first step, all evaluated neurocognitive domains were included. The PHS and MHS were then incorporated in the second step. When neurocognitive domains were included, only memory was significant, explaining 5.6% of the variance in HIV self-stigma. Subsequently, when PHS and MHS were added to the model, memory did not remain significant, and only MHS was revealed to be significant. In this step, the model explained 31.6% of the variance in HIV self-stigma ().
Table 3 Regression Analyses of Sociodemographic Variables, Memory, and HRQoL as Predictors of HIV Self-Stigma
Differences in HRQoL and HIV Self-Stigma According to the Performance Exhibited by Participants in Each Neurocognitive Domain
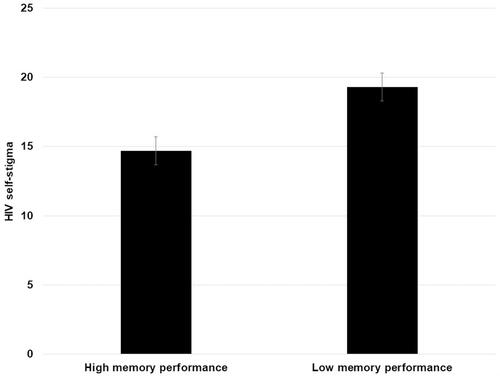
Participants were divided into two groups based on their performance in each evaluated domain. In this regard, following the instructions and normative data of the employed evaluation software for the assessment of cognitive variables, scores below 400 indicated low performance in each domain. Conversely, scores above 400 showed high performance in the neurocognitive domain. Based on this classification, differences in HRQoL and HIV self-stigma were evaluated. Except in the case of memory, no differences were found in any neurocognitive domain (p>0.05). In the case of memory, differences were found in HIV self-stigma t(67.418)=2.709, p=0.009, d=0.66, 95% IC [1.21, 8.02]. Results from the independent samples t test indicated that individuals with high memory performance (M=14.69, SD=4.95, N=23) scored much lower on HIV self-stigma than participants who exhibited lower memory performance (M=19.31, SD=9.30, N=47) (see ). No differences were found in the case of HRQoL between groups based on memory performance (p>0.05).
Multiple Mediating Effects of HRQoL on the Relationship Between Memory and HIV Self-Stigma
In order to analyse the possible mediating effect of PHS and MHS on the association of memory with HIV self-stigma, mediation analyses were performed employing a bootstrapping method.
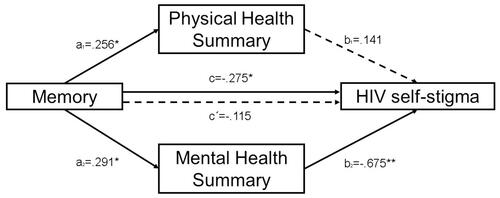
Memory was entered in the model as an independent variable, PHS and MHS as mediators and HIV self-stigma as dependent variable. First, memory predicted PHS (B=0.017, SE=0.008, p=0.03, IC95% [0.001, 0.034]) and MHS (B=0.026, SE=0.010, p=0.01, IC95% [0.005, 0.047]). With regard to the proposed mediators, only MHS predicted HIV self-stigma (B=−0.400, SE=0.097, p=0.0001, IC95% [−0.594, −0.206]). PHS did not reveal a significant effect on HIV self-stigma. The analysis of the indirect effect of memory on HIV self-stigma, through the MHS effect, showed a significant mediation (indirect effect=−0.010, SE=0.005, bias corrected 95%, confidence interval for the indirect effect: lower level=−0.023, upper level=−0.001). PHS did not reveal itself as a significant mediator (indirect effect=0.002, SE=0.003, bias corrected 95%, confidence interval for the indirect effect: lower level=−0.004, upper level=0.009). The total indirect effect estimated in the model was significant (indirect effect=−0.008, bias corrected 95%, confidence interval for the indirect effect: lower level=−0.019, upper level=−0.001). Given that a mediating effect is significant when the 95% confidence interval does not span zero, it could be concluded that memory had a significant indirect effect on HIV self-stigma, but only through the effect of the MHS of HRQoL as a significant mediator, unlike PHS. Hence, when MHS was introduced in the model as a mediator, the relationship between memory and HIV self-stigma did not show a statistically significant result (B=−0.006, SE=0.005, p=0.261), suggesting that MHS has a full mediating effect in that association. Overall, the model F(3, 66)=13.162, p=0.00001 predicted 37.4% of the variance in patients’ HIV self-stigma.
