Abstract
In the United States, budesonide/formoterol pressurized metered-dose inhaler (pMDI) is approved for treatment of asthma in patients aged ≥12 years whose asthma is not adequately controlled with an inhaled corticosteroid (ICS) or whose disease severity clearly warrants treatment with an ICS and a long-acting β2-adrenergic agonist. This article reviews studies of budesonide/formoterol pMDI in patients with persistent asthma, with a particular focus on patient-reported outcomes (eg, perceived onset of effect, patient satisfaction with treatment, health-related quality of life [HRQL], global assessments, sleep quality and quantity), as these measures reflect patient perceptions of asthma control and disease burden. A search of PubMed and respiratory meetings was performed to identify relevant studies. In two pivotal budesonide/formoterol pMDI studies in adolescents and adults, greater efficacy and similar tolerability were shown with budesonide/formoterol pMDI 160/9 μg and 320/9 μg twice daily versus its monocomponents or placebo. In those studies, improvements in HRQL, patient satisfaction, global assessments of asthma control, and quality of sleep also favored budesonide/formoterol pMDI compared with one or both of its monocomponents or placebo. Budesonide/formoterol pMDI has a rapid onset of effect (within 15 minutes) that patients can feel, an attribute that may have benefits for treatment adherence. In summary, budesonide/formoterol pMDI is effective and well tolerated and has additional therapeutic benefits that may be important from the patient’s perspective.
Introduction
Asthma is a chronic condition that affects approximately 23 million people in the United States alone and is associated with significant clinical morbidity and economic burden.Citation1 Poorly controlled asthma also imparts substantial burden to the patient, including decrements in health-related quality of life (HRQL) and interference with daily activities.Citation2 According to the National Asthma Education and Prevention Program (NAEPP) guidelines, the goal of treatment is to control asthma by reducing impairment (eg, preventing asthma symptoms, maintaining near normal pulmonary function and HRQL, achieving patient satisfaction with care) and risk (eg, preventing exacerbations, loss of pulmonary function, adverse events [AEs]).Citation3 The 2009 joint statement of the American Thoracic Society and the European Respiratory Society recommends the use of composite measures comprising multiple end points, including those that are patient-reported, for a more complete assessment of asthma control.Citation4 Thus, asthma control measures that provide objective clinical assessments of the disease, as well as those that measure the burden of disease from the patient’s perspective, are important for assessing the benefits and risks of asthma medications.
The NAEPP guidelines recommend a stepwise approach to treatment, with inhaled corticosteroids (ICSs) recommended as first-line treatment for patients with persistent asthma because of their potent anti-inflammatory effects.Citation3 Several long-term control medications are available for treating patients with persistent asthma, including ICS, long-acting β2-adenergic agonists (LABAs), leukotriene modifiers, cromolyn and nedocromil, immunomodulators, and methylxanthines.Citation3 The addition of a LABA to an ICS is recommended as one of the preferred treatment options in patients 5 years of age or older with persistent asthma that is not controlled with an ICS alone.Citation3
In the United States, the combination of the ICS budesonide and the LABA formoterol administered via one pressurized metered-dose inhaler (pMDI; Symbicort® Inhalation Aerosol, AstraZeneca LP, Wilmington, DE) is indicated for the treatment of asthma in patients aged ≥12 years who are not adequately controlled on a long-term asthma control medication, such as an ICS, or whose disease severity clearly warrants treatment with ICS/LABA combination therapy.Citation5 In countries outside of the United States, budesonide/formoterol administered via dry powder inhaler (DPI) (Symbicort® Turbuhaler®, AstraZeneca, Lund, Sweden) is indicated as maintenance therapy in patients aged ≥6 years with persistent asthma for whom combination therapy is appropriate or as maintenance and reliever therapy in patients aged ≥18 years with persistent asthma.Citation6 Budesonide/formoterol administered via pMDI is not approved for use as maintenance and reliever therapy in any country.Citation5 Budesonide/formoterol pMDI is approved for asthma in two dosage strengths (80/4.5 μg × 2 inhalations [160/9 μg] twice daily and 160/4.5 μg × 2 inhalations [320/9 μg] twice daily) in the United States.Citation5 This review describes the clinical profile of budesonide/formoterol pMDI for asthma, with a particular focus on patient-reported outcomes.
Clinical pharmacology
Budesonide is a potent corticosteroid that exhibits anti-inflammatory properties by acting on several inflammatory mediators (eg, histamine, leukotrienes, eicosanoids, cytokines) and cell types (eg, mast cells, eosinophils, lymphocytes, macrophages).Citation7 Budesonide acts locally and directly on the respiratory tract, reducing inflammation and decreasing airway hyperresponsiveness.Citation7,Citation8 Formoterol, a potent LABA and full agonist, has high aff inity and selectivity for β2-adrenergic receptors of the pulmonary smooth muscle.Citation9 These characteristics have translated to greater maximum relaxation of airway smooth muscle activity with formoterol compared with the partial agonist salmeterol in vitro.Citation9,Citation10 In humans, the magnitude of bronchodilation and duration of effect (∼12 hours) were shown to be similar with formoterol and salmeterol, but the onset of bronchodilation was faster with formoterol compared with salmeterol.Citation9,Citation11
Evidence suggests an additive effect of budesonide and formoterol when they are administered concomitantly.Citation12,Citation13 When coadministered, the bronchodilatory effects of a LABA complements the anti-inflammatory effects of an ICS, improving pulmonary function and reducing symptoms and exacerbations compared with increasing the ICS alone.Citation14 In addition, there may be a synergistic interaction between an ICS and a LABA.Citation13,Citation15,Citation16 Combination therapy with an ICS and a LABA has been shown to enhance nuclear translocation of the glucocorticoid receptor.Citation13 Studies also suggest that a LABA may enhance the inhibitory effect of an ICS on allergen-induced activation of certain inflammatory cytokines (eg, granulocyte-macrophage colony-stimulating factor, tumor necrosis factor α, interleukin-1β, interleukin-2).Citation15,Citation16 Specific details of the pharmacokinetic properties of budesonide and formoterol administered via pMDI have been described in detail previously.Citation17,Citation18
Clinical efficacy of budesonide/formoterol pMDI
The efficacy of budesonide/formoterol pMDI has been evaluated in clinical studies in patients with asthma across a range of disease severities and ages ().Citation19–Citation28 Budesonide/formoterol pMDI also has been evaluated across a range of doses (up to 640/18 μg twice daily [twice the US-approved maximum daily dose]) and treatment regimens (fixed-dose once or twice daily, adjustable dose) and in comparison with active treatments (fluticasone propionate/salmeterol, budesonide alone [similar or higher dose], formoterol alone) and placebo ().Citation19–Citation28
