Abstract
Aim
Negative symptoms of schizophrenia (NSS) have been linked with poor functional outcomes. A literature review was performed to identify instruments used to assess functional outcomes and quality of life in clinical trials and observational studies conducted in groups of people with NSS.
Methods
Literature search strings were designed using Medical Subject Headings combined with free-text terms and searches were performed using the PubMed, Embase and the Cochrane Library databases. For inclusion, articles were required to be published as full-text articles, in English, over the period 2011–2021, include at least one group or treatment arm of people with NSS and report either functional outcomes or quality of life (QoL).
Results
Literature searches identified a total of 3,268 unique hits. After two rounds of screening, 37 publications (covering 35 individual studies) were included in the review. A total of fourteen different instruments were used to assess functional outcomes and eleven different instruments were used to assess QoL. In studies in people with NSS, the most frequently used functional outcome measures were the Personal and Social Performance scale and the Global Assessment of Functioning. The most frequently used QoL instruments included the Manchester Short Assessment of Quality of Life, the Heinrich Carpenter Quality of Life Scale, the Schizophrenia Quality of Life Scale and the EQ-5D.
Conclusion
A large number of measures have been used to assess functional outcomes and QoL in people with NSS, these include both generic and condition-specific as well as both interviewer-administered and self-reported instruments.
Introduction
It is estimated that up to 60% of people with schizophrenia have negative symptoms to an extent that warrants treatment.Citation1 Negative symptoms of schizophrenia (NSS) include avolition, anhedonia, asociality, affective blunting/flattening and alogia.Citation2,Citation3 NSS may also be classified as either primary or secondary, with primary NSS being related to the disease process itself, whereas secondary NSS are related to other factors such as the presence of depression, social isolation or deprivation, or side effects of medication.Citation4 Currently available pharmacologic treatments are effective in terms of reducing the positive symptoms of schizophrenia (eg, hallucinations, delusions); however, NSS typically respond poorly to currently available treatmentsCitation3 and the effective management of NSS is acknowledged as an unmet need.Citation5 The use of measures that can quantify the magnitude and extent of NSS is important in terms of determining the severity of NSS and also in quantifying the treatment effects of interventions targeting NSS; however, there are several factors that may make the measurement of NSS (and treatment effects targeting NSS) challenging. For example, it may be difficult to delineate primary versus secondary NSSCitation1 and there may be potential for inter-observer variability in terms of quantifying NSS, although this may be reduced with training.Citation6
NSS can have a detrimental effect on everyday life and have been linked to poor quality of life (QoL) and impaired functional outcomes.Citation7,Citation8 Functional outcomes include activities of daily living, social relationships and QoLCitation9 and a greater severity of NSS has been linked with worse functional outcomes.Citation8 An improvement in functional outcomes is also increasingly recognized as an important metric in terms of recovery.Citation10
Several measures are available to assess QoL and functional outcomes, which may be of interest to investigators examining the efficacy of new interventions. These can be either condition-specific or generic measures and can be either self-reported or interviewer-administered, each of which are associated with relative merits and limitations. Given the importance of functional outcomes and the intricate association between NSS and functional outcomes, a literature review was conducted to identify measures that have been used to assess functional outcomes and QoL endpoints in clinical trials and observational studies conducted in people with NSS. The unmet clinical need for efficacious treatments specifically targeting NSS provided part of the rationale for focusing exclusively on studies conducted in people with NSS.
Methods
Literature search strategies were designed using high-level Medical Subject Headings (MeSH) terms combined with free-text terms and searches were run using the PubMed, Embase and Cochrane Library databases in June 2021. Full details of the search strategies for each database are available in the Supplementary Tables 1–3. The timeframe of the searches was limited to the previous 10 years and studies were required to be published in English as full-text articles.
Table 1 Summary of Included Publications
Table 2 Functional Outcome Measures Identified in the Literature Review
Table 3 Quality of Life Measures Identified in the Literature Review
Inclusion/exclusion criteria were developed. Specifically, for inclusion, studies were required to have at least one treatment arm or group of people with NSS (either by stating that patients had NSS or applying a minimum threshold level for NSS on a recognized scale, including but not limited to the Positive and Negative Syndrome Scale [PANSS], Scale for the Assessment of Negative Symptoms [SANS] or the Negative Symptom Assessment 16 [NSA-16]). The focus of the review was on studies where the presence of NSS was a prerequisite for inclusion rather than studies investigating the efficacy/effectiveness of pharmacologic or non-pharmacologic interventions in terms of a reduction in NSS as measured by scales such as the PANSS or SANS. Studies were also required to be conducted exclusively in people with schizophrenia; studies conducted in mixed groups of people with schizophrenia and other affective or non-affective disorders (eg, schizoaffective disorder, bipolar disorder, delusional disorder, etc.) were excluded. For inclusion, studies were also required to report at least one QoL or functional outcome endpoint, either as a primary, secondary or exploratory/ancillary endpoint. No exclusion criteria relating to type of intervention were applied; as such, studies on pharmacologic agents, non-pharmacologic interventions and psychological interventions were eligible for inclusion.