No other mediation models were analysed as the rest of the neurocognitive domains evaluated did not exhibit a significant association with HIV self-stigma. The magnitudes of the PHS and MHS mediating effects were not compared due to the PHS’s lack of significance as a mediator. Standardised regression coefficients and direct and indirect effects are represented in .
Figure 2 Results of multiple mediation analyses exploring the mediating effect of Physical Health Summary and Mental Health Summary on the association between memory and HIV self-stigma (**p < 0.01; *p < 0.05). The presented values are standardised regression coefficients where: “a1” indicates the standardised regression coefficient in the association between memory and Physical Health Summary; “a2” indicates the standardised regression coefficient in the association between memory and Mental Health Summary, “b1” indicates the standardised regression coefficient in the association between Physical Health Summary and HIV self-stigma; “b2” indicates the standardised regression coefficient in the association between Mental Health Summary and HIV self-stigma; “c” indicates an indirect effect and “c´” represents a direct effect in the association between memory and HIV self-stigma. Dashed lines represent non-significant effects.
Discussion
The objective of the present study was to understand the association between cognition, HRQoL, and HIV self-stigma in MSM with HIV. Our results present an explanatory model of how cognitive deficiencies influence self-stigma in MSM with HIV. More specifically, participants with low memory performance presented high HIV self-stigma. The performed mediation showed direct relationships between memory and HIV self-stigma. However, when we introduced HRQoL as a mediator in this relationship, the direct correlation disappeared. Moreover, the MHS of HRQoL mediated the relationship between cognition and HIV self-stigma.
Furthermore, we found that neurocognitive impairment influenced the quality of life of PLWHIV. This relationship has previously been described in the literature,Citation49–Citation51 but our study has further contributed to the field, thus addressing suggestions of future research in this line.Citation48 As such, our article supports the relevance of evaluating the quality of life and interacting factors for PLWHIV. Our work also supports HIV advocacy groups that are calling for its inclusion as an additional 90 in the 90-90-90 testing and treatment target introduced by the World Health Organization in 2016.Citation71
Nevertheless, the question remains as to how neurocognitive impairment influences HIV self-stigma in MSM with HIV. To our knowledge, the present study is the first to have analysed this relationship. It has produced an explanatory model, helping to understand how cognitive deficiencies influence self-stigma in this population. Our study also found that participants with low memory performance presented high HIV self-stigma. This direct relationship disappeared when we included HRQoL into the equation, and the MHS of HRQoL explained the relationship between cognition and HIV self-stigma.
In this sense, as mentioned earlier, the explanation could be that PLWHIV with neurocognitive impairment and low quality of life are not cognitively able to fight against their own HIV self-stigma. Moreover, they cannot reject the disqualifications associated with HIV in society, and as such, they internalise derogatory and erroneous beliefs as true, increasing their self-stigma. This has been described previously in people with schizophrenia, which also presents a high association with stigma. Nabors et al described how neurocognitive deficits limit the abilities of a person with schizophrenia to reject the stereotypes of mental illness held by society at large.Citation40 Nevertheless, although general cognitive functioning has been found to have a significant effect on self-stigma in previous studies, in our case, only memory seemed to be related to this factor. Memory is one of the main dimensions highly correlated with executive functioning, which in turn, is closely related to stigma resistance.Citation72 Adequate executive functioning seems to promote adequate coping strategies, resilience, and those metacognitive abilities needed to fight against self-stigma as proposed in recent research.Citation72 This fact has been replicated in other studies such as that conducted by Grover et al, in which executive dysfunction was found to be significantly related to higher levels of self-stigma in people with schizophrenia.Citation73 The conclusions obtained in these previous studies could explain the results of our work. Although executive functioning was not analysed in the present study, memory, one of its most related cognitive domains, was evaluated.Citation74 Indeed, some studies have found very strong correlations between memory domains and executive functions.Citation75 In this sense, memory deficits could be a major correlate of executive dysfunction. This latter fact could in turn explain how people exhibiting lower memory performance present higher executive dysfunctions and therefore, a lack of metacognitive abilities and resilience to develop higher stigma resistance. This mechanism may plausibly explain the development of higher levels of HIV self-stigma in MSM with HIV with memory deficits.