Table 1 Designs for studies of budesonide/formoterol pMDI
Budesonide/formoterol pMDI twice daily versus its monocomponents and placebo
Two pivotal 12-week US studies of similar design evaluated the efficacy of budesonide/formoterol pMDI (160/9 μg or 320/9 μg) twice daily in adolescents and adults with mild to moderateCitation20 or moderate to severeCitation19 persistent asthma who were previously receiving ICS therapy ().Citation19,Citation20 In those studies, patients had to be symptomatic during a 1- to 3-week run-in period on placebo (mild to moderate) or budesonide 160 μg twice daily (moderate to severe) to qualify for randomization.Citation19,Citation20 In both studies, the anti-inflammatory effect of budesonide and the bronchodilatory effect of formoterol each contributed to the efficacy of budesonide/formoterol pMDI, as shown by the significant improvements in predose forced expiratory volume in 1 second (FEV1) compared with formoterol DPI and in 12-hour postdose FEV1 compared with budesonide, respectively ().Citation19,Citation20 Treatment with budesonide/formoterol pMDI also resulted in significant (P ≤ 0.05) benefits in symptom-related variables compared with its monocomponents or placebo,Citation19,Citation20 with significant differences from budesonide observed only in patients with moderate to severe persistent asthma ().Citation19 The risk of asthma worsening also was assessed based on predefined criteria, including a decrease in morning predose FEV1 of ≥20% or <40% of predicted normal; use of ≥12 inhalations per day of albuterol or 20% decrease in morning peak expiratory flow [PEF] on ≥3 days within any consecutive 7-day period; ≥2 night-time awakenings requiring rescue medication use within any consecutive 7-day period; or a clinical exacerbation requiring emergency treatment, hospitalization, or nonprotocol treatment.Citation19,Citation20 The risk of having a predefined event of asthma worsening was significantly (P ≤ 0.05) reduced with budesonide/formoterol pMDI compared with its monocomponents and placebo in patients with moderate to severe persistent asthmaCitation19 and compared with formoterol and placebo in patients with mild to moderate persistent asthma.Citation20 The percentage of patients with moderate to severe persistent asthma who experienced ≥1 predefined asthma worsening event was 29.8% in those treated with budesonide/formoterol pMDI compared with 44.0%, 55.3%, and 67.2% in patients taking budesonide, formoterol, and placebo, respectively.Citation19 In patients with mild to moderate persistent asthma, the proportion of patients who experienced ≥1 predefined asthma worsening event was 18.7% with budesonide/formoterol pMDI compared with 21.5%, 42.1%, and 56.6% with budesonide, formoterol, and placebo, respectively.Citation20
Table 2 Efficacy of budesonide/formoterol pMDI in patients with asthma
Consistent with the study reported by Noonan et alCitation19 the results from a 1-year safety and efficacy study reported by Peters and colleagues, which also was conducted in patients with moderate to severe persistent asthma (), showed significant (P < 0.01) improvements in pulmonary function and symptom-related variables with budesonide/formoterol pMDI (320/9 μg or 640/18 μg twice daily) compared with budesonide pMDI (640 μg twice daily) ().Citation21 In that study, the proportion of patients with at least 1 asthma exacerbation also was significantly (P = 0.006) lower with budesonide/formoterol pMDI 640/18 μg twice daily (12.2%) and numerically lower with budesonide/formoterol pMDI 320/9 μg twice daily (14.4%) compared with budesonide 640 μg twice daily alone (21.8%).Citation21
Budesonide/formoterol pMDI is indicated in patients with persistent asthma aged ≥12 years; however, clinical studies of this combination product also have been conducted in children aged 6 to 15 yearsCitation25 and 6 to 11 yearsCitation26,Citation27 (). Results from these studies generally showed significant (P < 0.05) benefits to pulmonary function of treatment with budesonide/formoterol pMDI twice daily compared with budesonide twice daily alone.Citation25–Citation27 In the efficacy and safety study reported by Murphy et al treatment with budesonide/formoterol pMDI also significantly (P < 0.05) improved morning and evening PEF, decreased nighttime asthma symptoms and nighttime rescue medication use, and increased the percentage of rescue medication–free days compared with formoterol alone.Citation25
Twice-daily versus once-daily dosing
Two studies evaluated the efficacy of once-daily budesonide/formoterol pMDI, administered at half the daily formoterol dose (320/9 μg) or half the daily budesonide and formoterol doses (160/9 μg) compared with twice-daily budesonide/formoterol pMDI (320/18 μg daily), versus once-daily budesonide pMDI (320 μg) alone in adolescents and adults with mild to moderate persistent asthma previously stabilized during a 4- to 5-week run-in on twice-daily budesonide/formoterol pMDI (320/18 μg daily) ().Citation22,Citation23 Both studies showed that treatment with once- or twice-daily budesonide/formoterol pMDI generally was more effective on pulmonary function and symptom-related variables than treatment with budesonide pMDI alone ().Citation22,Citation23 However, twice-daily budesonide/formoterol pMDI maintained pulmonary function and asthma control more effectively than once-daily budesonide/formoterol pMDI in both studies ().Citation22,Citation23 Similar results were reported by Eid et al in a study including children and adolescents (6–15 years) ().Citation28
Budesonide/formoterol pMDI versus fluticasone propionate/salmeterol
In a 7-month open-label study, Busse et al compared the efficacy of fixed-dose budesonide/formoterol pMDI 320/9 μg twice daily with that of fixed-dose fluticasone propionate/salmeterol DPI 250/50 μg twice daily in adolescents and adults with moderate to severe persistent asthma.Citation24 Findings showed similar efficacy for all pulmonary function and symptom-related variables between the two treatments ().Citation24 In addition, the proportion of patients who experienced at least 1 exacerbation (primary variable) was not significantly different between the fixed-dose budesonide/formoterol pMDI and fixed-dose fluticasone propionate/salmeterol DPI groups (8.8% and 9.2%, respectively).Citation24
Patient-focused perspectives
In addition to objective measures of asthma control, outcomes reflecting the patient’s perception of disease burden and the effects of treatment are critical to effective asthma management. The poor correlation between clinical asthma status parameters and patient-reported outcomes, such as HRQL, suggest that such measures offer a unique assessment of the effects of the disease and of treatment from the perspective of the patient.Citation3,Citation4 Patient-reported outcomes have been assessed in several studies of budesonide/formoterol pMDI, including measures of the patient’s perception of onset of effect,Citation29,Citation30 patient satisfaction with treatment,Citation30–Citation32 and HRQL.Citation30–Citation32
Onset of effect
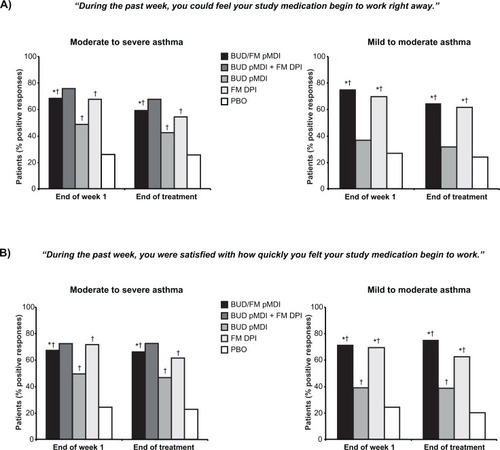
