Abstract
Objective
To investigate current status of quality of life and the association between depression and symptom burden in a sample of Chinese maintenance hemodialysis (MHD) patients.
Methods
A self-designed patient general information questionnaire, disease-related information questionnaire, dialysis patient symptom burden scale, depression scale, and quality of survival scale were used to investigate 380 maintenance haemodialysis patients in haemodialysis centres. A regression model of the factors affecting the quality of survival was established using structural equation modelling.
Results
The regression model data had a high goodness of fit: c2/df = 4.736, RMSEA = 0.099, GFI = 0.918, CFI = 0.972, TLI = 0.962, SRMR = 0.0469. Structural equation model analysis showed that depression had a positive predictive effect on symptom burden, β = 0.398, P < 0.001; Symptom burden had a negative predictive effect on the quality of life, β =−0.851, P < 0.001; and Depression had a negative predictive effect on the quality of life, β =−0.151, P < 0.001. Depression indirectly affects the quality of life through symptom burdens.
Conclusion
Depression and symptom burden directly or indirectly affect the quality of life in patients with maintenance hemodialysis. Symptom burden moderates the relationship between depression and quality of life as a mediating variable.
QR Code
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
© 2024 Xia et al. This work is published by Dove Medical Press Limited, and licensed under Creative Commons Attribution – Non Commercial (unported, v3.0) License. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed.
Introduction
In recent years, the global burden of kidney diseases has been increasing. About 10% of the global population suffers from chronic kidney disease. According to statistics, it is estimated that 14.5 million people worldwide will suffer from End-Stage Renal Disease (ESRD) by 2030.Citation1 Maintenance Hemodialysis (MHD) has become the main treatment for ESRD patients due to its high safety and efficacy. According to the Chinese Scientific Report on Kidney Diseases released in 2020, the prevalence of MHD in China is 402.18/ Per Million Population (PMP).Citation2
Although MHD treatment modalities can prolong the life span of patients, long-term dialysis treatment brings tremendous psychosocial stress and symptom burden to patients, threatening their seriously survival quality.Citation3 Survival quality has become a key outcome indicator in treating chronic diseases, assessing efficacy, predicting the risk of adverse outcomes, and predicting mortality, as well as an important prognostic indicator for ESRD and renal replacement therapy.Citation4,Citation5 Indeed, patients with MHD have poorer quality of life compared to healthy people.Citation6,Citation7 Severe Symptom burden, an important factor, refers to patients’ subjective perception of physical, emotional, or cognitive changes MHD only allows the passage of small molecules and some substances, which cannot completely replace the renal function. With the prolongation of treatment time, some molecules that cannot be completely removed accumulate in the patient’s body, and the effects of endotoxins and internal environmental disorders cause the patient to experience fatigue, nausea, itchiness, difficulty in falling asleep and other discomforts, thus resulting a severe symptom burden on the patient.Citation8,Citation9 Recently, there have been more studies on the studies of psychological factors among MHD patients. Depression often co-occurs with MHD patients as a negative psychological factor. In China, the prevalence of depression among MHD patients is close to 55.1%,Citation10,Citation11 influencing their lives and those of their families. High cost of treatment, long-term dietary restrictions, employment pressure, and progressive loss of social functioning continue to place a huge burden on the patient’s psyche. It has been found that patients undergoing haemodialysis experience significant psychological stress, resulting in increased rates of anxiety and depression, which are common problems affecting their quality of life.Citation12,Citation13 With the progress of the disease and the prolongation of the treatment time, patients suffer from a heavy symptom burden, depression and other negative emotions. This greatly reduces the patient’s adherence to treatment and treatment effects and is unfavorable for patients’ life.Citation4 In recent years, more and more studies have focus on the relationship between symptom burden, depression and quality of life in MHD patients. Some studies have stated that symptom burden is associated with life quality and negative emotions (eg anxiety and depression).Citation14,Citation15 However, there are few studies on the mechanism of symptom burden and depression on quality of life.
Therefore, this study aimed to explore the internal correlation and path of action between symptom burden and depression on the quality of life of MHD patients. This study will provide evidence for clinical medical staff to provide a valuable road to make targeted intervening measures, and improve depression and quality of life in MHD patients.
Methods
Ethical Statement
The study complies with the Declaration of Helsinki. Ethical approval was obtained from the institutional Ethics Committee at BenQ Hospital Affiliated to Nanjing Medical University, approval number[2022-KL010]. All participants in this study provided informed consent.