Search results from all three databases were uploaded into the web-based systematic literature review software Sourcerer (https://sourcerer.pro; Covalence Research Ltd, Harpenden, UK). Duplicates were removed and first-round screening of titles and abstracts was performed independently by two reviewers. Full-text second round screening and data extraction of relevant articles were then performed.
Results
Literature searches across all three databases identified a total of 4,297 hits, of which 1,029 were duplicates, resulting in a total of 3,268 unique hits. A total of 3,132 hits were excluded during first-round title and abstract screening and a further 99 were excluded during second-round full-text screening. Consequently, a total of 37 articles, covering 35 individual studies were included in the review ().
Figure 1 Summary of literature review process.
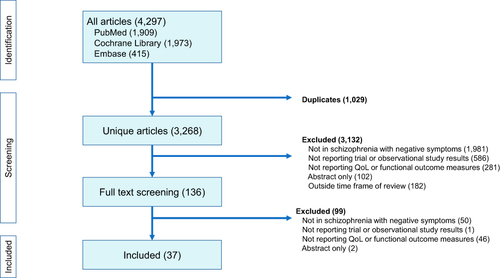
The studies identified were heterogeneous in terms of the sample group, with a small number of studies (n=3) conducted exclusively in inpatients but the majority were either conducted in outpatients or did not state whether the study group comprised inpatients or outpatients (). The definitions and threshold levels used to define the presence of negative symptoms were also heterogenous although the most commonly used method was to apply a minimum score on either the PANSS negative subscale (items N1–N7 of the PANSS scale) or the PANSS negative symptom factor (Marder) score (NSFS; items N1, N2, N3, N4, N6, G7 and G16 on the PANSS scale) (Supplementary Table 4). For the PANSS, the minimum negative symptom scores required for study entry ranged from ≥15Citation25 to ≥24Citation19,Citation20 although a score of ≥20 was the most commonly applied threshold using the PANSS negative symptom scores (see Supplementary Table 4). Other criteria used to define NSS included minimum scores on the SANS or Proxy for Deficit Syndrome.
Functional Outcome Assessment Measures
A total of fourteen different functional outcome measures were identified across the included studies (). A further two studies included functional outcome as an endpoint measure but did not use a specific instrument to assess this. Instead, Novick et alCitation33 assessed the level of social functioning by quantifying the number of activities performed with friends/social groups in the preceding 4 weeks and Liu et alCitation31 classified patients as either high or low functioning based on assessment of several factors including personal relationships, family life, achievement and time planning. In the included articles, functional outcome was typically either a secondary or exploratory/ancillary endpoint.
The instruments used to assess functional outcomes included both self-reported and interviewer-administered measures as well as measures specifically designed for use in mental health (eg, the Personal and Social Performance Scale [PSP], Social and Occupational Functioning Assessment Scale [SOFAS] and Global Assessment of Functioning [GAF]) as well as more generic measures of functioning such as the Sheehan Disability Scale (SDS) and the World Health Organization Disability Assessment Scale 2.0 (WHODAS 2.0).
The most frequently used measure was the PSP, which was used in a total of eleven different publications covering ten individual studies.Citation14–20,Citation24,Citation25,Citation39,Citation44 The PSP was developed from the SOFAS, which was in turn developed from the GAF. The PSP was developed by Morosini et alCitation48 to improve and overcome some of the limitations of the GAF and is therefore similar in structure to both the SOFAS and the GAF. It is reported to have better face validity relative to the SOFAS.Citation48 The PSP is an investigator-reported measure (a self-reported version is also available)Citation49 that takes approximately 5−10 minutes to complete and assesses functioning across four different areas (socially useful activities, personal and social relationships, self-care, and disturbing and aggressive behaviors). The output of the PSP is a single score ranging from 0 to 100, with a higher score indicating a higher level of functioning.Citation48 Validation studies of the PSP have been performed across a number of different countries and patient groups, including those with stable schizophrenia, acute schizophrenia, inpatients and outpatients and both adults and adolescents. The PSP has consistently demonstrated satisfactory psychometric properties across validation studies.Citation50–58
For functional outcomes, the secondly most commonly used instrument identified was the GAF, which was used in a total of seven publicationsCitation21–23,Citation28,Citation29,Citation42,Citation46 covering five individual studies. However, in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) released in 2013, revisions included the removal of the GAF as an assessment of functioning, with the WHODAS 2.0 now recommended instead.Citation59 Despite this recommendation, the review only identified one article that cited the use of the WHODAS 2.0 (Bryl et al 2020),Citation12 although this may partially reflect the fact that the time frame of the review means that many of the included trials may have been designed prior to the publication of this recommendation.