Onset of effect is an important concept in asthma management, as evidence suggests that asthma controller medications that have a rapid onset of effect may contribute to improved patient adherence.Citation33 In addition, study findings indicate that the majority of patients prefer a reliever medication with a fast onset and a long duration of effect.Citation34 Measured onset of effect of budesonide/formoterol pMDI has been compared with that of its monocomponents and placebo in the two pivotal studies of budesonide/formoterol pMDI in patients aged ≥12 yearsCitation19,Citation20,Citation29 and with that of fluticasone propionate/salmeterol DPI in two crossover studies in patients aged ≥18 yearsCitation35 based on serial spirometry. Patient perception of the onset of effect of budesonide/formoterol pMDI was evaluated based on the Onset of Effect Questionnaire (OEQ) in the two pivotal budesonide/formoterol pMDI studies in patients aged ≥18 years with mild to moderate or moderate to severe persistent asthmaCitation29 and in the study comparing fixed-dose budesonide/formoterol pMDI with fluticasone propionate/salmeterol DPI.Citation30 The 5-item OEQ has been validated in patients with asthma aged ≥18 years and provides an assessment of patients’ ability to perceive an asthma therapy working right away (item 2) and satisfaction with how quickly they feel their medication begins to work (item 5) based on a 1-week recall, with responses scored on a 5-point Likert-type scale.Citation36 These items were identified previously as being important to patientsCitation37 and as the items that best capture patients’ perceptions of onset of effect.Citation36
In the two pivotal trials of budesonide/formoterol pMDI, the percentage of patients who experienced clinically significant bronchodilation (eg, ≥15% FEV1 improvement) within 15 minutes of administering study medication on the day of randomization was significantly (P < 0.05) higher in patients treated with budesonide/formoterol pMDI (49%–57%) compared with budesonide pMDI (6% for both studies) or placebo (6%–8%) on the day of randomization.Citation29 Combining data from both studies, the median time to onset of clinically significant bronchodilation (achievement of ≥15% FEV1 improvement in 50% of patients) after administration of study medication was 13 minutes for budesonide/formoterol pMDI.Citation29
Patient-perceived onset of effect, assessed based on the OEQ in patients aged ≥18 years in the two pivotal studies, was consistent with the findings for measured onset of effect in those studies.Citation29 In both studies, a significantly greater percentage of patients in the budesonide/formoterol pMDI groups indicated that they could feel their study medication begin to work right away and that they were satisfied with how quickly they felt their medication begin to work compared with the budesonide pMDI (P ≤ 0.04) or placebo groups (P < 0.001) at the end of the first treatment week and at the end of treatment ().Citation29 In a separate study, a consensus panel of 12 community-based health care professionals blinded to the study drug names agreed that the observed differences between the treatment groups for patient perceptions of onset of effect and satisfaction in the two pivotal studies were clinically important.Citation38 Moreover, all of the panelists agreed that if a patient is able to perceive onset of effect, it would improve adherence to maintenance asthma treatment.Citation38 The findings from this study further suggest that improved adherence to treatment may be facilitated by a medication with a rapid onset of effect.
Figure 1 Onset of Effect Questionnaire: Percentage of patients who indicated that they could feel their study medication begin to work right away (A) and that they were satisfied with how quickly they felt their study medication begin to work (B).Citation29 Statistical analyses comparing FM DPI vs BUD pMDI and BUD pMDI + FM DPI vs BUD pMDI and PBO not performed in study I.
Abbreviations: BUD, budesonide; DPI, dry powder inhaler; FM, formoterol; PBO, placebo; pMDI, pressurized metered-dose inhaler.
In two single-dose, randomized, active- and placebo-controlled crossover studies (NCT00646620 and NCT00646009) of identical design, Hampel et al evaluated the onset of effect of budesonide/formoterol pMDI 160/9 μg compared with that of fluticasone propionate/salmeterol DPI 250/50 μg, albuterol pMDI 180 μg, or placebo in patients aged ≥18 years with persistent asthma.Citation35 In a combined analysis of both studies, a significantly (P ≤ 0.001) greater percentage of patients in the budesonide/formoterol pMDI group (40%) achieved a ≥15% improvement in FEV1 within 15 minutes compared with fluticasone propionate/salmeterol DPI (19%) or placebo (2%).Citation35 Budesonide/formoterol pMDI treatment also resulted in significantly (P < 0.001) greater mean adjusted FEV1 values at 3 minutes postdose compared with fluticasone propionate/salmeterol DPI and placebo (2.71 L vs 2.52 L and 2.45 L, respectively), and similar improvement compared with albuterol pMDI (2.69 L).Citation35
OEQ findings from the open-label study comparing fixed-dose budesonide/formoterol pMDI with fixed-dose fluticasone propionate/salmeterol DPICitation24,Citation30 were consistent with the results of measured onset of effect reported in the crossover studies by Hampel et al.Citation35 Compared with fixed-dose fluticasone propionate/salmeterol DPI, a significantly higher (P ≤ 0.025) percentage of patients in the fixed-dose budesonide/formoterol pMDI group indicated that they could feel their study medication begin to work right away (71% vs 59%, respectively) and that they were satisfied with how quickly they felt their medication begin to work (80% vs 73%, respectively) at the end of treatment.Citation30 These findings show that budesonide/formoterol pMDI has a more rapid onset of bronchodilatory effect compared with fluticasone propionate/salmeterol DPI that patients can perceive.
Satisfaction
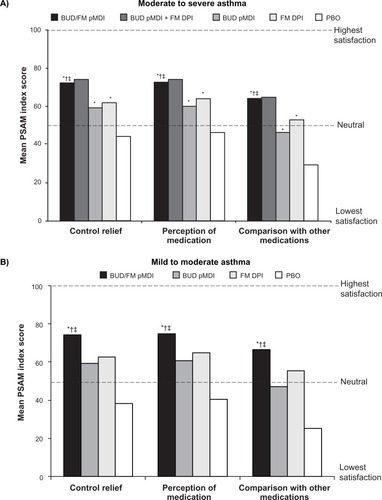
One of the goals of asthma therapy according to the NAEPP asthma management guidelines is to maintain patient satisfaction with asthma care, as improvements in patient satisfaction have been associated with improved treatment adherence.Citation3 Satisfaction with budesonide/formoterol pMDI treatment was assessed in a subset of patients aged ≥18 years in the two previously described pivotal studies (553 patients with moderate to severe asthma;Citation31 405 patients with mild to moderate asthmaCitation32) using three domains of the Patient Satisfaction with Asthma Medication (PSAM) questionnaire (Control Relief, Perception of Medication, and Comparison With Other Medications).Citation31,Citation32 The 23-item PSAM questionnaire has demonstrated reliability and validity in patients aged ≥18 years with asthma,Citation31,Citation32,Citation39 and is scored on a scale from 0–100, with 0 representing the lowest level of satisfaction and 100 representing the highest level of satisfaction.Citation31,Citation32,Citation39 In both pivotal studies, mean PSAM scores at the end of treatment were significantly (P ≤ 0.004) higher with budesonide/formoterol pMDI compared with its monocomponents or placebo ().Citation31,Citation32,Citation40
Figure 2 Mean PSAM scores at the end of treatment in patients with (A) moderate to severeCitation31 or (B) mild or moderateCitation32 persistent asthma.
Abbreviations: BUD, budesonide; DPI, dry powder inhaler; FM, formoterol; PBO, placebo; pMDI, pressurized metered-dose inhaler; PSAM, Patient Satisfaction with Asthma Medication.