Study Design and Population
This is an observational cross-sectional study. Sample size was calculated using G*Power 3.1.9.7 software, taking α = 0.05, power = 0.95, and effect size f2 = 0.15. A calculated sample size of at least 107 cases was obtained. The inclusion criteria were as follows: ① Age≥18 years old; ② MHD≥3 months; normal cognitive function; and ③ Informed consent and voluntary participation. Exclusion criteria were as follows: ① Language and hearing disorder; ② ESRD from acute kidney disease or other malignant tumors; ③ Alzheimer’s patients. Finally, 392 patients were collected from a single center of hemodialysis in the BenQ Hospital in Nanjing. This study was conducted between March 2022 and May 2022.
Measures and Data Collection
The questionnaires were distributed by the members of the team after uniform training. The researchers distributed all the questionnaires. The purpose of the research was explained to the respondents first, and then a face-to-face survey was adopted after informed consent was obtained. Questionnaires were distributed and collected on the spot. Researchers eliminated the invalid questionnaires with incomplete answers by checking the completeness of the questionnaires one by one. Two-person input method was used to ensure the accuracy of data input. A total of 392 questionnaires were sent out, of which 4 patients did not complete the survey due to kidney transplantation, 8 patients had incomplete entries due to unwillingness to answer, and finally 380 patients completed the survey, with a valid recovery rate of 96.9%.
General Data Questionnaire
Researchers designed a general data questionnaire. Main indicators included gender, age, marital status, working status, monthly income, education level, payment method, primary disease, dialysis age, blood calcium, blood phosphorus, parathyroid hormone and Kt/V and Antidepressants durg use.
Dialysis Frequency, Severity, and Symptom Burden Index (DFSSBI)
DFSSBICitation17 was used to assess patient’s symptom burden. The index covers 30 items, including 5 items on psychological symptoms and 25 items on physiological symptoms. Each item included four aspects: occurrence, frequency, severity, and distress degree. 0 was marked for no symptoms, and the following options were set up for symptoms. Based on the frequency of occurrence, 1~4 was respectively marked for occasional, sometimes, often, and always symptoms. Moreover, 1–4 was marked for mild, moderate, severe, and very serious conditions according to the severity level, respectively. In symptom distress, 0 means no distress, and 1–4 means mild, moderate, severe, and very serious distress. The total score of the scale was 360, and the higher the score, the heavier the symptom burden of the patients. The retest reliability of the scale was 0.91, and the Cronbach’s α coefficient of internal consistency was 0.89.Citation17 In this study, Cronbach’s α coefficient of this scale was 0.967. This scale has been widely employed to assess symptom burden in China.Citation18
Patient Health Questionnaire (PHQ-9)
PHQ-9Citation20 was used to assess depression. The questionnaire is a simple and effective self-assessment tool for depression based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). It does not require professionals, is simple and efficient, and is currently the most commonly-used self-assessment scale for depression screening. Subjects fill out the scale according to their comprehensive situation in the past 2 weeks. The scale includes 9 items, and the answers for each item consist of 4 choices, respectively: not at all, several days, more than 1 week, and almost every day, corresponding to a score of 0~3, and a total score of 27 points. The higher the score, the greater likelihood of depression. Cronbach’s coefficient of this scale is 0.839.Citation20 In this study, 0~4 was indicated no depression, and ≥5 was indicated depression. In this study, Cronbach’s α coefficient was 0.836. This scale has been extensively adopted to assess depression status in China.
Health-Related Quality of Life (KDQOL-36) Scale
KDQOL-36Citation22 contains five subscales: symptoms and the problem of kidney disease (SPKD subscale), effects of kidney disease (EKD) subscale, the burden of kidney disease (BKD) subscale, SF-12 physical component summary (PCS) subscale, SF-12 mental component summary (MCS) subscale. There was no total score for the KDQOL-36 scale. Each subscale score was separately calculated, ranging from 0 to 100. A higher score indicates a better quality of life. KDQOL-36 scale was scored using an Excel rating template at the website of Rand Corporation in the United States (www.rand.org/health/surveys_tools/kdqol). This scale has been widely used to evaluate Quality of Life status in China.
