Abstract
Platelets play a central role in atherothrombosis and subsequent development of acute coronary syndromes (ACS). The understanding of this process has driven a large body of evidence demonstrating the mortality and morbidity benefits of antiplatelet agents in the ACS population. As expected, however, these agents come with an intrinsically increased risk of bleeding which underlies the vast majority of their complications and adverse effects. In today’s setting of compounding comorbidities and broadening indications, finding the balance between thrombosis prevention and bleeding risk remains the challenge for all clinicians considering these medications. This article reviews the current main antiplatelet agents that are available for clinical use and outlines their impact on ACS outcome. We also outline factors which affect the response to these agents and discuss strategies to optimize clinical outcomes.
Introduction
Atherosclerosis is a progressive and systemic disease process potentially resulting in grave cardiovascular, neurological, and peripheral vascular complications. Following the spontaneous rupture of an atherosclerotic plaque during an acute coronary syndrome (ACS) or controlled endothelial disruption during percutaneous coronary intervention (PCI), platelets are simultaneously exposed to numerous agonists promoting the process of thrombus formation. The platelet undergoes a morphological change through the process of thrombus formation. Initially, the platelet adheres to the damaged subendothelial matrix via binding of glycoprotein Ib/IX to von Willebrand factor. Once adhered to the subcellular matrix, the platelet is activated by collagen via further glycoprotein receptors as well as by thrombin.Citation1 Finally, platelet aggregation is initiated by thromboxane A2 and adenosine diphosphate (ADP) with subsequent release of further aggregating factors from the platelet- dense granulesCitation2 resulting in a procoagulant surface required for clot formation. Given their capacity to ablate these above pathways (), antiplatelet agents have become the cornerstone of therapy in both ACS and PCI. The potential benefit on patient outcomes is proportional to the degree to which their current antithrombotic potential outweighs the associated current bleeding risk. In this review, we focus on the impact that antiplatelet agents, to date, have had on patient outcomes in ACS. We then address how clinicians and health systems can best utilize these agents to optimize patient outcomes.
Figure 1 Mechanism of action of current antiplatelet agents.Citation135
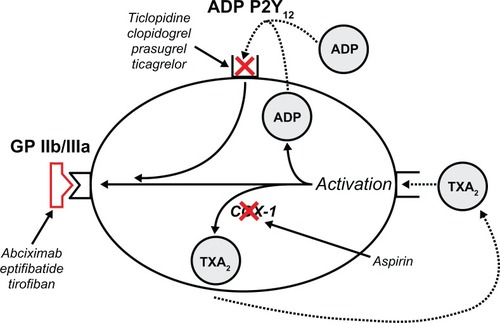
Antiplatelet agents
Aspirin
Aspirin has been the mainstay of antithrombotic therapy for many years. When used in doses of 75–300 mg, aspirin irreversibly acetylates serine 530 of cyclooxygenase-1 (COX-1), thereby permanently inhibiting platelet transformation of arachidonic acid (AA) into thromboxane A2, a potent inducer of platelet aggregation.Citation3 This COX-1-dependent pathway appears to be dose independent with maximal effect occurring at doses as low as 50 mg. Gurbel et al demonstrated a dose response in platelet aggregation in the presence of near-complete (AA) inhibition, suggesting that further antiplatelet effects could occur through COX-1-independent mechanisms.Citation4 Furthermore, some of aspirin’s benefit may occur downstream from platelet inhibition through mechanisms such as the enhancement of fibrin clot permeability and some weak anti-inflammatory activity and promotion of nitric oxide production in platelets.Citation5
Aspirin causes gastrointestinal (GI) side effects in a dose-dependent manner.Citation6 It is otherwise well tolerated with only a minority of patients experiencing side effects such as asthma (2%–4%),Citation7 rhinitis, urticaria, and angioedema (0.07%–0.2%).Citation8 The second International Study of Infarct Survival is the seminal aspirin trial which compared placebo with 160 mg aspirin daily for the treatment of acute myocardial infarction (MI).Citation9 For every 1000 patients, 1 month of at least 162 mg aspirin daily prevented 25 deaths and 10–15 nonfatal MI or strokes.Citation10 As aspirin blocks only one of several pathways implicated in platelet activation and aggregation, it is of no surprise that the majority of cardiovascular events are not prevented by aspirin.Citation11 To further improve patient outcomes, numerous antiplatelet agents blocking COX-1-independent pathways have been developed over the last three decades leading to further significant reductions in thrombotic events across the cardiovascular disease continuum.