Another reason could be that PLWHIV with symptomatic cognitive impairment associated with HIV view their quality of life as low because they perceive themselves as sick. This self-definition of sick person reaffirms the beliefs established by society that having the disease holds serious consequences, which, in turn, increases a person’s HIV self-stigma. Terpstra et al interviewed PLWHIV with neurocognitive disorders: several participants reported that they commonly experienced frustration, embarrassment, as well as worry associated with cognitive difficulties and potential decline. Moreover, when their cognitive difficulties caused problems, they decided not to ask for help because they were concerned about being judged negatively.Citation30
Beyond the effects of neurocognitive impairment on HIV self-stigma, a number of recent studies are indicating that HIV self-stigma may also influence cognitive performance and quality of life, especially in mental health domains.Citation61 In this sense, it has been found that HIV-related stigma is a significant predictor of poorer performance in cognitive testing or disturbances in mental health, such as higher levels of anxiety and depression.Citation61 Some authors explain the negative effect of HIV-related stigma on brain structures, probably mediated by chronic stress, isolation or negative social experiences directly related to HIV-related stigma. The effect of HIV self-stigma on cognitive functioning or HRQoL is likely to be bidirectional, and additional research is necessary to clarify this issue.
In any event, the findings of this study point to the necessity of implementing several interventions strategies to prevent neurocognitive and HRQoL deterioration, and therefore, HIV self-stigma in this population. Considering the conclusions derived from this research, a key approach following an HIV diagnosis could be to maintain adequate neurocognitive functioning. It seems that optimal neurocognitive functioning could prevent a reduction in HRQoL, preserving the patients’ autonomy and adequate independence in daily life. Patients’ self-perceptions of being sick could be mitigated, thus preventing the development of higher levels of self-stigma. In this sense, a recent review has identified that cognitive interventions have shown to be effective in this population.Citation76
Moreover, strategies directly aimed at reducing HIV self-stigma could be very effective when internalised stigma levels are high. Several studies have identified the effectiveness of a variety of interventions directed towards diminishing HIV self-stigma in this collective.Citation42,Citation44,Citation45 Although psycho-educational interventions are the most developed and evaluated strategies, it is necessary to analyse the efficacy of other holistic approaches. In this vein, based on the multiple variables involved in HIV self-stigma, multicomponent approach interventions may be the most effective strategies. According to previous research and the results of the present study, HIV self-stigma is influenced by multiple variables of a psychological, social, cognitive and health nature. Implementing multicomponent interventions could thus be a plausible mechanism to prevent and reduce this manifestation in PLWHIV.
Despite advancing our understanding of the association between cognition, HRQoL, and HIV self-stigma in PLWHIV, the present study presented some limitations. Its design only allowed measuring the association between the variables, it did not enable establishing any causality. Furthermore, the questionnaire was self-administered and as such, some participants may not have been sincere in their responses. Caution must therefore be applied in the interpretation of the results considering desirability biases. Moreover, the number of HIV-positive people in the study sample was small. We were nonetheless able to confirm our results as the participants were representative of the target population. On the other hand, no social HIV determinants were measured in the present study, and these variables could be possible confounders of the obtained results. It has been demonstrated that risky sexual behaviours,Citation77 low monthly income,Citation78 lower social supportCitation78 or isolation,Citation79 among others, significantly influence neurocognitive deterioration in PLWHIV. Finally, the fact that the study was performed in a Spanish region may limit the generalisability of its conclusions. Futures studies should be conducted based on larger samples, longitudinal designs, considering possible social confounders of neurocognitive impairment in this population, and contemplating the need to analyse possible differences based on ethnicity or cultural dimensions.