Statistical Analysis
Two-person input was conducted using Excel 2016, and the data were analyzed with SPSS 26.0. The counting data were described through frequency and percentage. Measurement data were described by mean ± standard deviation or median and quartile. Non-parametric test was performed single factor analysis of data that did not conform to normal distribution. Spearman correlation coefficient analysis was carried out for correlation analysis, and mediating effects were analyzed by structural equation analysis using AMOS 26 software. Furthermore, the bootstrap method was repeated 5000 times. The mediation effect was established if the 95% deviation correction confidence interval did not contain 0. P < 0.05 was considered statistically significant.
Results
shows the characteristics of the study population. Among the 380 patients, 222 patients were detected with depressive symptoms, with an incidence of 58.4%; 158 cases (41.6%) had no depressive symptoms; 153 cases (40.3%) had mild depression, and 49 cases (12.9%) had moderate depression; 14 cases (3.7%) had moderate to severe depression, and 6 cases (1.6%) had severe depression. Symptom burden scores were 77.41 ± 45.74; there were 13.11 ± 6.01 symptoms, and frequency scores were 26.71 ± 15.31; severity scores were 25.43 ± 15.29; and distress scores were 25.22 ± 15.42. Quality of life scores were 279.92 ± 60.76; symptoms and problems scores were 79.24 ± 13.03; kidney disease impact scores were 66.50 ± 16.22; kidney disease burden scores were 43.45 ± 27.79; total physiological scores were 43.71 ± 9.31; and total psychological scores were 47.55 ± 9.55.
Table 1 Characteristics of the Study Population (n=380)
According to , symptom burden is positively correlated with depression score in the MHD patients (P < 0.01) but negatively correlated with quality of life (P < 0.01). Life quality is negatively correlated with depression (P < 0.01).
Table 2 Correlation Analysis of Symptom Burden, Depression, and Quality of Life in MHD Patients (n=380, r-Value)
According to the professional knowledge and the results of correlation analysis, it was assumed that depression directly affected the quality of life and indirectly affected the quality of life through symptom burden. The route between symptom burden, depression, and quality of life were mapped through a structural equation model, and the mediating effect of symptom burden on depression and quality of life was observed. The model parameters were evaluated using the maximum likelihood method (ML), and the model fitting results were as follows: c2/df = 4.736 (1 <χ2/df < 5), RMSEA = 0.099 (< 0.10), GFI = 0.918 (> 0.90), CFI = 0.972 (> 0.90), TLI = 0.962 (> 0.90), and SRMR = 0.0469 (< 0.08). As shown in and , the model fit met the standard, indicating that the model had higher goodness of fit to the data. Based on the path coefficient results, depression had a positive predictive effect on symptom burden, β = 0.398, P < 0.001; symptom burden had a negative predictive effect on the quality of life, β = −0.851, P < 0.001; and depression had a negative predictive effect on the quality of life, β = −0.151, P < 0.001.
Table 3 Path Analysis of Factors Influencing the Life Quality of MHD Patients
Figure 1 Mediating effects of symptom burden, depression, and quality of life in MHD patients.
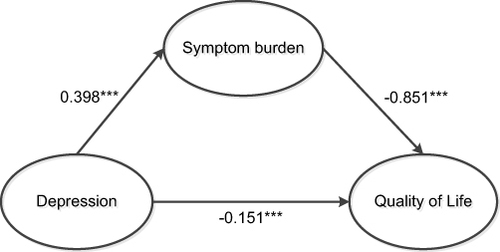
According to the mediating effect analysis table, symptom burden had a significant mediating effect on the relationship between depression and life quality. The value of this mediating effect reached −0.339 (95% confidence interval of [−0.440, −0.245]), and did not include 0. This suggested that the effect of symptom burden was established ().