ADP receptor antagonists
ADP is a platelet activator released from red blood cells, activated platelets, and damaged endothelial cells, which induces platelet adhesion and aggregation.Citation12 Adenine nucleotides interact with P2 receptors, which are distributed in many different cell types including endothelial, smooth muscle, and epithelial cells as well as in platelets. These receptors can be subdivided into the P2X ligand-gated ion channel and the two P2Y G protein-coupled receptors (P2Y1 and P2Y12) both of which have to be coactivated for normal ADP-induced platelet aggregation to occur.Citation12 A well-conducted in vitro study has shown that even in conditions of near complete P2Y12 inhibition by thienopyridines, ADP is still capable of inducing platelet conformational change and residual aggregation via the P2Y1 receptor.Citation13 The currently available ADP receptor antagonists i) ticlopidine, ii) clopidogrel, iii) prasugrel, and iv) ticagrelor will be discussed in detail.
Ticlopidine
Ticlopidine was the first commercially available thienopyridine-derivative ADP receptor antagonist gaining marketing approval in 1991. Its use increased significantly after numerous trials demonstrated the superiority of the combination of ticlopidine and aspirin in maintaining coronary stent patency following PCI.Citation14,Citation15 It is a prodrug that is metabolized in the liver into an active metabolite which irreversibly blocks the P2Y12 ADP receptor for the lifetime of the platelet (7–10 days). Clinically relevant antiplatelet activity at the standard dose (250 mg twice daily, oral) occurs at 24–48 h, peaking at 3–5 days. The unacceptably high incidence of GI side effects (30%–50% vomiting, nausea, and diarrhea) precluded the use of a higher loading dose (500 mg daily, oral).Citation16,Citation17 Neutropenia as a side effect of ticlopidine was first noted in phase III trials and was subsequently shown to be as high as 2.4%.Citation18 Furthermore, ticlopidine use was associated with aplastic anemia, thrombotic thrombocytopenic purpura, agranulocytosis, and pancytopenia. These sometimes turned fatal within the first 3 months, with a median recovery time of 15 days upon cessation of agent.Citation19 Hence, it is no surprise that ticlopidine was superseded by the second-generation thienopyridine derivative clopidogrel. A meta-analysis comparing the two agents showed that clopidogrel led to a reduction in major adverse cardiac events (MACE) including mortality, with better tolerability and a favorable side effect profile.Citation20 At present, use of ticlopidine is limited to cases of clopidogrel intolerance and in settings where the use of the newer antiplatelet agents may not be economically feasible. The use of ticlopidine requires 2-weekly blood counts during the first 3 months of therapy, although the optimal frequency and utility of subsequent monitoring are not well defined.