Patient satisfaction with f ixed-dose budesonide/formoterol pMDI also has been evaluated in comparison with fixed-dose fluticasone propionate/salmeterol DPI using the Asthma Treatment Satisfaction Measure (ATSM) in patients aged ≥18 years.Citation30 The ATSM assessment has been validated in patients aged ≥18 years and contains four parts: expectations for treatment, importance rating of treatment attributes, outcomes of treatment, and satisfaction with asthma treatment.Citation41 It evaluates 11 predefined medication attributes: timely relief of symptoms, level of symptom relief, rescue medication use, asthma attack frequency, medication worked, feel medication working, daily activity, leisure activity, dosing management, medication convenience, and side effects.Citation30,Citation41 ATSM responses are scored on a scale ranging from 0–100, with 0 representing the lowest level of satisfaction and 100 representing the highest level of satisfaction.Citation30,Citation41 In the study reported by O’Connor et al there was no significant difference between fixed-dose budesonide/formoterol pMDI and fixed-dose fluticasone propionate/salmeterol DPI in mean overall ATSM score at the end of treatment (47.7 and 46.7, respectively).Citation30 However, treatment with fixed-dose budesonide/formoterol pMDI resulted in significantly (P < 0.05) greater treatment satisfaction scores compared with fixed-dose fluticasone propionate/salmeterol DPI for the attributes of timely relief of symptoms (52.9 vs 47.7, respectively) and feel medication working (36.6 vs 32.8, respectively) at the end of treatment.Citation30
Health-related quality of life
Clinical asthma parameters provide important information about disease status and the effects of treatment, but the information derived from each measure may be applicable only to specific domains of the disease.Citation3,Citation4 Assessment of HRQL from the patient’s perspective provides a more global view of treatment effectiveness, as well as potentially unique information about the effects of treatment that may be identified only by the patient.Citation4 The effect of budesonide/formoterol pMDI on HRQL has been evaluated in several clinical studiesCitation22,Citation23,Citation27,Citation28,Citation30–Citation32 using validated instruments that are asthma-specific and recommended by the NAEPP guidelines.Citation3
The validated, standardized Asthma Quality of Life Questionnaire (AQLQ[S])Citation42 was included in several clinical studies of budesonide/formoterol pMDI in subsets of patients aged ≥18 years.Citation22,Citation23,Citation30–Citation32 The 32-item AQLQ(S) comprises four domains: symptoms, emotional function, activity limitation, and exposure to environmental stimuli.Citation30–Citation32,Citation43 Overall and domain scores range from 1 (greatest impairment) to 7 (least impairment).Citation30–Citation32,Citation43 A change of ≥0.5 points in overall or domain scores has been defined as a clinically meaningful change for the AQLQ(S).Citation44
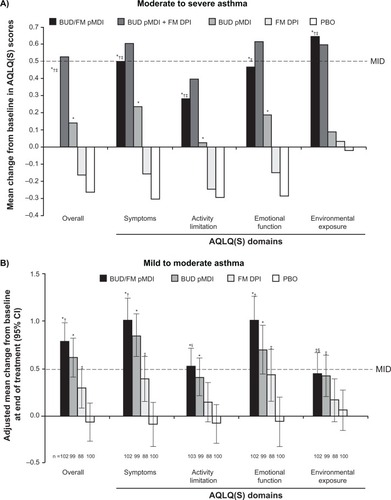
Results from the two pivotal studies showed mean improvements in AQLQ(S) overall and domain scores from baseline to the end of treatment that were significantly (P ≤ 0.042) greater with budesonide/formoterol pMDI compared with formoterol DPI or placebo in patients with mild to moderateCitation32 or moderate to severeCitation31 persistent asthma (). In addition, in patients with moderate to severe persistent asthma, treatment with budesonide/formoterol pMDI resulted in significantly (P ≤ 0.047) greater improvements in the AQLQ(S) overall score and all domain scores except emotional function compared with budesonide pMDI alone.Citation31 A significantly (P ≤ 0.006) higher percentage of patients achieved clinically meaningful improvements from baseline in AQLQ(S) overall score with budesonide/formoterol pMDI compared with placebo in patients with mild to moderate (63% vs 35%)Citation32 or moderate to severe (44% vs 23%)Citation31 persistent asthma at the end of treatment.
Figure 3 Adjusted mean change from baseline to end of treatment in AQLQ(S) overall and domain scores in patients with (A) moderate to severeCitation31 or (B) mild to moderateCitation32 persistent asthma.
Notes: A: *P < 0.01 vs PBO; †P < 0.05 vs BUD; ‡P < 0.001 vs FM. B: *P < 0.001 vs PBO; †P < 0.001 vs FM; ‡P < 0.05 vs PBO; §P < 0.05 vs FM. Copyright © 2008. Elsevier. Reprinted with permission from Chervinsky P, Baker J, Bensch G, et al. Patient-reported outcomes in adults with moderate to severe asthma after use of budesonide and formoterol administered via 1 pressurized metered-dose inhaler. Ann Allergy Asthma Immunol. 2008;101(5):463–473.Citation31 Copyright © 2008. Informa Healthcare. Murphy K, Nelson H, Parasuraman B, Boggs R, Miller C, O’Dowd L. The effect of budesonide and formoterol in one pressurized metered-dose inhaler on patient-reported outcomes in adults with mild-to-moderate persistent asthma. Curr Med Res Opin. 2008;24(3): 879–894.Citation32
Quality of life based on the AQLQ(S) also was assessed in the studies evaluating budesonide/formoterol pMDI once or twice daily compared with budesonide pMDI once daily in patients with mild to moderate persistent asthma who were previously stabilized on twice-daily budesonide/formoterol pMDI ().Citation22,Citation23 In the study by Kerwin et al quality of life was better maintained with twice-daily budesonide/formoterol pMDI (320/18-μg total daily dose) compared with once-daily budesonide pMDI (320-μg daily dose) based on AQLQ(S) overall and all domain scores (P < 0.05); however benefits of once-daily budesonide/formoterol pMDI (320/9-μg or 160/9-μg daily dose) relative to once-daily budesonide pMDI were less apparent and significantly better only on the AQLQ(S) environmental exposure domain score (P < 0.05).Citation22 In the study by Berger et al quality of life was significantly better maintained with budesonide/formoterol pMDI twice daily (320/18-μg daily dose) and once daily (320/9-μg daily dose) compared with once-daily budesonide pMDI (P < 0.05), except for the environmental exposure domain, for which differences were significant only for twice-daily budesonide/formoterol pMDI.Citation23 These results were consistent with the overall efficacy results from those studies showing generally more favorable results with twice-daily budesonide/formoterol pMDI relative to once-daily budesonide/formoterol pMDI and once-daily budesonide pMDI.Citation22,Citation23
In a study directly comparing fixed-dose budesonide/formoterol pMDI twice daily with fluticasone propionate/salmeterol DPI twice daily, mean improvements from baseline to end of treatment in AQLQ(S) overall and domain scores were not significantly different.Citation30 Similarly, the percentages of patients who achieved a clinically meaningful change from baseline to the end of treatment in AQLQ(S) overall score were similar in the fixed-dose budesonide/formoterol pMDI (63%) and fixed-dose fluticasone propionate/salmeterol DPI (62%) groups.Citation30
In pediatric studies of budesonide/formoterol pMDI,Citation27,Citation28 HRQL was evaluated in children with asthma using the standardized Pediatric Asthma Quality of Life Questionnaire (PAQLQ[S])Citation45 and in their caregivers using the Pediatric Asthma Caregiver’s Quality of Life Questionnaire (PACQLQ).Citation45 The 23-item PAQLQ(S) has been validated in children aged 7 to 17 yearsCitation46 and the 13-item PACQLQ has been validated in caregivers of children aged 7 to 17 years.Citation46 Scores for both questionnaires are based on a 7-point scale ranging from 1 (greatest possible impairment) to 7 (least impairment).Citation45,Citation46 A mean change from baseline of ≥0.5 points in overall PAQLQ(S) and PACQLQ scores has been defined as a clinically meaningful change.Citation27,Citation28,Citation46
In the 26-week study by Berger et al budesonide/formoterol pMDI resulted in significantly greater (P ≤ 0.006) mean improvements from baseline to end of treatment in the overall PAQLQ(S) and PACQLQ scores compared with budesonide DPI.Citation27 Improvements from baseline in the PAQLQ(S) score were clinically meaningful (eg, >0.5) for patients in the budesonide/formoterol pMDI group; however, the differences between the two treatment groups did not reach the minimally important difference for the PAQLQ(S) or the PACQLQ.Citation27 In that study, clinically meaningful differences might have been difficult to achieve because baseline scores for both the PAQLQ(S) and the PACQLQ were high (5.84–6.21 out of 7 points).Citation27 In the 12-week study by Eid et al the PAQLQ(S) and PACQLQ overall scores were stable at baseline and maintained throughout the randomized study period in all treatment groups, with no significant or clinically meaningful differences observed.Citation28 Similar to the 26-week study reported by Berger et al baseline overall PAQLQ(S) and PACQLQ scores were high (6.34–6.62 out of 7 points) and clinically meaningful changes difficult to show.Citation28