Table 4 Mediating Effects Analysis Table of Symptom Burden, Depression, and Quality of Life in MHD Patients
Discussion
In this study, the prevalence of depression in 380 MHD patients was 58.4%, which was much higher than the prevalence in the general population (3.3%).Citation22 Globally, the prevalence of depressive symptoms in patients undergoing dialysis ranges from 13.1% to 76.3%.Citation23 Indian studies report that 21.76%-83.5% of patients with CKD have depressive symptoms.Citation24 This wide variation in the range may be explained by the different methodologies. MHD is the main treatment method for ESRD and requires dialysis 2–3 times a week for 3.5–5.5 hours each time.Citation6,Citation7 Treatment cycle is long and lasts for life. Long-term dialysis affects both the career and family life of patients. Patients will face psychological states such as loneliness and helplessness while experiencing physical symptoms. As a result, they are more prone to negative emotions such as depression than the non-dialysis population. Symptom burden refers to the number of symptoms in the course of illness, including frequency, severity, and distress of symptoms.Citation8 Recently, research has shown that patients on maintenance hemodialysis experience various symptoms and face greater symptom burdens instead of just one symptom.Citation25 The symptom burden scores of dialysis patients were (77.41 ± 45.74), which was higher than that reported by Liu Yan et al.Citation26 This may stem from a fact that the dialysis age of patients in this study was (4.75 ± 4.52 years) lower. It was believed that the dialysis age of patients was positively correlated with symptom burden. The longer the dialysis age, the more likely adverse reactions and complications would occur, and the higher the symptom burden scores. For MHD patients, because the glomerular filtration function was reduced and the residual nephrons could not effectively excrete metabolites, toxins accumulated in the body and caused various symptoms. With the change in medical models, there is also a focus on the quality of life of patients rather than the disease itself. In this study, the life quality scores of MHD patients were (279.92 ± 60.76), similar to the results of Dena E.Citation27 However, the kidney disease impact scores, burden scores, and total psychological scores were lower than those of foreign studies. Therefore, patients with MHD had heavier symptom burdens and a high incidence of depression, which affected their quality of life. Attention should be paid to the most frequent symptom groups of patients in the nursing process to timely detect patients’ depression, and provide early interventions.
According to a past study,Citation14 there is a significant negative correlation between depression and life quality of patients with MHD. This implies that the more severe the depression, the lower the quality of life experienced by the patients. These findings are consistent with the results of a previous study conducted by Xiong et al.Citation28 Patients with MHD depression tend to focus on their experiences and feelings, repeatedly think about related symptoms and the possible causes and consequences of these symptoms, and frequently focus on their negative emotions, leading to decreased sleep and life quality. Symptom burden was significantly negatively correlated with quality of life, verifying the important role of improving symptom burden on the life quality of dialysis patients. Psychological and physiological effects, independently or in combination, reduce treatment compliance and body function status of hemodialysis patients and negatively affect the quality of life. There was a significant positive correlation between depression and symptom burden. The higher the degree of depression, the higher the symptom burdens. Many studies have shown that physical discomfort symptoms mainly manifest depression, and patients with depression have obvious negative attention bias.Citation29 Depression levels increase the occurrence of other symptoms through somatization, increasing symptom burden. The nursing staff can continuously evaluate the symptom burden level of patients with MHD, timely detect the change of their symptom burdens, distinguish the source of physical symptoms, identify the depressed patients with MHD, and give early psychological intervention.
It has been shown thatCitation30 screening patients for depression prior to admission to MHD is beneficial in reducing mortality [hazard ratio (95% confidence interval): 0.94 (0.90–0.99)] and hospitalization [incidence rate ratio (95% confidence interval): 0.97 (0.9–0.99)], and improved patient survival. Depressed MHD patients have a poor quality of life with a high and complex symptom burden, with up to 58.4% of patients screened for depressive symptoms in this study, so we explored how depression affects quality of survival. We hypothesised that depression would have a direct effect on quality of life, as well as an indirect effect on quality of life through the mediating effect of symptom burden. In accordance with modelling, the effect of symptom burden on depression and quality of life was −0.339, accounting for 69.2% of the total effect. Depression not only directly affects the quality of life in patients with MHD but also indirectly predicts the quality of life through symptom burdens. Hence, for MHD patients with depression, the symptom burden has a more serious impact on the quality of life although depression and symptom burdens can reduce their quality of life. The possible reason was thatCitation31,Citation32 the central release of corticotrophin releasing hormone in depressed persons may activate the hypothalamic pituitary adrenal axis, and subsequent increased inflammation could modify or interfere with the intrarenal microcirculatory regulation and perfusion distribution and can induce renal damage, thus enhancing CKD progression. On the other hand, such individuals usually focus on itself, magnifying the physical and psychological burdens,Citation33 in addition to patients’ difficulties in objectively describing their true symptom experience during treatment. This phenomenon suggests that efforts should be invested into the symptomatic distress of depressed MHD patients, and that healthcare workers should screen for depression before patients enter haemodialysis and intervene in advance to improve the survival quality of patients. Therefore, the symptom management of depressed MHD patients is more important than in ordinary hemodialysis patients. By strengthening symptom management, patients’ symptom burdens can be relieved, and their confidence in controlling and relieving symptoms can be enhanced, thus decreasing the level of depression in MHD patients and improving their quality of life.