Clopidogrel
Like its predecessor, clopidogrel is a prodrug that requires hepatic cytochrome P450-dependent biotransformation into an active metabolite, which irreversibly blocks the P2Y12 ADP receptor. It undergoes intestinal absorption which is unaffected by food or antacids.Citation21,Citation22 Clopidogrel absorption is controlled by the ABCB1 gene, which exhibits genetic polymorphism and codifies for the intestinal P-glycoprotein multidrug resistance transporter (MDR1). The impact of polymorphism at this locus on overall platelet aggregation and patient outcomes remains controversial with two well-conducted studies showing conflicting results.Citation23,Citation24 Once it reaches the bloodstream, 85% of the parent drug is metabolized into an inactive form. The remaining 15% is metabolized via a two-step process with the participation of several CYP450 isoenzymes. The CYP2C19 isoenzyme is involved in both steps, and recent studies have shown a strong association between allelic variations at this locus and increased cardiovascular events despite clopidogrel treatment.Citation23–Citation27 Not surprisingly, the pharmacodynamic response to clopidogrel shows significant interpatient variability across a normal distribution.Citation28 Without the administration of a loading dose, maximal platelet inhibition occurs after 3–5 days at the standard oral daily dose of 75 mg. Loading doses of 300 and 600 mg result in maximal inhibition of platelet aggregation (IPA) at 6 and 2 h, respectively.Citation29 The minimum dose of clopidogrel required to maintain maximal platelet inhibition in most subjects is 60 mg; thus, the standard daily dose of 75 mg exposes patients to incomplete platelet inhibition if compliance is unreliable.Citation30
Clopidogrel has been extensively studied in both the non-ST elevation myocardial infarction (NSTEMI) and ST elevation myocardial infarction (STEMI) populations. The CURE study randomized 12,562 patients suffering from NSTE–ACS to receive aspirin and either clopidogrel (300/75 mg) or placebo for an average of 9 months.Citation31 Primary outcome (death, MI, or stroke at 12 months) was significantly less in the clopidogrel arm (9.3% vs 11.4%; relative risk [RR] = 0.8; P < 0.001), although at the expense of increased major (3.7% vs 2.7%; P = 0.001) and minor bleeding (5.1% vs 2.4%; P < 0.001). A subset of 2658 patients who underwent an invasive strategy was studied in PCI-CURE.Citation32 Despite significant crossover, composite endpoints of death, MI, or urgent target vessel revascularization within 30 days were 6.1% in the control group versus 3.5% in clopidogrel arm (P = 0.016) with similar bleeding outcomes. COMMIT/CCS-2Citation33 and CLARITY-TIMI 28Citation34 both confirmed the superiority of dual antiplatelet therapy (DAT) over aspirin monotherapy in STEMI patients.
Rates of clopidogrel-induced neutropenia in the early trials were extremely low varying between 0%Citation35 and 0.12%.Citation31 Thrombotic thrombocytopenic purpura,Citation36 suppression in all bone marrow lineages,Citation37 and various allergic reactionsCitation38,Citation39 have all been reported (rate < 0.1%) in association with clopidogrel use mostly occurring in the first month of therapy.
Prasugrel
The third-generation thienopyridine, prasugrel, is a prodrug whose active metabolite R-138727 irreversibly binds to the P2Y12 receptor. Its activation occurs in a two-step process with initial rapid hydrolysis to a thiolactone with a further conversion to its thiol-containing pharmacologically active metabolite R-138727 by oxidation via P450 cytochromes.Citation40 Absorption of prasugrel is decreased by factors which increase gastric pH. Coadministration with the proton pump inhibitor (PPI) lansoprazole, however, does not alter prasugrel’s efficacy as measured by IPA.Citation41 Furthermore, a US Food and Drug Administration (FDA) analysis suggested that antiacid use did not affect prasugrel’s clinical efficacy.Citation42
The maximal concentration of the active metabolite is seen after 30 min of oral dosing,Citation43 with maximal platelet inhibition occurring at 1 h with a 60-mg loading dose.Citation44 Prasugrel was found to be ∼10-fold more potent than clopidogrel in inhibiting thrombus formation and increasing bleeding time.Citation45 This pharmacodynamic superiority is most likely a consequence of the more extensive and rapid formation of the equipotent active metabolite.Citation46