Patient perspectives of asthma control
The NAEPP recommends using patients’ self-assessments and caregivers’ assessments as one of the principal methods to monitor asthma control.Citation3 The effects of budesonide/formoterol pMDI on patient global assessmentsCitation22,Citation31,Citation32 and caregiver global assessmentsCitation27,Citation28 have been assessed in clinical studies. In studies evaluating budesonide/formoterol pMDI in adult patients, a 5-point scale was used by patients to rate their overall health (a great deal better, somewhat better, unchanged, somewhat worse, and a great deal worse) and ability to manage their asthma (a great deal easier, somewhat easier, unchanged, somewhat more difficult, and a great deal more difficult) at the end of the study compared with the start of the study.Citation22,Citation31,Citation32 Similarly, in pediatric studies of budesonide/formoterol pMDI, caregivers rated their child’s health and their own ability to manage the child’s asthma at the end of treatment on a 5-point scale.Citation27,Citation28
Findings in patients aged ≥18 years from the two pivotal trials showed significantly (P ≤ 0.01) higher percentages of patients reporting improvement in overall health with budesonide/formoterol pMDI (61%) compared with placebo (20%) in patients with mild to moderate asthmaCitation32 and with budesonide/formoterol pMDI (59%) compared with formoterol DPI (40%) or placebo (13%) in patients with moderate to severe asthma (59% vs 40%).Citation31 A significantly (P ≤ 0.03) higher percentage of patients reported easier management of their asthma with budesonide/formoterol pMDI compared with budesonide pMDI or placebo in patients with mild to moderate (62% vs 46% and 21%, respectively)Citation32 or moderate to severe (62% vs 46% and 19%, respectively)Citation31 persistent asthma.
Kerwin et al reported a significantly (P ≤ 0.033) higher percentage of patients reporting overall health improvements with budesonide/formoterol pMDI twice daily (320/18-μg daily dose) (63%) and budesonide/formoterol pMDI once daily (160/9-μg daily dose) (60%) compared with budesonide pMDI once daily (320 μg) (48%) and a significantly (P = 0.008) higher percentage of patients reporting easier management of their asthma with budesonide/formoterol pMDI twice daily (66%) compared with budesonide pMDI once daily (51%).Citation22 In the pediatric study by Eid et al the percentages of caregivers who reported improvements in their child’s health or ease of asthma management were similar across all treatment groups (57%–60%).Citation27 However, Berger et al reported a significantly (P ≤ 0.048) higher percentage of caregivers indicating improvements in their child’s health and easier management of their child’s asthma with budesonide/formoterol pMDI (69% and 70%, respectively) compared with budesonide DPI (52% for each question).Citation27
Patient perspectives of sleep quantity and quality
Impaired quality and quantity of sleep, including difficulty in falling asleep, difficulty in maintaining sleep, and increased daytime sleepiness, is common in patients with asthma.Citation47 The NAEPP recommends the periodic assessment of patients for key areas of quality of life, including sleep disturbances due to asthma.Citation3 Sleep quality and quantity with budesonide/formoterol pMDI treatment was assessed in the two pivotal studies using the Medical Outcomes Study (MOS) Sleep Scale, including the Long Index and the “awaken during sleep” and “awaken short of breath or with a headache” questions (scored on a scale from 0 [best sleep] to 100 [worst sleep]).Citation31,Citation32 The 12-item MOS Sleep Scale has been validated in a general population of adults aged ≥18 years in the United States.Citation48
In patients with moderate to severe asthma, there were no significant differences across treatment groups in mean changes from baseline in the Long Index or “awaken during sleep” question scores.Citation31 However, patients reported awakening short of breath or with a headache significantly (P < 0.01) less frequently with budesonide/formoterol pMDI (–8.87) compared with formoterol (–1.49) or placebo (4.00).Citation31 Patients with mild to moderate asthma experienced significant improvements in overall quality of sleep, as assessed by the Long Index, and a lower likelihood of awakening during sleep and awakening short of breath or with a headache with budesonide/formoterol pMDI (mean change from baseline in Long Index score, −7.1, −5.7, −13.7, respectively) compared with placebo (−1.8, 1.1, −2.6, respectively) (P ≤ 0.033).Citation32
Safety and tolerability
The benefits of ICSs and LABAs are well established in patients with asthma;Citation3 however, each class of medication is associated with potential risks.Citation3 Common local drug-related AEs for ICSs may include oral candidiasis and hoarseness, and in very rare cases, systemic effects (eg, reduction in growth velocity in children, reduced bone mineral density, increased risk of cataracts or glaucoma) may occur.Citation3 Common drug-related AEs for LABAs are similar to those observed with short-acting β2-adrenergic agonists (SABAs) (eg, cardiovascular AEs, tremor).Citation3,Citation9
The tolerability of budesonide/formoterol pMDI has been evaluated in 10 active- and placebo-controlled studies with 3393 patients aged ≥12 years with varying severities of asthmaCitation5 and in three studies in children and adolescents.Citation26–Citation28 Findings from the two 12-week pivotal studies reported by Noonan et al and Corren et al and from a 1-year safety study reported by Peters et al show that budesonide/formoterol pMDI is well tolerated at doses of 160/9 μg, 320/9 μg, and 640/18 μg twice daily in adolescents and adults with persistent asthma, with safety findings consistent with the known safety profiles of ICS and LABA medications.Citation19–Citation21 In the long-term safety study, which evaluated two doses of budesonide/formoterol pMDI (320/9 μg twice daily and 640/18 μg twice daily – twice the highest Food and Drug Administration [FDA]-approved dose) compared with budesonide pMDI 640 μg twice daily, dose-related AEs were few, and the authors reported no unexpected patterns of abnormalities for safety findings.Citation21 In the 6-month study in children (aged 6–11 years) reported by Berger et al the safety profile of budesonide/formoterol pMDI 320/9 μg twice daily was similar to that of budesonide administered at the same dose, with no new safety concerns identified.Citation27 Fixed-dose budesonide/formoterol pMDI 320/9 μg twice daily also has shown a safety profile similar to that of fixed-dose fluticasone propionate/salmeterol DPI 250/50 μg twice daily in the 7-month study reported by Busse et al.Citation24
Recently, the FDA issued new requirements for LABA-containing product labelingCitation49,Citation50 based on a meta-analysis conducted by the US FDA Office of Safety and EpidemiologyCitation51 and two previous studies of salmeterol showing an increased risk of asthma-related death or life-threatening experience compared with placebo in one studyCitation52 and an increased number of respiratory and asthma-related deaths compared with albuterol in another study.Citation53 The new labeling states that combination ICS/LABA therapy should be used only in patients whose asthma is not adequately controlled with a long-term asthma control medication (eg, an ICS) and that the LABA should be discontinued if possible once asthma control is achieved.Citation50 In addition, the new FDA guidance states that data are inadequate to determine whether concomitant administration with an ICS mitigates these safety risks.Citation50 Because of differences in methodologies used, conflicting results have been reported in different analyses and meta-analyses.Citation54–Citation63 In addition, because of the rarity of serious asthma-related events in clinical trials, results of meta-analyses assessing the risk of such events with LABAs, alone or with an ICS, have been inconclusive.Citation54–Citation62 To more clearly determine the safety of ICS/LABA combination therapies, the FDA is requiring LABA manufacturers to conduct additional large clinical trials.Citation64 Until more definitive findings on the safety of LABA-containing products are available, budesonide/formoterol pMDI should be used in accordance with the product label and current asthma guidelines.