Conclusion
In conclusion, our study highlights how cognitive deficiencies in PLWHIV affect their quality of life and how this relationship increases HIV self-stigma. The results of this study improve our understanding of the processes underlying HIV self-stigma and the factors involved. Such knowledge plays a key role in the development of potential intervention strategies. These interventions, which aim at reducing HIV self-stigma and improving the quality of life of PLWHIV, should include strategies that specifically address neurocognitive impairment as a relevant variable.
Acknowledgments
This study was funded by the Office of the Vice President of Research and Knowledge Transfer of the University of Alicante, Grant Number: GRE-18-17B.
Disclosure
The authors report no conflicts of interest in this work.
References
- Global HIV & AIDS statistics — 2020 fact sheet; 2020. Available from: https://www.unaids.org/en/resources/fact-sheet. Accessed December 7, 2021.
- UNAIDS data; 2020. Available from: https://www.unaids.org/en/resources/documents/2020/unaids-data. Accessed December 10, 2020
- Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355. doi:10.1371/journal.pone.0081355
- Wandeler G, Johnson LF, Egger M. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: comparisons with general population. Curr Opin HIV AIDS. 2016;11(5):492–500. doi:10.1097/COH.0000000000000298
- Tu W, Chen PA, Koenig N, et al. Machine learning models reveal neurocognitive impairment type and prevalence are associated with distinct variables in HIV/AIDS. J Neurovirol. 2020;26(1):41–51. doi:10.1007/s13365-019-00791-6
- Wang Y, Liu M, Lu Q, et al. Global prevalence and burden of HIV-associated neurocognitive disorder: a meta-analysis. Neurology. 2020;95(19):e2610–e2621. doi:10.1212/WNL.0000000000010752
- Xiao X, Zeng H, Feng C, et al. Cognitive impairment among aging people living with HIV on antiretroviral therapy: a Cross-Sectional Study in Hunan, China. J Assoc Nurses AIDS Care JANAC. 2020;31(3):301–311. doi:10.1097/JNC.0000000000000122
- Portilla I, Reus S, León R, et al. Neurocognitive impairment in well-controlled HIV-infected patients: a Cross-Sectional Study. AIDS Res Hum Retroviruses. 2019;35(7):634–641. doi:10.1089/AID.2018.0279
- DeFleur ML. Stigma: notes on the management of spoiled identity. By Erving Goffman. Englewood Cliffs, New Jersey: prentice-Hall, 1963. 147 pp. Cloth, $4.50; paper, $1.95. Soc Forces. 1964;43(1):127–128. doi:10.1093/sf/43.1.127
- Crawford AM. Stigma associated with AIDS: A meta-analysis1. J Appl Soc Psychol. 1996;26(5):398–416. doi:10.1111/j.1559-1816.1996.tb01856.x
- Bos AER, Dijker AJM, Koomen W. Sex differences in emotional and behavioral responses to HIV + individuals’ expression of distress. Psychol Health. 2007;22(4):493–511. doi:10.1080/14768320600976257
- Dijker AJ, Kok G, Koomen W. Emotional reactions to people with AIDS. J Appl Soc Psychol. 1996;26(8):731–748. doi:10.1111/j.1559-1816.1996.tb02741.x
- Herek GM. AIDS and stigma. Am Behav Sci. 1999;42(7):1106–1116. doi:10.1177/0002764299042007004
- Lau JT, Tang AS, Tsui HY. The relationship between condom use, sexually transmitted diseases, and location of commercial sex transaction among male Hong Kong clients. AIDS. 2003;17(1):105–112. doi:10.1097/00002030-200301030-00014
- Mahajan AP, Sayles JN, Patel VA, et al. Stigma in the HIV/AIDS epidemic: a review of the literature and recommendations for the way forward. AIDS. 2008;22(Suppl 2):S67–S79. doi:10.1097/01.aids.0000327438.13291.62