The TRITON-TIMI 38 compared a 60-mg loading dose of prasugrel followed by 10 mg daily dosing with standard clopidogrel dosing in high-risk ACS patients undergoing PCI. Importantly, randomization only occurred once coronary anatomy was known; hence, the study did not test the two agents as upstream therapy given in the emergency department to ACS patients prior to proceeding to cardiac catheterization.Citation47 Prasugrel use resulted in a 19% relative risk reduction (9.9% for prasugrel vs 12.1% for clopidogrel; hazard ratio (HR) = 0.81; P < 0.001) for the composite primary efficacy endpoint of death from cardiovascular causes, nonfatal MI, or nonfatal stroke. This benefit occurred at the expense of an increase in the rate of noncoronary artery bypass graft (CABG)-related major bleeding (HR = 1.32; 95% confidence interval (CI): 1.03–1.68; number needed to harm (NNH) = 167; P = 0.03) and a significantly higher rate of CABG-related bleeding in the prasugrel group (13.4% vs 3.2%; NNH = 10). Of note, the majority of the benefit was accrued in the first 3 days, and when adjudicated episodes of MI were removed from the analysis, no further separation of the Kaplan–Meier curves occurred after 30 days.Citation48,Citation49 Prasugrel use did not decrease all-cause mortality. A post hoc subgroup analysis by the TRITON authors identified the elderly (age >75 years), patients weighing <60 kg, and those with past history of stroke or transient ischemic attack as having unfavorable bleeding risk–benefit profiles. Currently, the FDA has approved prasugrel use for ACS patients undergoing PCI when coronary anatomy is known and likelihood of undergoing CABG is low. The clinical efficacy of prasugrel in other patient groups such as medically managed patients with unstable angina/NSTEMI is currently being evaluated in the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial (NCT00699998; TRILOGY ACS).
Ticagrelor
Ticagrelor is an orally administered nonthienopyridine, which directly and reversibly inhibits the P2Y12 receptor.Citation50 Animal studies have shown that irreversible P2Y12 inhibition with ticagrelor can attenuate ADP-mediated vascular vasoconstrictionCitation51 and inhibit adenosine uptake by red cells, thereby increasing circulating ADP levels which augment the hyperemic response following arterial occlusion.Citation52 A loading dose of 180 mg provides similar rates of platelet inhibition within 30 min that a 600-mg loading dose of clopidogrel provides at 8 h.Citation53 Maximal IPA occurs within 2 h of dosing, with a dose of 100 mg twice daily maintaining near complete IPA and limited added inhibition from increasing doses. The half-life is ∼7 h, and there is minimal residual antiplatelet effect 48 h after last dose.Citation54 Dose-related dyspnea is a common adverse event occurring in 10%–20% of patients.Citation54,Citation55 Among patients experiencing dyspnea, no changes were noted in any cardiopulmonary function parameters at baseline and up to 6 weeks,Citation56 and resulted in discontinuation in about 0.8% of patients.Citation57 The PLATO trial showed that among 13,000 ACS patients managed with an early invasive approach, ticagrelor use resulted in a significant decrease (12.3% vs 10.2%; HR = 0.84; P = 0.0001) in the composite endpoint (death from vascular causes, MI, or stroke) at 12 months. What distinguishes ticagrelor from other anti-platelet and antithrombotic agents is that the overall mortality benefit (4.5% vs 5.9%; P < 0.001) was mainly achieved by decreasing rates of MI (2.8% vs 2.2%; P = 0.03) without increasing major non-CABG-related bleeding events using the TRITON Trial definition.Citation58 Ticagrelor acts directly in a dose-dependent manner with a rapid onset and offset of its antiplatelet effect. These characteristics make it ideally suited to the acute setting of ACS when coronary anatomy is not known and in cohorts where semielective/urgent surgery necessitates discontinuation of antiplatelet therapy. In July 2010, the FDA Cardiovascular and Renal Advisory Committee voted 7:1 in favor of approving this medication for the indication of a troponin-positive ACS. The time for review, however, has recently been extended as the FDA was uncertain how to evaluate the lack of effect of this drug in the PLATO study in patients enrolled in sites from the USA.Citation59