Three articles identified in the review assessed functional outcome using the brief version of the University of California San Diego Performance-Based Skills Assessment (UPSA).Citation11,Citation44,Citation47 The UPSA brief version was developed by Mausbach et alCitation60 who describe it as a “performance-based functional outcome measure”. The participant is requested to role-play a number of everyday tasks such as making an appointment or paying a bill and the brief version takes around 10−15 minutes to complete. However, the UPSA may be considered as a measure of functional capacity (the ability to perform a task) rather than a measure of functional outcome (whether the task is performed in a real-world environment).Citation61 Further, functional capacity may not always be an accurate reflection of functional performance.Citation62 However, one advantage of this type of measurement is that it is not subject to recall bias.Citation63
Two articles (covering one study) reported using three different functional outcome measures, which were the Objective Social Outcomes Index, Social Network Schedule and the Time Use Survey.Citation35,Citation36 The use of the Sheehan Disability Scale was also reported in two articles (covering three studies).Citation12,Citation15 The use of seven further measures was reported in one article each (the Global Assessment Scale [GAS],Citation41 the Index of Functioning,Citation38 the SOFAS,Citation30 the Social Functioning Scale [SFS],Citation40 the Social Skills Inventory,Citation27 the WHODAS 2.0,Citation12 and the Work and Social adjustment Scale).Citation32
Quality of Life Assessment Measures
The literature review identified a total of eleven different measures of QoL that have been used in studies in people with NSS (). The identified measures included both generic scales such as the EQ-5D as well as measures more specific to schizophrenia such as the Heinrich Carpenter Quality of Life Scale (QLS) and the Schizophrenia Quality of Life Scale (SQLS). The most frequently used measures of QoL identified in the review included the Manchester Short Assessment of Quality of Life (MANSA), the use of which was reported in four articles covering two studies.Citation35–37,Citation40, the QLS (reported in three articles covering six studies),Citation13,Citation23,Citation26 with an abbreviated version of the QLS developed by Bilker et alCitation64 used in one further article,Citation11 the EQ-5D (reported in three articles covering two studies),Citation35,Citation36,Citation44 the SQLS (reported in three articles covering a single study)Citation16–18 and the long- and short-form versions of the Subjective Well Being Under Neuroleptic TreatmentCitation34,Citation44 (used in one study each). Of these, the MANSA, QLS and the SLQS are specific to people with schizophrenia. The MANSA was developed by Priebe et al as an abbreviated and modified version of the Lancashire Quality of Life Profile. It is an interviewer-administered scale that consists of sixteen questions and takes approximately 3–5 minutes to administer.Citation65 The QLS, developed by Heinrichs et al, is also an interviewer-administered scale and takes approximately 45 minutes to administer.Citation66 It consists of 21 items covering four areas (interpersonal relations, instrumental role, intrapsychic foundations and common objects and activities) and is aimed primarily at outpatients as it contains items relating to work. The SQLS was developed by Wilkinson et al and is a 30-item self-reported questionnaire that takes approximately 5–10 minutes to complete.Citation67 In contrast, the EQ-5D is a generic measure that assesses QoL across five domains (mobility, self-care, usual activities, pain/discomfort and anxiety/depression). Two versions of the EQ-5D exist, the EQ-5D-3L, wherein responses are measured on a three-point scale, and the EQ-5D-5L, wherein responses are measured on a 5-point scale. The 5L version was introduced in 2009 with the aim of improving sensitivity and reducing ceiling effects.Citation68 EQ-5D validation studies have been performed in mental health conditions including schizophrenia. The authors of a 2004 validation study reported that the 3L version showed “acceptable construct validity” in people with schizophrenia.Citation69 However, validation studies have also suggested that there may be a ceiling effect in the EQ-5D index score, suggesting that it may have limited sensitivity in those with less severe disease.Citation70
The other QoL measures identified in the review were the Psychological General Well-Being Schedule,Citation43 World Health Organization Quality of life Scale Brief Version,Citation45 the Quality of Life Enjoyment and Satisfaction Questionnaire,Citation15 SF-12,Citation38 and the SF-6D.Citation38
Discussion
Patient-reported outcomes including functional outcome and QoL are increasingly recognized as important endpoints.Citation71 In people with NSS, a greater severity of negative symptoms has been linked with worse functional outcomeCitation8 and there has been some discussion as to whether functional outcome should be included as a co-primary endpoint in trials of interventions targeting NSS.Citation72 In the studies identified in the current review functional outcome was typically presented as a secondary, exploratory or ancillary endpoint rather than a primary endpoint.