- McDoom MM, Bokhour B, Sullivan M, Drainoni M-L. How older black women perceive the effects of stigma and social support on engagement in HIV care. AIDS Patient Care STDs. 2015;29(2):95–101. doi:10.1089/apc.2014.0184
- Simbayi LC, Kalichman SC, Strebel A, Cloete A, Henda N, Mqeketo A. Disclosure of HIV status to sex partners and sexual risk behaviours among HIV-positive men and women, Cape Town, South Africa. Sex Transm Infect. 2006;83(1):29–34. doi:10.1136/sti.2006.019893
- Visser MJ, Kershaw T, Makin JD, Forsyth BWC. Development of parallel scales to measure HIV-related stigma. AIDS Behav. 2008;12(5):759–771. doi:10.1007/s10461-008-9363-7
- Adimora AA, Schoenbach VJ, Doherty IA. HIV and African Americans in the Southern United States: sexual networks and social context. Sex Transm Dis. 2006;33(7):S39–S45. doi:10.1097/01.olq.0000228298.07826.68
- Adimora AA, Ramirez C, Schoenbach VJ, Cohen MS. Policies and politics that promote HIV infection in the Southern United States. AIDS. 2014;28(10):1393–1397. doi:10.1097/QAD.0000000000000225
- Livingston JD, Boyd JE. Correlates and consequences of internalized stigma for people living with mental illness: a systematic review and meta-analysis. Soc Sci Med. 2010;71(12):2150–2161. doi:10.1016/j.socscimed.2010.09.030
- Corrigan PW, Watson AC, Barr L. The self–stigma of mental illness: implications for self–esteem and self–efficacy. J Soc Clin Psychol. 2006;25(8):875–884. doi:10.1521/jscp.2006.25.8.875
- Earnshaw VA, Smith LR, Chaudoir SR, Amico KR, Copenhaver MM. HIV stigma mechanisms and well-being among PLWH: a test of the HIV stigma framework. AIDS Behav. 2013;17(5):1785–1795. doi:10.1007/s10461-013-0437-9
- Pantelic M, Boyes M, Cluver L, Meinck F. HIV, violence, blame and shame: pathways of risk to internalized HIV stigma among South African adolescents living with HIV. J Int AIDS Soc. 2017;20(1):21771. doi:10.7448/IAS.20.1.21771
- Turan B, Budhwani H, Fazeli PL, et al. How does stigma affect people living with HIV? The mediating roles of internalized and anticipated HIV stigma in the effects of perceived community stigma on health and psychosocial outcomes. AIDS Behav. 2017;21(1):283–291. doi:10.1007/s10461-016-1451-5
- Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc. 2013;16:18640. doi:10.7448/IAS.16.3.18640
- Sweeney SM, Vanable PA. The association of HIV-related stigma to HIV medication adherence: a systematic review and synthesis of the literature. AIDS Behav. 2016;20(1):29–50. doi:10.1007/s10461-015-1164-1
- Treves-Kagan S, El Ayadi AM, Pettifor A, et al. Gender, HIV testing and stigma: the association of HIV testing behaviors and community-level and individual-level stigma in rural South Africa differ for men and women. AIDS Behav. 2017;21(9):2579–2588. doi:10.1007/s10461-016-1671-8
- Leserman J, Petitto JM, Gu H, et al. Progression to AIDS, a clinical AIDS condition and mortality: psychosocial and physiological predictors. Psychol Med. 2002;32(6):1059–1073. doi:10.1017/S0033291702005949
- Terpstra AR, Worthington C, Ibáñez-Carrasco F, et al. “I’m just forgetting and I don’t know why”: exploring how people living with HIV-associated neurocognitive disorder view, manage, and obtain support for their cognitive difficulties. Qual Health Res. 2018;28(6):859–872. doi:10.1177/1049732318761364