Glycoprotein IIb/IIIa inhibitors
Glycoprotein IIb/IIIa inhibitors (GPIs) are intravenous agents that inhibit fibrinogen-mediated platelet aggregation and block the expression of the prothrombotic CD40 ligand.Citation60 Abciximab, tirofiban, and eptifibatide are the three GPIs currently available for clinical use. At optimal doses, these agents result in prompt, uniform, and very potent IPA when compared to oral antiplatelet agents.Citation16 The major risk with their use is increased bleeding episodes, especially in certain at-risk subgroups (diabetics, chronic kidney disease, and elderly) and when inappropriate dosing occurs.Citation61 Thrombocytopenia is associated with abciximab and tirofiban use and occurs at a frequency of 2.4% and 0.5%, respectively. It occurs within the first 24 h and is associated with adverse outcomes.Citation62 GPI use is recommended by the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines in selected patients with NSTEMI/UA,Citation63 STEMI,Citation64 and those undergoing PCI.Citation65 The Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction trial compared bivalirudin (a direct thrombin inhibitor) alone with heparin plus a GPI in patients with STEMI undergoing primary PCI. Bivalirudin alone led to a significant decrease in overall mortality at 30 days (2.1% vs 3.1%; P = 0.047). This study has led many to question the clinical utility of GPIs in STEMI patients.
The Early Glycoprotein IIb/IIIa inhibition in Non-ST-Segment Elevation Acute Coronary Syndrome trial compared routine eptifibatide use with delayed provisional use in NSTEMI in whom an invasive strategy is pursued (N = 9492). Routine use did not affect the primary composite endpoint (death, MI, recurrent ischemia necessitating urgent revascularization, or thrombotic bailout at 96 h) and resulted in a higher incidence in bleeding events and transfusions.Citation66 The routine use of GPIs in ACS cannot be justified. Selective downstream use in high-risk ACS patients and following complicated PCI is likely to continue.
A summary of the important clinically used antiplatelet agents is shown in .
Table 1 Summary of current antiplatelet agents approved for clinical use in the acute coronary syndromes population
Impact on patient outcomes
An individual patient is best served by antiplatelet agents if the thrombotic risk significantly outweighs the resultant risk of bleeding. For any particular disease process, this critical balance depends on a myriad of factors including age, patient comorbodities, stage of disease process, pharmacogenetics, and choice of treatment modality. It is through i) maximizing adherence to therapy, ii) evaluating bleeding risk, iii) applying methods to reduce bleeding risk, iv) ensuring pharmacodynamic efficacy, and v) minimizing drug interactions that clinicians can optimize treatment outcomes with antiplatelet agents in ACS.
Adherence
Adherence can be viewed as a shared process where the care provider and patient work together to ensure that evidence-based treatment is administered during the patient’s hospitalization and subsequently taken regularly for the recommended duration of treatment upon discharge. Adherence to ACC/ AHA guidelines during index hospitalization in ACS has been shown in numerous studies to correlate with decreased morbidity and mortality.Citation67 An observational study of more than 65,000 patients demonstrated that for every 10% increase in composite adherence to nine ACC/AHA Class I recommended therapies, a 10% reduction in in-hospital mortality ensued (adjusted OR = 0.90; 95% CI: 0.84–0.97).Citation68 Societal and governmental quality improvement programs,Citation69,Citation70 registries,Citation71 guideline-based tools,Citation72 and the use of standardized patient pathway medication formsCitation73 have all been shown to improve care providers adherence to guidelines.