Decisions relating to which instruments to use to assess functional outcomes and QoL may be influenced by several factors including, the recall period, the total time required for completion, whether the instrument is generic or disease-specific and whether it is self-reported or interviewer-administered. With regard to the choice of self-reported versus interviewer-reported measures, some advantages associated with self-reported measures include low cost and low time requirements for investigators. However, limitations include the risk of recall bias, particularly over longer recall periodsCitation73 and reliability.Citation74 A strength of interviewer-administered questionnaires is higher response ratesCitation75 but limitations include the potential for inter-rater variability and longer time requirements. For example, the full version of the UPSA may take up to 30 minutes to complete and such a lengthy time requirement may limit the practicality of such measures for large studies. Additionally, instruments used to assess QoL and functional outcome may be either generic or specific to particular conditions. The use of generic measures allows comparison with other disease areas, and some generic QoL instruments such as the EQ-5D or SF-6D allow utility scores to be determined, which can then be used to inform health economic modeling analyses. However, a limitation of generic measures is that they may not be nuanced enough to detect small but meaningful changes in signs or symptoms specific to particular conditions.Citation76
An earlier review by Burns and Patrick (2007)Citation77 also investigated functional outcome measures used in schizophrenia studies (not limited to individuals with NSS), published over the period 1990–2006. Burns and Patrick identified a total of 87 different measures used across a total of 301 studies with the GAF, GAS and SFS being the most frequently used measures. The PSP was only used in three studies included in the earlier review; however, this likely reflects the timeframe of the review, which largely covered a period prior to the introduction of the PSP in 2001. Burns and Patrick also noted that “a striking lack of data on psychometric properties was observed”. This situation is now being remedied as validation studies have become available for the majority of measures identified in the present review, although the number of published validation studies varied considerably between different measures. However, validation studies specific to groups of people with prominent NSS are still lacking.
Another review by Karow et alCitation78 characterized QoL measures used in schizophrenia studies published over the period 2009–2013. Karow et al identified a total of 35 different QoL measures used across a total of 432 studies. In this earlier review, the most commonly used condition-specific measures were the Heinrich Carpenter Quality of Life Scale and the Q-LES-Q-18. The most frequently used generic measures were the WHOQOL-BREF, SF-36 and SF-12. Notably, Karow et al reported that over 50% of the studies included in their review listed QoL as a primary endpoint. This contrasts with the findings of the current review wherein QoL was typically reported as a secondary or exploratory/ancillary endpoint. In another more recent review, Azaiez et alCitation79 identified a total of nineteen different QoL measures used across schizophrenia studies. However, they noted that none of the studies included in the review were specific to people with NSS and also highlighted a lack of validation studies for QoL measures specifically in groups of people with NSS.
The focus of the present review was to identify instruments used to measure QoL and functional outcomes in studies conducted in people with NSS. However, ecological momentary assessment (EMA) methods, also known as experience sampling assessment have been used to assess day-to-day activity and functioning in people with schizophrenia. EMA typically utilizes smartphones or wearable activity trackers to either passively or actively monitor activity. Passive monitoring involves the discreet collection of data such as GPS data whereas active monitoring involves collection of self-reported data via questions relating to current or recent activities, interactions or mood. As such, EMA can provide real-time information relating to a number of different aspects of functioning and daily life (eg, social activities, employment or education, self-care and home care).Citation80 A key strength of EMA is that it largely overcomes the recall bias that self-reported measures are susceptible to, as these can require the respondent to accurately recall events over the preceding week or month.Citation81 In a 2020 study, Granholm et alCitation80 used a smartphone-based system to ask questions (at a frequency of seven times per day) relating to activities and functioning in the previous hour. The questions posed included activities such as shopping, using a bank or ATM, grooming, and interactions with family, friends or colleagues. The authors reported that EMA was a reliable and valid method for assessing functioning in people with schizophrenia.Citation80 However, limitations of EMA are that active EMA requires the cooperation of the respondent, potentially several times per day, for the duration of the study,Citation81 and also, EMA alone may not sufficient to capture subtle changes or nuances of NSS. Lopez-Morinigo et alCitation82 also draw attention to the role of monetary incentives in terms of influencing acceptability. Lopez-Morinigo et al also reported that in their study, which offered no monetary incentive for responses, the acceptability of EMA in people with schizophrenia spectrum disorders was low at just 31%, and that acceptability was influenced by age, educational level, early adolescent premorbid adjustment, insight and executive functioning.Citation82