Place in therapy
The 2009 Global Initiative for Asthma (GINA) guidelines recommend adjustment of asthma therapy in a continuous cycle, driven by the asthma control status of each patient.Citation65 In treatment-naïve patients with persistent asthma, GINA guidelines recommend initiating therapy with low-dose ICS (eg, 200 to 500 μg beclomethasone dipropionate [BDP] or equivalent) as the preferred therapy, unless patients have very symptomatic (uncontrolled) asthma, for whom the recommended initial treatment consists of the combination of low-dose ICS plus LABA (eg, budesonide/formoterol pMDI 160/9 μg twice daily) as the preferred therapy.Citation65 The 2007 NAEPP guidelines recommend initiating treatment based on the patient’s asthma severity, which is assessed using measures of impairment (eg, asthma symptoms, pulmonary function, HRQL) and risk (eg, exacerbations).Citation3 The 2007 NAEPP guidelines recommend that adolescents and adults with mild asthma initiate step 2 treatment (low-dose ICS as preferred treatment), while patients with asthma of moderate severity should initiate step 3 treatment (low-dose ICS plus LABA or medium-dose ICS as equally preferred treatment options).Citation3
Subsequently, asthma therapy should be adjusted using a stepwise approach according to the patient’s level of asthma control.Citation3,Citation65 According to the 2007 NAEPP guidelines, patients whose asthma is not well controlled on low-dose ICS therapy (step 2) should be stepped up to low-dose ICS plus LABA or medium-dose ICS therapy (step 3).Citation3 The 2009 GINA guidelines show a preference for stepping up to low-dose ICS plus LABA therapy in patients whose asthma is not controlled on a low-dose ICS, with step-up to a medium- or high-dose ICS listed as alternative step-up treatments.Citation65 Stepping down therapy also is recommended once asthma control is achieved and maintained for approximately 3 months.Citation3,Citation65 Thus, according to the 2007 NAEPP guidelines,Citation3 if a patient has been well controlled on step 4 therapy (medium-dose ICS/LABA [eg, budesonide/formoterol pMDI 320/9 μg twice daily]) for at least 3 months, stepping down to step-3 therapy (either medium-dose ICS or low-dose ICS/LABA [budesonide/formoterol pMDI 160/9 μg twice daily]) may be appropriate. According to the updated recommendations from the FDA in February 2010, LABA should be discontinued, if possible, once asthma control is achieved, and patients should be maintained on an asthma control medication, such as an ICS.Citation64 As noted in the NAEPP guidelines, physicians also must base treatment decisions on the individual patient’s needs and circumstances and on his or her response to treatment.Citation3
Conclusions
The efficacy and tolerability of budesonide/formoterol pMDI have been shown in several clinical studies of children, adolescents, and adults with mild to moderate and moderate to severe persistent asthma.Citation19–Citation28 Greater efficacy for pulmonary function and asthma control measures was shown with budesonide/formoterol pMDI compared with its monocomponents or placebo.Citation19–Citation23,Citation25–Citation28 Findings from patient-reported outcomes further support the benefits of budesonide/formoterol pMDI in patients with asthma.Citation29–Citation32 In adults with asthma, treatment with budesonide/formoterol pMDI was associated with patient satisfaction and HRQL benefits that were greater than that of its monocomponents or placeboCitation31,Citation32 and similar to that of fluticasone propionate/salmeterol.Citation30 In addition, global assessments of asthma control suggested significant benefits of budesonide/formoterol pMDI relative to one or both monocomponents and placebo for overall health and the ability to manage asthma.Citation22,Citation27,Citation31,Citation32 Measures of sleep quality and quantity also suggested benefits of budesonide/formoterol pMDI relative to placebo in adults with mild to moderateCitation32 or moderate to severeCitation31 persistent asthma.
In addition, more patients receiving budesonide/formoterol pMDI perceived their medication working right away and were satisfied with how quickly they felt their medication begin to work compared with patients receiving budesonide pMDI or placebo.Citation29 In addition, patient perception of onset of effect and satisfaction with timely relief of symptoms with treatment favored budesonide/formoterol pMDI compared with fluticasone propionate/salmeterol DPI.Citation30 The findings for patient perception of onset of effect were consistent with those observed for measured onset of effect.Citation29,Citation35 Evidence from studies that have not directly evaluated the effects of budesonide/formoterol pMDI suggest that patients prefer and are more satisfied with medications with a rapid onset of effectCitation33,Citation37 and that the ability of patients to perceive their medication working right away may contribute to improved patient adherence.Citation33
Studies also have shown that budesonide/formoterol pMDI is well tolerated, with a safety profile similar to that of its monocomponentsCitation19–Citation21,Citation23,Citation28 and fluticasone propionate/salmeterol.Citation24 Across studies, budesonide/formoterol pMDI showed an acceptable safety profile across a range of assessments.Citation19–Citation21,Citation23,Citation28 Even in patients receiving twice the maximum daily recommended dose (640/18 μg twice daily) of budesonide/formoterol pMDI for up to 1 year of treatment, there were no unexpected abnormalities in safety assessments.Citation21
In summary, budesonide/formoterol pMDI is an effective and well tolerated maintenance therapy that provides benefits of treatment from the patient’s perspective, including improved treatment satisfaction, HRQL, asthma control, and sleep quality and quantity. Compared with its monocomponents and placebo, budesonide/formoterol pMDI has been shown to be more effective in achieving the established goals of therapy (eg, preventing asthma symptoms, maintaining pulmonary function, HRQL, satisfaction with care), particularly in patients with moderate to severe persistent asthma. In addition, its rapid onset of effect (within 15 minutes), combined with the ability of patients to perceive their medication working right away, may have benefits for treatment adherence. Overall, the findings from studies of budesonide/formoterol pMDI support its use in patients with persistent asthma that is not adequately controlled with an ICS alone, or whose disease severity warrants initiation of treatment with both an ICS and a LABA.