The transition period from hospital discharge to the outpatient setting is the period where the majority of patient-initiated drug discontinuation occurs.Citation74,Citation75 A study by Ho et al found that one in six patients delayed filling in their index clopidogrel script following drug-eluting stent (DES) implantation and subsequently went on to have long gaps between future clopidogrel refills. This group of patients was at increased risk (HR = 1.53; 95% CI: 1.25–1.87) of mortality and future cardiovascular events.Citation76 Patient factors known to predict poor adherence to pharmacotherapy following ACS include insurance status, level of education, number of medications, and older age.Citation77 The utility and cost-effectiveness requires targeting at-risk patients and patients who delay filling their scripts with intensive education, reminder tools, and more regular follow-up appointments, although intuitive will need to be investigated in randomized trials. A newly identified factor specifically predictive of inappropriate cessation of antiplatelet therapy is that of minor (nuisance) bleeding. Up to 28% of patients on DAT experience nuisance bleeding, resulting in high cessation rates of one (5%) or both (1%) antiplatelet agents.Citation78 Of note, patients who experience a bleed and receive follow-up care from a cardiologist are more likely to maintain antiplatelet therapy than those who receive follow-up from other health professionals.Citation79
Of concern is that 32% of cases of inappropriate discontinuation of antiplatelet agents were the result of a health professional’s recommendation.Citation76 An observational study in a noncardiac preoperative clinic setting showed that following coronary stent implantation, the majority of patients had very poor understanding of the rationale, duration, and risk of discontinuation of antiplatelet therapy. Surgical instructions regarding antiplatelet therapy were provided in 57% of patients. Alarmingly, however, cardiology input was only documented in 17% of cases.Citation80 For improved outcomes in the perioperative setting, providers of cardiovascular care must ensure adequate patient education and collaboration with other health professionals in the decision-making process of antiplatelet therapy management.
Bleeding risk
Antiplatelet agents prevent death, MI, and other ischemic events through their antithrombotic properties. This benefit is attenuated by an increase in bleeding risk. In PCI, it is known that with increasing levels of antithrombotic therapy, the anti-thrombotic beneficial effect eventually plateaus.Citation81,Citation82 The risk of bleeding in patients with cardiovascular disease is the result of complex interactions between baseline characteristics, comorbidities, type and stage of disease process, drug combinations, and dosing. The delicate risk/benefit balance of antiplatelet therapy is summarized in .
Figure 2 The delicate balance of an individual’s comorbidities: risk of thrombosis versus risk of bleeding.
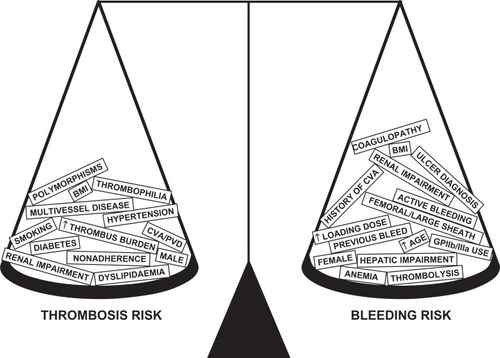
In ACS, bleeding in the acute phase is a strong, stepwise independent predictor of death.Citation83–Citation85 This risk is more marked in the acute period (first 30 days),Citation84–Citation87 and in some studies, it surpasses the risk of MI.Citation84 However, unlike MI, the long-term risk remains significant and persists for up to a year.Citation79–Citation82
Two recent scoring systems have been validated to predict early bleeding in ACS.Citation84,Citation88 The Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines (CRUSADE) score used the following eight admission variables: female sex, diabetes, vascular disease, heart rate, abnormal systolic blood pressure, congestive heart failure, baseline hematocrit, and creatinine clearance to predict inpatient bleeding in ACS patients.Citation88 The risk score proposed by Mehran et al relied on age, sex, creatinine clearance, hematocrit, white cell count, type of ACS presentation, and antithrombotic regimen used to predict bleeding within 30 days of presentation.Citation84 A novel finding from this study was that CABG-related bleeding did not increase mortality, a finding that is likely to generate controversy about the pros and cons of initiating antiplatelet therapy prior to knowing the coronary anatomy. A bleeding obesity paradox has recently been observed where patients with mild (Class I) obesity had the lowest rates of bleeding following PCI after risk adjustment compared to underweight or severely overweight patients.Citation89