Overall, the findings of the review show that in studies conducted in people with NSS and published from 2011 onwards a total of fourteen different instruments used to assess functional outcomes and eleven different measures used to assess QoL were identified. The large number of measures available permits study designers to select an instrument that meets the requirements of the study. For example, whether to use a generic scale to allow greater comparability or a condition-specific scale that is more nuanced and may have greater sensitivity in terms of changes in condition-specific signs and symptoms. Similarly, if time constraints are a factor, study designers may opt for either self-reported measures or abbreviated versions of interviewer-rated scales. The current review showed that the PSP was the most frequently used functional outcome measure used in recent studies conducted in people with NSS. Further, multiple linguistic and cultural validation studies of the PSP report that it has satisfactory psychometric properties. Similarly, the most frequently used measures for assessing QoL including the MANSA, the QLS, the SQLS and the EQ-5D. The most extensively validated QoL measure specific to people with schizophrenia is the SQLS. However, across both functional outcome and QoL measures there is a general paucity of validation studies specific to groups of people with prominent NSS.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, have provided final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
DHB, SH and DB are current employees of Otsuka Pharmaceutical Development & Commercialization Inc. JSP, JP and RFP are current employees of Covalence Research Ltd. Covalence Research Ltd received consulting fees from Otsuka Pharmaceutical Development & Commercialization Inc to undertake the systematic literature review and prepare the current manuscript. The authors report no other conflicts of interest in this work.
Acknowledgments
This work has been presented in abstract/poster format at the 2022 Psych Congress, 17–20 September, 2022, New Orleans, USA.
Additional information
Funding
References
- Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. 2020;16:519–534.
- Foussias G, Agid O, Fervaha G, Remington G. Negative symptoms of schizophrenia: clinical features, relevance to real world functioning and specificity versus other CNS disorders. Eur Neuropsychopharmacol. 2014;24:693–709.
- Veerman SRT, Schulte PFJ, de Haan L. Treatment for negative symptoms in schizophrenia: a comprehensive review. Drugs. 2017;77:1423–1459.
- Mosolov SN, Yaltonskaya PA. Primary and secondary negative symptoms in Schizophrenia. Front Psychiatry. 2022;12:766692.
- Marder SR, Galderisi S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry. 2017;16:14–24.
- Gupta M, Holshausen K, Gou L, Bowie C. Measuring negative symptom change in schizophrenia: considering alternatives to self-report. Expert Rev Neurother. 2014;14(8):911–922.
- Kirkpatrick B, Fenton WS, Carpenter WT, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–219.
- Fervaha G, Foussias G, Agid O, Remington G. Impact of primary negative symptoms on functional outcomes in schizophrenia. Eur Psychiatry. 2014;29:449–455.
- Andreasen NC, Carpenter WT, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162:441–449.
- Vita A, Barlati S. Recovery from schizophrenia: is it possible? Curr Opin Psychiatry. 2018;31:246–255.
- Beck AT, Grant PM, Huh GA, Perivoliotis D, Chang NA. Dysfunctional attitudes and expectancies in deficit syndrome schizophrenia. Schizophr Bull. 2013;39:43–51.
- Bryl K, Bradt J, Cechnicki A, Fisher K, Sossin KM, Goodill S. The role of dance/movement therapy in the treatment of negative symptoms in schizophrenia: a mixed methods pilot study. J Ment Health. 2020;1:1–11.
- Buchanan RW, Panagides J, Zhao J, et al. Asenapine versus olanzapine in people with persistent negative symptoms of schizophrenia. J Clin Psychopharmacol. 2012;32:36–45.
- Bugarski-Kirola D, Blaettler T, Arango C, et al. Bitopertin in negative symptoms of schizophrenia-results from the Phase III flashlyte and daylyte studies. Biol Psychiatry. 2017;82:8–16.
- Dunayevich E, Buchanan RW, Chen CY, et al. Efficacy and safety of the glycine transporter type-1 inhibitor AMG 747 for the treatment of negative symptoms associated with schizophrenia. Schizophr Res. 2017;182:90–97.
- Edgar CJ, Blaettler T, Bugarski-Kirola D, Le Scouiller S, Garibaldi GM, Marder SR. Reliability, validity and ability to detect change of the PANSS negative symptom factor score in outpatients with schizophrenia on select antipsychotics and with prominent negative or disorganized thought symptoms. Psychiatry Res. 2014;218:219–224.
- Rofail D, Regnault A, le Scouiller S, Berardo CG, Umbricht D, Fitzpatrick R. Health-related quality of life in patients with prominent negative symptoms: results from a multicenter randomized Phase II trial on bitopertin. Qual Life Res. 2016;25:201–211.