Acknowledgements
The authors acknowledge Anny Wu, PharmD, and Cynthia Gobbel, PhD, of Scientific Connexions, Newtown PA, for providing medical writing support funded by Astra-Zeneca LP.
Disclosure
Dr. O’Connor has served on advisory boards for GlaxoSmithKline and Sepracor.
References
- American Lung AssociationTrends in asthma morbidity and mortality Epidemiology and Statistics Unit, Research and Program Services Division. 2010 Feb report. Available at: http://www.lungusa.org/finding-cures/our-research/trend-reports/asthma-trend-report.pdf. Accessed Aug 11 2010.
- WertzDAPollackMRodgersKBohnRLSaccoPSullivanSDImpact of asthma control on sleep, attendance at work, normal activities, and disease burdenAnn Allergy Asthma Immunol2010105211812320674821
- National Asthma Education and Prevention Program Expert panel report 3: guidelines for the diagnosis and management of asthma – Full report. NIH Publication Number 08-4051, 2007.
- ReddelHKTaylorDRBatemanEDAn official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practiceAm J Respir Crit Care Med20091801599919535666
- Symbicort® Inhalation Aerosol (budesonide/formoterol pMDI) prescribing information Available at: http://www1.astrazeneca-us.com/pi/symbicort.pdf. Accessed Jun 23 2010.
- Symbicort Turbohaler (budesonide/formoterol DPI) prescribing information Available at: http://www.medicines.org.uk/EMC/medicine/4821/SPC/Symbicort+Turbohaler+100+6+Inhalation+powder/. Accessed Dec 1 2010.
- SzeflerSJEigenHBudesonide inhalation suspension: a nebulized corticosteroid for persistent asthmaJ Allergy Clin Immunol2002109473074211941331
- SzeflerSJPharmacodynamics and pharmacokinetics of budesonide: a new nebulized corticosteroidJ Allergy Clin Immunol1999104S175S183
- BergerWEThe use of inhaled formoterol in the treatment of asthmaAnn Allergy Asthma Immunol2006971243316892777
- JeppssonABKällströmBLWaldeckBStudies on the interaction between formoterol and salmeterol in guinea-pig trachea in vitroPharmacol Toxicol19927142722771360657
- PalmqvistMPerssonGLazerLRosenborgJLarssonPLötvallJInhaled dry-powder formoterol and salmeterol in asthmatic patients: onset of action, duration of effect and potencyEur Respir J19971011248424899426083
- GoldsmithDRKeatingGMBudesonide/formoterol: a review of its use in asthmaDrugs200464141597161815233594
- UsmaniOSItoKManeechoetesuwanKGlucocorticoid receptor nuclear translocation in airway cells after inhaled combination therapyAm J Respir Crit Care Med2005172670471215860753
- McCormackPLLyseng-WilliamsonKABudesonide/formoterol: a review of its use as maintenance and reliever inhalation therapy in asthmaDrugs200767162407243117983258
- OdderaSSilvestriMTestiRRossiGASalmeterol enhances the inhibitor activity of dexamethasone on allergen-induced blood mononuclear cell activationRespiration19986531992049670302
- BarnesPJScientific rationale for inhaled combination therapy with long-acting β2-agonists and corticosteroidsEur Respir J200219118219111843317
- TrondeAGillenMBorgströmLLötvallJAnkerstJPharmacokinetics of budesonide and formoterol administered via 1 pressurized metered-dose inhaler in patients with asthma and COPDJ Clin Pharmacol200848111300130818974284
- EklundATrondeAJohannes-HellbergIGillenMBorgströmLPharmacokinetics of budesonide and formoterol administered via a series of single-drug and combination inhalers: four open-label, randomized, crossover studies in healthy adultsBiopharm Drug Dispos200829738239518623040
- NoonanMRosenwasserLJMartinPO’BrienCDO’DowdLEfficacy and safety of budesonide and formoterol in one pressurized metered-dose inhaler in adults and adolescents with moderate to severe asthma: a randomised clinical trialDrugs200666172235225417137405
- CorrenJKorenblatPEMillerCJO’BrienCOMezzanotteWSTwelve-week, randomized, placebo-controlled, multicenter study of the efficacy and tolerability of budesonide and formoterol in one metered-dose inhaler compared with budesonide alone and formoterol alone in adolescents and adults with asthmaClin Ther200729582384317697902
- PetersSPPrennerBMMezzanotteWSMartinPO’BrienCDLong-term safety and asthma control with budesonide/formoterol versus budesonide pressurized metered-dose inhaler in asthma patientsAllergy Asthma Proc200829549951618694544
- KerwinEMOppenheimerJJLaForceCEfficacy and tolerability of once-daily budesonide/formoterol pressurized meted-dose inhaler in adults and adolescents with asthma previously stable with twice-daily budesonide/formoterol dosingAnn Allergy Asthma Immunol20091031627219663129
- BergerWEBleeckerERO’DowdDMillerCJMezzanotteWEfficacy and safety of budesonide/formoterol pressurized metered-dose inhaler: randomized controlled trial comparing once- and twice-daily dosing in patients with asthmaAllergy Asthma Proc2010311495920167145
- BusseWWShahSSSomervilleLParasuramanBMartinPGoldmanMComparison of adjustable- and fixed-dose budesonide/formoterol pressurized metered-dose inhaler and fixed-dose fluticasone propionate/salmeterol dry powder inhaler in asthma patientsJ Allergy Clin Immunol200812161407141418455221
- MurphyKPearlmanDSUryniakTO’BrienCDMezzanotteWSEfficacy of budesonide/formoterol pressurized metered-dose inhaler (BUD/FM pMDI) in children with asthma previously treated with inhaled corticosteroids (ICSs)Am J Respir Crit Care Med.2008177A710 [Abstract]
- MoriceAHPetersonSBeckmanOKubovaZEfficacy and safety of a new pressurized metered-dose inhaler formulation of budesonide/formoterol in children with asthma: a superiority and therapeutic equivalence studyPul Pharmacol Ther2008211152159
- BergerWELefleinJGGellerDEThe safety and clinical benefit of budesonide/formoterol pressurized metered-dose inhaler versus budesonide alone in childrenAllergy Asthma Proc2010311263920167143
- EidNSNoonanMJChippsBParasuramanBMillerCJO’BrienCDOnce- vs twice-daily budesonide/formoterol in 6- to 15-year-old patients with stable asthmaPediatrics20101263e565e57520713475
- KaiserHParasuramanBBoggsRMillerCJLeidyNKO’DowdLOnset of effect of budesonide and formoterol administered via one pressurized metered-dose inhaler in patients with asthma previously treated with inhaled corticosteroidsAnn Allergy Asthma Immunol2008101329530318814453
- O’ConnorRDPatrickDLParasuramanBMartinPGoldmanMComparison of patient-reported outcomes during treatment with adjustable- and fixed-dose budesonide/formoterol pressurized metered-dose inhaler versus fixed-dose fluticasone propionate/salmeterol dry powder inhaler in patients with asthmaJ Asthma201047221722320170333