Limitations
This study is a single-center cross-sectional survey with a limited sample representative sample, susceptible to selection bias, respondent bias, and may lead to highly biased study results. More multi-center studies are needed for further discussion.
Conclusion
Maintenance haemodialysis patients suffer from a high symptom burden and severe depressive symptoms, which affects the quality of life of these patients. This study indicates that depression and symptom burden, directly and indirectly, affect the quality of life in patients with maintenance hemodialysis. Symptom burden as a mediating variable moderates the relationship between depression and quality of life.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Bharati J, Jha V. Global kidney health atlas: a spotlight on the Asia-Pacific Sector. Kidney Res Clin Pract. 2022;41(1):22–30. doi:10.23876/j.krcp.21.236
- Zhang L, Zhao MH, Zuo L, et al. China Kidney Disease Network (CK-NET) 2016 annual data report. Kidney Int Suppl. 2020;10(2):e97–e185. doi:10.1016/j.kisu.2020.09.001
- Chen JY, Wan EYF, Choi EPH, et al. The health-related quality of life of Chinese patients on hemodialysis and peritoneal dialysis. Patient. 2017;10(6):799–808. doi:10.1007/s40271-017-0256-6
- Kontodimopoulos N, Pappa E, Niakas D. Gender- and age-related benefit of renal replacement therapy on health-related quality of life. Scand J Caring Sci. 2009;23(4):721–729. doi:10.1111/j.1471-6712.2008.00670.x
- Zazzeroni L, Pasquinelli G, Nanni E, Cremonini V, Rubbi I. Comparison of quality of life in patients undergoing hemodialysis and peritoneal dialysis: a systematic review and meta-analysis. Kidney Blood Press Res. 2017;42(4):717–727. doi:10.1159/000484115
- Ma SJ, Wang WJ, Tang M, Chen H, Ding F. Mental health status and quality of life in patients with end-stage renal disease undergoing maintenance hemodialysis. Ann Palliat Med. 2021;10(6):6112–6121. doi:10.21037/apm-20-2211
- Al-Rajhi W, Al Salmi I. Quality of life and health-related quality of life in patients with end-stage kidney disease undergoing hemodialysis: a literature review. Saudi J Kidney Dis Transpl. 2022;33(Supplement):S184–S230. doi:10.4103/1319-2442.384191
- Brown EA, Zhao J, McCullough K, et al. Burden of kidney disease, health-related quality of life, and employment among patients receiving peritoneal dialysis and in-center hemodialysis: findings from the DOPPS program. Am J Kidney Dis. 2021;78(4):489–500.e1. doi:10.1053/j.ajkd.2021.02.327
- He S, Zhu J, Jiang W, Ma J, Li G, He Y. Sleep disturbance, negative affect and health-related quality of life in patients with maintenance hemodialysis. Psychol Health Med. 2019;24(3):294–304. doi:10.1080/13548506.2018.1515493
- Ye W, Wang L, Wang Y, Wang C, Zeng J. Depression and anxiety symptoms among patients receiving maintenance hemodialysis: a single center cross-sectional study. BMC Nephrol. 2022;23(1):417. doi:10.1186/s12882-022-03051-8
- Meng Y, Wu HT, Niu JL, et al. Prevalence of depression and anxiety and their predictors among patients undergoing maintenance hemodialysis in Northern China: a cross-sectional study. Ren Fail. 2022;44(1):933–944. doi:10.1080/0886022X.2022.2077761
- Wang SY, Zang XY, Liu JD, Cheng M, Shi YX, Zhao Y. Indicators and correlates of psychological disturbance in Chinese patients receiving maintenance hemodialysis: a cross-sectional study. Int Urol Nephrol. 2015;47(4):679–689. doi:10.1007/s11255-015-0910-7
- Yuan H, Zhang Y, Xue G, Yang Y, Yu S, Fu P. Exploring psychosocial factors associated with frailty incidence among patients undergoing maintenance hemodialysis. J Clin Nurs. 2020;29(9–10):1695–1703. doi:10.1111/jocn
- Rikos N, Kassotaki A, Frantzeskaki C, et al. Investigation of perception of quality of life and psychological burden of patients undergoing hemodialysis-quality of life of hemodialysis patients. Nurs Rep. 2023;13(3):1331–1341. doi:10.3390/nursrep13030112
- Zhang M, Kim JC, Li Y, et al. Relation between anxiety, depression, and physical activity and performance in maintenance hemodialysis patients. J Ren Nutr. 2014;24(4):252–260. doi:10.1053/j.jrn.2014.03.002
- Weisbord SD, Fried LF, Arnold RM, et al. Development of a symptom assessment instrument for chronic hemodialysis patients: the dialysis symptom index. J Pain Symptom Manage. 2004;27(3):226–240. doi:10.1016/j.jpainsymman.2003.07.004
- Chen ZW The relationship between symptom burden, self-management behavior, and quality of life in maintenance hemodialysis patientss [ Dissertation]. Yanji: Yanbian University; 2018.