- Umbricht D, Alberati D, Martin-Facklam M, et al. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: a randomized, double-blind, proof-of-concept study. JAMA Psychiatry. 2014;71:637–646.
- Fleischhacker W, Galderisi S, Laszlovszky I, et al. The efficacy of cariprazine in negative symptoms of schizophrenia: post hoc analyses of PANSS individual items and PANSS-derived factors. Eur Psychiatry. 2019;58:1–9.
- Németh G, Laszlovszky I, Czobor P, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet. 2017;389:1103–1113.
- Hasan A, Wobrock T, Guse B, et al. Structural brain changes are associated with response of negative symptoms to prefrontal repetitive transcranial magnetic stimulation in patients with schizophrenia. Mol Psychiatry. 2017;22:857–864.
- Wobrock T, Guse B, Cordes J, et al. Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: a sham-controlled, randomized multicenter trial. Biol Psychiatry. 2015;77:979–988.
- Hill M, Shannahan K, Jasinski S, et al. Folate supplementation in schizophrenia: a possible role for MTHFR genotype. Schizophr Res. 2011;127:41–45.
- Hirayasu Y, Sato S, Takahashi H, et al. A double-blind randomized study assessing safety and efficacy following one-year adjunctive treatment with bitopertin, a glycine reuptake inhibitor, in Japanese patients with schizophrenia. BMC Psychiatry. 2016;16:66.
- Kane JM, Yang R, Youakim JM. Adjunctive armodafinil for negative symptoms in adults with schizophrenia: a double-blind, placebo-controlled study. Schizophr Res. 2012;135:116–122.
- Kaphzan H, Ben-Shachar D, Klein E. Entacapone augmentation of antipsychotic treatment in schizophrenic patients with negative symptoms; a double-blind placebo-controlled study. Int J Neuropsychopharmacol. 2014;17:337–340.
- Kayo M, Scemes S, Savoia MG, et al. A randomized controlled trial of social skills training for patients with treatment-resistant schizophrenia with predominantly negative symptoms. Psychiatry Res. 2020;287:112914.
- Klingberg S, Wölwer W, Engel C, et al. Negative symptoms of schizophrenia as primary target of cognitive behavioral therapy: results of the randomized clinical TONES study. Schizophr Bull. 2011;37(Suppl 2):S98–110.
- Klingberg S, Herrlich J, Wiedemann G, et al. Adverse effects of cognitive behavioral therapy and cognitive remediation in schizophrenia: results of the treatment of negative symptoms study. J Nerv Ment Dis. 2012;200:569–576.
- Levkovitz Y, Rabany L, Harel EV, Zangen A. Deep transcranial magnetic stimulation add-on for treatment of negative symptoms and cognitive deficits of schizophrenia: a feasibility study. Int J Neuropsychopharmacol. 2011;14:991–996.
- Liu CC, Chen CH, Hwu HG, et al. Medium-term course and outcome of schizophrenia depicted by the sixth-month subtype after an acute episode. J Formos Med Assoc. 2012;111:265–274.
- Mairs H, Lovell K, Campbell M, Keeley P. Development and pilot investigation of behavioral activation for negative symptoms. Behav Modif. 2011;35:486–506.
- Novick D, Montgomery W, Treuer T, Moneta MV, Haro JM. Real-world effectiveness of antipsychotics for the treatment of negative symptoms in patients with schizophrenia with predominantly negative symptoms. Pharmacopsychiatry. 2017;50:56–63.
- Palm U, Keeser D, Hasan A, et al. Prefrontal transcranial direct current stimulation for treatment of schizophrenia with predominant negative symptoms: a double-blind, sham-controlled proof-of-concept study. Schizophr Bull. 2016;42:1253–1261.
- Priebe S, Savill M, Wykes T, et al. Effectiveness of group body psychotherapy for negative symptoms of schizophrenia: multicentre randomised controlled trial. Br J Psychiatry. 2016;209:54–61.
- Priebe S, Savill M, Wykes T, et al.; NESS team. Clinical effectiveness and cost-effectiveness of body psychotherapy in the treatment of negative symptoms of schizophrenia: a multicentre randomised controlled trial. Health Technol Assess. 2016;20(11):vii–xxiii, 1–100.
- Savill M, Orfanos S, Reininghaus U, Wykes T, Bentall R, Priebe S. The relationship between experiential deficits of negative symptoms and subjective quality of life in schizophrenia. Schizophr Res. 2016;176:387–391.
- Rabinowitz J, Berardo CG, Bugarski-Kirola D, Marder S. Association of prominent positive and prominent negative symptoms and functional health, well-being, healthcare-related quality of life and family burden: a CATIE analysis. Schizophr Res. 2013;150:339–342.