- Chan RCH, Mak WWS. Cognitive, regulatory, and interpersonal mechanisms of HIV stigma on the mental and social health of men who have sex with men living with HIV. Am J Mens Health. 2019;13(5):1557988319873778. doi:10.1177/1557988319873778
- Holzemer WL, Uys LR, Chirwa ML, et al. Validation of the HIV/AIDS stigma instrument—PLWA (HASI-P). AIDS Care. 2007;19(8):1002–1012. doi:10.1080/09540120701245999
- Pantelic M, Boyes M, Cluver L, Thabeng M. “They say HIV is a punishment from god or from ancestors”: cross-cultural adaptation and psychometric assessment of an HIV stigma scale for South African Adolescents Living with HIV (ALHIV-SS). Child Indic Res. 2018;11(1):207–223. doi:10.1007/s12187-016-9428-5
- Lee RS, Kochman A, Sikkema KJ. Predictors of HIV related stigma among young people living with HIV/AIDS. AIDS Behav. 2002;6(4):309–319. doi:10.1023/A:1021144511957
- Vanable PA, Carey MP, Blair DC, Littlewood RA. Impact of HIV-related stigma on health behaviors and psychological adjustment among HIV-positive men and women. AIDS Behav. 2006;10(5):473–482. doi:10.1007/s10461-006-9099-1
- Mak WWS, Cheung RYM, Law RW, Woo J, Li PCK, Chung RWY. Examining attribution model of self-stigma on social support and psychological well-being among people with HIV+/AIDS. Soc Sci Med. 2007;64(8):1549–1559. doi:10.1016/j.socscimed.2006.12.003
- Corrigan P, Markowitz FE, Watson A, Rowan D, Kubiak MA. An attribution model of public discrimination towards persons with mental illness. J Health Soc Behav. 2003;44(2):162–179. doi:10.2307/1519806
- Corrigan PW. Mental health stigma as social attribution: implications for research methods and attitude change. Clin Psychol Sci Pract. 2000;7(1):48–67. doi:10.1093/clipsy.7.1.48
- Chan RCH, Mak WWS, Ma GYK, Cheung M. Interpersonal and intrapersonal manifestations of HIV stigma and their impacts on psychological distress and life satisfaction among people living with HIV: toward a dual-process model. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2021;30(1):145–156. doi:10.1007/s11136-020-02618-y
- Nabors LM, Yanos PT, Roe D, et al. Stereotype endorsement, metacognitive capacity, and self-esteem as predictors of stigma resistance in persons with schizophrenia. Compr Psychiatry. 2014;55(4):792–798. doi:10.1016/j.comppsych.2014.01.011
- Nyamayaro P, Gouse H, Hakim J, Robbins RN, Chibanda D. Neurocognitive impairment in treatment-experienced adults living with HIV attending primary care clinics in Zimbabwe. BMC Infect Dis. 2020;20(1):383. doi:10.1186/s12879-020-05090-8
- Ma PHX, Chan ZCY, Loke AY. Self-stigma reduction interventions for people living with HIV/AIDS and their families: a systematic review. AIDS Behav. 2019;23(3):707–741. doi:10.1007/s10461-018-2304-1
- Stangl AL, Lloyd JK, Brady LM, Holland CE, Baral S. A systematic review of interventions to reduce HIV-related stigma and discrimination from 2002 to 2013: how far have we come? J Int AIDS Soc. 2013;16:18734. doi:10.7448/IAS.16.3.18734
- Feyissa GT, Lockwood C, Woldie M, Munn Z. Reducing HIV-related stigma and discrimination in healthcare settings: a systematic review of quantitative evidence. PLoS One. 2019;14(1):e0211298. doi:10.1371/journal.pone.0211298
- Soro DB, Isabel JAC, García MA. Reducción del estigma, la depresión y la ansiedad en personas con VIH mediante un grupo terapéutico cognitivo conductual. Psicol Conduct Behav Psychol Rev Int Psicol Clínica Salud. 2021;29(2):237–257.