- ChervinskyPBakerJBenschGPatient-reported outcomes in adults with moderate to severe asthma after use of budesonide and formoterol administered via 1 pressurized metered-dose inhalerAnn Allergy Asthma Immunol2008101546347319055199
- MurphyKNelsonHParasuramanBBoggsRMillerCO’DowdLThe effect of budesonide and formoterol in one pressurized metered-dose inhaler on patient-reported outcomes in adults with mild-to-moderate persistent asthmaCurr Med Res Opin200824387989418267051
- BenderBGLongAParasuramanBTranZVFactors influencing patient decisions about the use of asthma controller medicationAnn Allergy Asthma Immunol200798432232817458427
- JohanssonGStällbergBTornlingGAsthma treatment preference study: a conjoint analysis of preferred drug treatmentsChest2004125391692315006950
- HampelFCMartinPMezzanotteWSEarly bronchodilatory effects of budesonide/formoterol pMDI compared with fluticasone/salmeterol DPI and albuterol pMDI: 2 randomized controlled trials in adults with persistent asthma previously treated with inhaled corticosteroidsJ Asthma200845426527218446589
- LeidyNKMathiasSDParasuramanBMPatrikDLPathakDDevelopment and validation of an onset of effect questionnaire for patients with asthmaAllergy Asthma Proc200829659059918775104
- HauberABMohamedAFJohnsonRMeddisDWagnerSO’DowdLQuantifying asthma patient preferences for onset of effect of combination inhaled corticosteroids and long-acting beta2-agnoist maintenance medicationsAllergy Asthma Proc200930213914719463204
- HardingGLeidyNKMeddisDKleinmanLWagnerSO’BrienCDInterpreting clinical trial results of patient-perceived onset of effect in asthma: methods and results of a Delphi panelCurr Med Res Opin20092561563157119445651
- MathiasSDWarrneEHColwellHHSungJCYA new treatment satisfaction measure for asthmatics: a validation studyQual Life Res20009787388211297030
- KorenblatPERosenwasserLJBudesonide/formoterol pressurized metered-dose inhaler for patients with persistent asthmaAllergy Asthma Proc201031319020220482961
- MartinMLPatrickDLBushnellDMMeltzeEOGutierrezBParasuramanBDevelopment of the asthma treatment satisfaction measureCurr Med Res Opin200925102495250619689222
- JuniperEFBuistASCoxFMFerriePKKingDRValidation of a standardized version of the asthma quality of life questionnaireChest19991151265127010334138
- JuniperEFGuyattGHEpsteinRSFerriePJJaeschkeRHillerTKEvaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trialsThorax199247276831549827
- JuniperEFGuyattGHWillanAGriffithLEDetermining a minimal important change in a disease-specific Quality of Life QuestionnaireJ Clin Epidemiol199447181878283197
- JuniperEFGuyattGHFeenyDHFerriePJGriffithLETownsendMMeasuring quality of life in children with asthmaQual Life Res19965135468901365
- JuniperEFGuyattGHFeenyDHFerriePJGriffithLETownsendMMeasuring quality of life in the parents of children with asthmaQual Life Res19965127348901364
- JansonCGislasonTBomanGHettaJRoosBESleep disturbances in patients with asthmaRepir Med19908413742
- HaysRDStewardALSleep measures: definitions and issuesStewardALWareJEMeasuring Functioning and Well-being: The Medical Outcomes Study ApproachDurham, NCDuke University Press1992235259
- FDA Drug Safety Communication: New safety requirements for long-acting inhaled asthma medications called Long-Acting Beta-Agonists (LABAs)2010218 Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm200776.htm. Accessed Aug 13 2010.
- FDA Drug Safety Communication: Drug labels now contain updated recommendations on the appropriate use of long-acting inhaled asthma medications called Long-Acting Beta-Agonists (LABAs)201062 Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm213836.htm. Accessed Aug 13 2010.
- LevensonMLong-acting beta-agonists and adverse asthma events meta-analysis: statistical briefing package for joint meeting of the Pulmonary-Allergy Drugs Advisory Committee, Drug Safety and Risk Management Advisory Committee, and Pediatric Advisory Committee on 2008 Dec 10–11 http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4398b1-01-FDA.pdf. Accessed Aug 13 2010.
- NelsonHSWeissSTBleeckerERYanceySWDorinskyPMThe salmeterol multicenter asthma research trialChest20061291152616424409
- CastleWFullerRHallJPalmerJSerevent nationwide surveillance study: comparison of salmeterol with salbutamol in asthmatic patients who require regular bronchodilator treatmentBMJ19933066884103410378098238
- CatesCJCatesMJRegular treatment with salmeterol for chronic asthma: serious adverse eventsCochrane Database Syst Rev.20087163CD00636318646149
- BatemanENelsonHBousquetJMeta-analysis: effects of adding salmeterol to inhaled corticosteroids on serious asthma-related eventsAnn Intern Med20081491334218523132
- SearsMROttossonARadnerFLong-acting beta-agonists: a review of formoterol safety data from asthma clinical trialsEur Respir J2009331213218768573
- NelsonHSBonuccelliCRadnerFSafety of formoterol in patients with asthma: combined analysis of data from double-blind, randomized controlled trialsJ Allergy Clin Immunol2010125239039620159250
- SalpeterSRWallAJBuckleyNSLong-acting beta-agonists with and without inhaled corticosteroids and catastrophic asthma eventsAm J Med20101234322328.e220176343
- SalpeterSRBuckleyNSOrmistonTMSalpeterEEMeta-analysis: effect of long-acting β-agonists on severe asthma exacerbations and asthma-related deathsAnn Intern Med20061441290491216754916
- JaeschkeRO’ByrnePMMejzaFThe safety of long acting beta agonists among patients with asthma using inhaled corticosteroids: systematic review and meta-analysisAm J Respir Crit Care Med2008178101009101618776152
- PriceJFRadnerFLenneyWLindbergBSafety of formoterol in children and adolescents: experience from asthma clinical trialsArch Dis Child201095121047105321030368
- WijesingheMWeatherallMPerrinKHarwoodMBeasleyRRisk of mortality associated with formoterol: a systematic review and meta-analysisEur Respir J200934480381119797669
- ButlandBKAndersonHRCatesCJBronchodilator treatment and asthma death: a new analysis of a British case-control studyRespir Med20101027 [Epub ahead of print]
- ChowdhuryBADal PanGThe FDA and safe use of long-acting beta-agonists in the treatment of asthmaN Engl J Med2010362131169117120181964
- Global Initiative for AsthmaGlobal Strategy for Asthma Management and Prevention Updated 2009. Available at: http://www.ginasthma.org. Accessed Dec 13 2010.