- Rabinowitz J, Badescu S, Palamarchuk P, et al. Personal and social adjustment effects of roluperidone in patients with schizophrenia and negative symptoms: results from an exploratory outcome of a randomized placebo-controlled trial. Schizophr Res. 2019;211:103–104.
- Röhricht F, Clarke T, Priebe S. Therapeutic processes and clinical outcomes of body psychotherapy in chronic schizophrenia – an open clinical trial. Arts Psychotherapy. 2011;38:196–203.
- Schaefer M, Sarkar S, Theophil I, Leopold K, Heinz A, Gallinat J. Acute and long-term memantine add-on treatment to risperidone improves cognitive dysfunction in patients with acute and chronic schizophrenia. Pharmacopsychiatry. 2020;53:21–29.
- Schoemaker JH, Jansen WT, Schipper J, Szegedi A. The selective glycine uptake inhibitor org 25935 as an adjunctive treatment to atypical antipsychotics in predominant persistent negative symptoms of schizophrenia: results from the GIANT trial. J Clin Psychopharmacol. 2014;34:190–198.
- Shoja Shafti S, Akbari S. Intractability of deficit syndrome of schizophrenia against adjunctive modafinil. J Clin Psychopharmacol. 2016;36:45–49.
- Stauffer VL, Millen BA, Andersen S, et al. Pomaglumetad methionil: no significant difference as an adjunctive treatment for patients with prominent negative symptoms of schizophrenia compared to placebo. Schizophr Res. 2013;150:434–441.
- Sum MY, Tay KH, Sengupta S, Sim K. Neurocognitive functioning and quality of life in patients with and without deficit syndrome of schizophrenia. Psychiatry Res. 2018;263:54–60.
- Valiengo LDCL, Goerigk S, Gordon PC, et al. Efficacy and safety of transcranial direct current stimulation for treating negative symptoms in schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77:121–129.
- Walling D, Marder SR, Kane J, et al. Phase 2 Trial of an Alpha-7 Nicotinic Receptor Agonist (TC-5619) in negative and cognitive symptoms of Schizophrenia. Schizophr Bull. 2016;42:335–343.
- Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101:323–329.
- Bai YM, Hsiao CY, Chen KC, et al. The development of a self-reported scale for measuring functionality in patients with schizophrenia--self-reported version of the graphic Personal and Social Performance (SRG-PSP) scale. Schizophr Res. 2014;159:546–551.
- Brissos S, Palhavã F, Marques JG, et al. The Portuguese version of the Personal and Social Performance Scale (PSP): reliability, validity, and relationship with cognitive measures in hospitalized and community schizophrenia patients. Soc Psychiatry Psychiatr Epidemiol. 2012;47:1077–1086.
- Wu BJ, Lin CH, Tseng HF, et al. Validation of the Taiwanese Mandarin version of the Personal and Social Performance scale in a sample of 655 stable schizophrenic patients. Schizophr Res. 2013;146:34–39.
- Apiquian R, Elena Ulloa R, Herrera-Estrella M, et al. Validity of the Spanish version of the Personal and Social Performance scale in schizophrenia. Schizophr Res. 2009;112:181–186.
- Kawata AK, Revicki DA. Psychometric properties of the Personal and Social Performance scale (PSP) among individuals with schizophrenia living in the community. Qual Life Res. 2008;17:1247–1256.
- Garcia-Portilla MP, Saiz PA, Bousoño M, Bascaran MT, Guzmán-Quilo C, Bobes J. en nombre del grupo de validación de la versión española de la escala de Funcionamiento Personal y Social (PSP). Validation of the Spanish Personal and Social Performance scale (PSP) in outpatients with stable and unstable schizophrenia. Rev Psiquiatr Salud Ment. 2011;4:9–18.
- Juckel G, Schaub D, Fuchs N, et al. Validation of the Personal and Social Performance (PSP) Scale in a German sample of acutely ill patients with schizophrenia. Schizophr Res. 2008;104:287–293.
- Nafees B, van Hanswijck de Jonge P, Stull D, et al. Reliability and validity of the Personal and Social Performance scale in patients with schizophrenia. Schizophr Res. 2012;140:71–76.
- Patrick DL, Burns T, Morosini P, et al. Reliability, validity and ability to detect change of the clinician-rated Personal and Social Performance scale in patients with acute symptoms of schizophrenia. Curr Med Res Opin. 2009;25:325–338.
- Tianmei S, Liang S, Yun’ai S, et al. The Chinese version of the Personal and Social Performance Scale (PSP): validity and reliability. Psychiatry Res. 2011;185:275–279.