- Barroso J, Relf MV, Williams MS, et al. A randomized controlled trial of the efficacy of a stigma reduction intervention for HIV-infected women in the Deep South. AIDS Patient Care STDs. 2014;28(9):489–498. doi:10.1089/apc.2014.0014
- Pantelic M, Steinert JI, Park J, Mellors S, Murau F. “Management of a spoiled identity”: systematic review of interventions to address self-stigma among people living with and affected by HIV. BMJ Glob Health. 2019;4(2):e001285. doi:10.1136/bmjgh-2018-001285
- Alford K, Daley S, Banerjee S, Vera JH. Quality of life in people living with HIV-associated neurocognitive disorder: a scoping review study. PLoS One. 2021;16(5):e0251944. doi:10.1371/journal.pone.0251944
- Rubtsova AA, Sabbag S, Sundermann E, et al. Frailty and neurocognitive impairment: impacts on quality of life in HIV. J Assoc Nurses AIDS Care JANAC. 2020;31(3):290–300. doi:10.1097/JNC.0000000000000142
- Amara PS, Naveed Z, Wichman CS, Fox HS, Baccaglini L. Neurocognitive impairment and health-related quality of life among people living with Human Immunodeficiency Virus (HIV). PLoS One. 2021;16(4):e0248802. doi:10.1371/journal.pone.0248802
- Tozzi V, Balestra P, Murri R, et al. Neurocognitive impairment influences quality of life in HIV-infected patients receiving HAART. Int J STD AIDS. 2004;15(4):254–259. doi:10.1258/095646204773557794
- Degroote S, Vogelaers D, Vandijck DM. What determines health-related quality of life among people living with HIV: an updated review of the literature. Arch Public Health. 2014;72(1):40. doi:10.1186/2049-3258-72-40
- Mayo NE, Brouillette M-J, Scott SC, et al. Relationships between cognition, function, and quality of life among HIV+ Canadian men. Qual Life Res. 2020;29(1):37–55. doi:10.1007/s11136-019-02291-w
- Abo-Rass F, Shinan-Altman S, Werner P. Health-related quality of life among Israeli Arabs diagnosed with depression: the role of illness representations, self-stigma, self-esteem, and age. J Affect Disord. 2020;274:282–288. doi:10.1016/j.jad.2020.05.125
- Alexová A, Kågström A, Winkler P, Kondrátová L, Janoušková M. Correlates of internalized stigma levels in people with psychosis in the Czech Republic. Int J Soc Psychiatry. 2019;65(5):347–353. doi:10.1177/0020764019850204
- Morgades-Bamba CI, Jose Fuster-Ruizdeapodaca M, Molero F. Internalized stigma and its impact on schizophrenia quality of life. Psychol Health Med. 2019;24(8):992–1004. doi:10.1080/13548506.2019.1612076
- Wong CCY, Pan-Weisz BM, Pan-Weisz TM, Yeung NCY, Mak WWS, Lu Q. Self-stigma predicts lower quality of life in Chinese American breast cancer survivors: exploring the mediating role of intrusive thoughts and posttraumatic growth. Qual Life Res. 2019;28(10):2753–2760. doi:10.1007/s11136-019-02213-w
- Caqueo-Urizar A, Urzua A, Habib J, et al. Relationships between social stigma, stigma experience and self-stigma and impaired quality of life in schizophrenia across three Latin-American countries. Eur Arch Psychiatry Clin Neurosci. 2020;270(5):513–520. doi:10.1007/s00406-019-01035-8
- Guo Y, Qu S, Qin H. Study of the relationship between self-stigma and subjective quality of life for individuals with chronic schizophrenia in the community. Gen Psychiatry. 2018;31(3). doi:10.1136/gpsych-2018-100037
- Fuster-RuizdeApodaca MJ, Molero F, Ubillos S. Evaluación de una intervención dirigida a reducir el impacto del estigma en las personas con VIH capacitándolas para afrontarlo. An Psicol Ann Psychol. 2016;32(1):39–48. doi:10.6018/analesps.32.1.192121
- Lam A, Mayo NE, Scott S, Brouillette M-J, Fellows LK. HIV-Related stigma affects cognition in older men living with HIV. JAIDS J Acquir Immune Defic Syndr. 2019;80(2):198–204. doi:10.1097/QAI.0000000000001898
- Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi:10.3758/BF03193146
- Hernansaiz-Garrido H, Alonso-Tapia J. Internalized HIV stigma and disclosure concerns: development and validation of two scales in Spanish-speaking populations. AIDS Behav. 2017;21(1):93–105. doi:10.1007/s10461-016-1305-1
- Badía X, Podzamczer D, López-Lavid C, García M. [Evidence-based medicine and the validation of quality-of-life questionnaires: the Spanish version of the MOS-HIV questionnaire for the evaluation of the quality of life in patients infected by HIV]. Enferm Infecc Microbiol Clin. 1999;17(Suppl 2):103–113. Spanish.