- Li H, Xie L, Yang J, Pang X. Symptom burden amongst patients suffering from end-stage renal disease and receiving dialysis: a literature review. Int J Nurs Sci. 2018;5(4):427–431. Chinese. doi:10.1016/j.ijnss.2018.09.010
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi:10.1046/j.1525-1497.2001.016009606.x
- Chen R, Wang Y, Yu JY, Zhang L. Citic validity of PHQ-9 in inpatients of general hospital. Sichuan Ment Health. 2017;30(02):149–153. Chinese. doi:10.11886/j.issn.1007-3256.2017.02.013
- Tao X, Chow SK, Wong FK. Determining the validity and reliability of the Chinese version of the Kidney Disease Quality of Life Questionnaire (KDQOL-36™). BMC Nephrol. 2014;15:115. doi:10.1186/1471-2369-15-115
- India State-Level Disease Burden Initiative Mental Disorders Collaborators. The burden of mental disorders across the states of India: the global burden of disease study 1990–2017. Lancet Psychiatry. 2020;7(2):148–161. doi:10.1016/S2215-0366(19)30475-4
- Tian N, Chen N, Li PK. Depression in dialysis. Curr Opin Nephrol Hypertens. 2021;30(6):600–612. doi:10.1097/MNH.0000000000000741
- Gupta S, Patil NM, Karishetti M, Tekkalaki BV. Prevalence and clinical correlates of depression in chronic kidney disease patients in a tertiary care hospital. Indian J Psychiatry. 2018;60(4):485–488. doi:10.4103/psychiatry
- Zhou M, Gu X, Cheng K, Wang Y, Zhang N. Exploration of symptom clusters during hemodialysis and symptom network analysis of older maintenance hemodialysis patients: a cross-sectional study. BMC Nephrol. 2023;24(1):115. doi:10.1186/s12882-023-03176-4
- Liu Y, Peng YM, Cheng JZ. Symptom burden of maintenance hemodialysis patients and its influencing factors. Chin J Med Doctors. 2017;19(08):1180–1183. Chinese. doi:10.3760/cma.j.issn.1008-1372.2017.08.015
- Cohen DE, Lee A, Sibbel S, Benner D, Brunelli SM, Tentori F. Use of the KDQOL-36™ for assessment of health-related quality of life among dialysis patients in the United States. BMC Nephrol. 2019;20(1):461. doi:10.1186/s12882-019-1630-5
- Xiong L, Wu YZ, Mu QY, et al. The depression and quality of life in 356 patients with chronic renal failure without dialysis. J Third Mil Med Univ. 2019;41(02):163–169. doi:10.16016/j.1000-5404.201808033
- Ren QT, Lu YZ, Tian MP. Analysis of misdiagnosis of depression with physical discomfort as a main symptom. Chin J Neuropsychiatr Disord. 2001;27(6):453–454. Chinese. doi:10.3969/j.issn.1002-0152.2001.06.022
- Fischer MJ, Streja E, Hsiung JT, et al. Depression screening and clinical outcomes among adults initiating maintenance hemodialysis. Clin Kidney J. 2021;14(12):2548–2555. doi:10.1093/ckj/sfab097
- Mihai S, Codrici E, Popescu ID, et al. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J Immunol Res. 2018;2018:2180373. doi:10.1155/2018/2180373
- Liu M, Zhang Y, Yang S, et al. Bidirectional relations between depression symptoms and chronic kidney disease. J Affect Disord. 2022;311:224–230. doi:10.1016/j.jad.2022.05.104
- Tian M, Qian Z, Long Y, Yu F, Yuan J, Zha Y. Decreased intracellular to total body water ratio and depressive symptoms in patients with maintenance hemodialysis. Psychol Res Behav Manag. 2023;16:4367–4376. doi:10.2147/PRBM.S436574