- Gspandl S, Peirson RP, Nahhas RW, Skale TG, Lehrer DS. Comparing Global Assessment of Functioning (GAF) and World Health Organization Disability Assessment Schedule (WHODAS) 2.0 in schizophrenia. Psychiatry Res. 2018;259:251–253.
- Mausbach BT, Harvey PD, Goldman SR, Jeste DV, Patterson TL. Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophr Bull. 2007;33:1364–1372.
- Heinrichs RW, Statucka M, Goldberg J, McDermid Vaz S. The University of California Performance Skills Assessment (UPSA) in schizophrenia. Schizophr Res. 2006;88:135–141.
- Uchino T, Nemoto T, Yamaguchi T, et al. Associations of personality traits with the capacity-performance discrepancy of functional outcome in patients with schizophrenia. Neuropsychiatr Dis Treat. 2019;15:2869–2877.
- Bromley E, Brekke JS. Assessing function and functional outcome in schizophrenia. Curr Top Behav Neurosci. 2010;4:3–21.
- Bilker WB, Brensinger C, Kurtz MM, et al. Development of an abbreviated schizophrenia quality of life scale using a new method. Neuropsychopharmacology. 2003;28:773–777.
- Priebe S, Huxley P, Knight S, Evans S. Application and results of the Manchester Short Assessment of Quality of Life (MANSA). Int J Soc Psychiatry. 1999;45:7–12.
- Heinrichs DW, Hanlon TE, Carpenter WT. The quality of life scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10:388–398.
- Wilkinson G, Hesdon B, Wild D, et al. Self-report quality of life measure for people with schizophrenia: the SQLS. Br J Psychiatry. 2000;177:42–46.
- EUROQOL. EuroQol instruments. Available from: https://euroqol.org/information-and-support/euroqol-instruments/. Accessed May 29, 2024.
- Prieto L, Sacristán JA, Hormaechea JA, Casado A, Badia X, Gómez JC. Psychometric validation of a generic health-related quality of life measure (EQ-5D) in a sample of schizophrenic patients. Curr Med Res Opin. 2004;20:827–835.
- König HH, Roick C, Angermeyer MC. Validity of the EQ-5D in assessing and valuing health status in patients with schizophrenic, schizotypal or delusional disorders. Eur Psychiatry. 2007;22:177–187.
- Cruz Rivera S, McMullan C, Jones L, Kyte D, Slade A, Calvert M. The impact of patient-reported outcome data from clinical trials: perspectives from international stakeholders. J Patient Rep Outcomes. 2020;4:51.
- Marder SR, Daniel DG, Alphs L, Awad AG, Keefe RS. Methodological issues in negative symptom trials. Schizophr Bull. 2011;37:250–254.
- Topp J, Andrees V, Heesen C, Augustin M, Blome C. Recall of health-related quality of life: how does memory affect the SF-6D in patients with psoriasis or multiple sclerosis? A prospective observational study in Germany. BMJ Open. 2019;9:e032859.
- Bellack AS, Green MF, Cook JA, et al. Assessment of community functioning in people with schizophrenia and other severe mental illnesses: a white paper based on an NIMH-sponsored workshop. Schizophr Bull. 2007;33:805–822.
- Tsakos G, Bernabé E, O’Brien K, Sheiham A, de Oliveira C. Comparison of the self-administered and interviewer-administered modes of the child-OIDP. Health Qual Life Outcomes. 2008;6:40.
- Wiebe S, Guyatt G, Weaver B, Matijevic S, Sidwell C. Comparative responsiveness of generic and specific quality-of-life instruments. J Clin Epidemiol. 2003;56:52–60.
- Burns T, Patrick D. Social functioning as an outcome measure in schizophrenia studies. Acta Psychiatr Scand. 2007;116(6):403–418.
- Karow A, Wittmann L, Schöttle D, Schäfer I, Lambert M. The assessment of quality of life in clinical practice in patients with schizophrenia. Dialogues Clin Neurosci. 2014;16:185–195.
- Azaiez C, Millier A, Lançon C, et al. Health related quality of life in patients having schizophrenia negative symptoms - a systematic review. J Mark Access Health Policy. 2018;6(1):1517573.
- Granholm E, Holden JL, Mikhael T, et al. What do people with schizophrenia do all day? Ecological momentary assessment of real-world functioning in schizophrenia. Schizophr Bull. 2020;46:242–251.
- Fulford D, Mote J, Gonzalez R, et al. Smartphone sensing of social interactions in people with and without schizophrenia. J Psychiatr Res. 2021;137:613–662.
- Lopez-Morinigo JD, Barrigón ML, Porras-Segovia A, et al. Use of ecological momentary assessment through a passive smartphone-based app (eB2) by patients with schizophrenia: acceptability study. J Med Internet Res. 2021;23:e26548.