- Alcocer‐Bruno C, Ferrer‐Cascales R, Rubio‐Aparicio M, Ruiz‐Robledillo N. The medical outcome study-HIV health survey: a systematic review and reliability generalization meta-analysis. Res Nurs Health. 2020;43(6):610–620. doi:10.1002/nur.22070
- Haimov I, Hanuka E, Horowitz Y. Chronic insomnia and cognitive functioning among older adults. Behav Sleep Med. 2008;6:32–54. doi:10.1080/15402000701796080
- Thompson HJ, Demiris G, Rue T, et al. A holistic approach to assess older adults’ wellness using e-health technologies. Telemed E-Health. 2011;17(10):794–800. doi:10.1089/tmj.2011.0059
- Miller A, Shatil E. Personalized on-line (computer-based) cognitive training (CogniFit) for patients with MS. Mult Scler J. 2013;19(7):976.
- Yaneva A, Massaldjieva R, Mateva N. Initial adaptation of the general cognitive assessment battery by cognifittm for Bulgarian older adults. Exp Aging Res. 2021;1–15. doi:10.1080/0361073X.2021.1981096
- Hayes AF, Rockwood NJ. Regression-based statistical mediation and moderation analysis in clinical research: observations, recommendations, and implementation. Behav Res Ther. 2017;98:39–57. doi:10.1016/j.brat.2016.11.001
- Lazarus JV, Safreed-Harmon K, Barton SE, et al. Beyond viral suppression of HIV – the new quality of life frontier. BMC Med. 2016;14(1):94, s12916-016-0640-0644. doi:10.1186/s12916-016-0640-4
- Dubreucq J, Plasse J, Gabayet F, et al. Stigma resistance is associated with advanced stages of personal recovery in serious mental illness patients enrolled in psychiatric rehabilitation. Psychol Med. 2020:1–11. doi:10.1017/S0033291720004055
- Grover S, Sahoo S, Nehra R. A comparative study of childhood/adolescent and adult onset schizophrenia: does the neurocognitive and psychosocial outcome differ? Asian J Psychiatry. 2019;43:160–169. doi:10.1016/j.ajp.2019.05.031
- Tirapu-Ustárroz J, Muñoz-Céspedes JM. [Memory and the executive functions]. Rev Neurol. 2005;41(8):475–484. Spanish.
- McCabe DP, Roediger III HL, McDaniel MA, Balota DA, Hambrick DZ. The relationship between working memory capacity and executive functioning: evidence for a common executive attention construct. Neuropsychology. 2010;24(2):222–243. doi:10.1037/a0017619
- Chan T, Marta M, Hawkins C, Rackstraw S. Cognitive and neurologic rehabilitation strategies for central nervous system HIV infection. Curr HIV/AIDS Rep. 2020;17(5):514–521. doi:10.1007/s11904-020-00515-0
- Anand P, Springer SA, Copenhaver MM, Altice FL. Neurocognitive impairment and HIV risk factors: a reciprocal relationship. AIDS Behav. 2010;14(6):1213–1226. doi:10.1007/s10461-010-9684-1
- Wubetu AD, Asefa KK, Gebregiorgis BG. Prevalence of neurocognitive impairment and associated factors among people living with HIV on highly active antiretroviral treatment, Ethiopia. HIVAIDS Auckl NZ. 2021;13:425–433. doi:10.2147/HIV.S298141
- Eaton P, Lewis T, Kellett-Wright J, et al. Risk factors for symptomatic HIV-associated neurocognitive disorder in adults aged 50 and over attending a HIV clinic in Tanzania. Int J Geriatr Psychiatry. 2020;35(10):1198–1208. doi:10.1002/gps.5357