Interventions to minimize bleeding risk
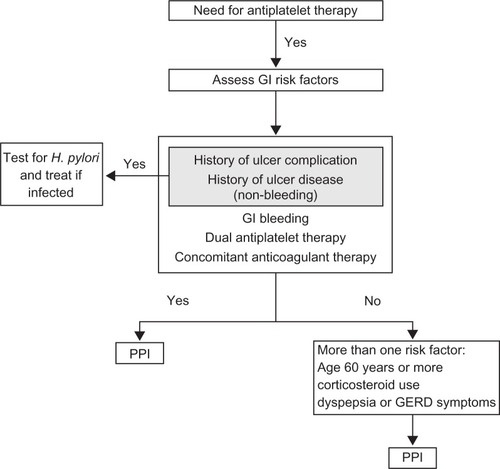
Antiplatelet agents increase the risk of gastrointestinal bleeding (GIB): aspirin predominantly by promoting ulcer formationCitation90,Citation91 and preventing ulcer healing,Citation92 whereas the thienopyridines are believed to promote bleeding at sites of existing lesions caused by nonsteroidal anti-inflammatory drugs (NSAIDs) or infection with Helicobacter pylori.Citation93 Ancillary use of other medications such as corticosteroids and anticoagulants further compounds this risk. Of importance, GIB following ACS is independently associated with mortality.Citation94 Consensus now exists that upper GI bleeding may be reduced in the setting of antiplatelet use by suppressing gastric acid production and thus promoting the healing of ulcers and erosions as well as potentially stabilizing thrombi. Histamine H2 receptor antagonists, although suppressing acid production by up to 68%, have shown only a modest benefit in patients taking aspirinCitation95 and no benefit in those on clopidogrel.Citation96 The data for PPIs are far more convincing with one trial showing a 50% relative risk reduction in patients’ baseline risk of GIB (absolute risk 1.2%) and an absolute risk reduction of 2.8% per year in patients with ≥3 risk factors for GIB.Citation97 These findings and other smaller studiesCitation98 are supported by the most recent trial and largest randomized controlled trial (RCT) which looked at clopidogrel plus omeprazole versus clopidogrel alone.Citation99 In the composite outcome of overt or occult bleeding, symptomatic gastroduodenal ulcer, or erosion, a hazard ratio of 0.34 (95% CI: 0.18–0.63) was observed in the PPI combination arm. An expert consensus document advocates the use of PPI in all patients on DAT and patients with high-risk features.Citation100 Patients with a history of peptic ulcer disease should have testing for H. pylori and treatment when indicated. A flow diagram summarizing key clinical issues in this area is shown in .
Figure 3 Algorithm proposing an approach to cost effective utilization of PPI cotherapy for the prevention of gastrointestial bleeding.
Copyright © 2008, American College of Cardiology. Reproduced with permission from Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52(18):1502–1517.
A wealth of evidence is accumulating supporting the superior safety of the radial approach for PCI in all groups of ACS patients, particularly regarding bleeding risk.Citation101,Citation102 It increases the safety of aggressive platelet inhibition in the acute periprocedural phase and minimizes bleeding and vascular access complications, without significantly increasing procedural time.Citation103–Citation105 Nonrandomized evidence now exists for a mortality benefit using radial compared to femoral approach for both stableCitation101 and ACSCitation105 patients. We await results of the randomized radial versus femoral access for coronary intervention study due for completion at the end of 2010 for definitive evidence regarding this issue (Clinical Trial: NCT01014273).
An increasing number of patients require long-term anticoagulation with warfarin predominantly for prevention of thromboembolic events in atrial fibrillation. Coexisting indications for concurrent antiplatelet therapy such as ACS and previous PCI are common in this patient population. A recent large, Danish cohort study showed that combining clopidogrel and warfarin carries a bleeding HR of 3.08 (2.32–3.91) compared with warfarin monotherapy. Additional use of aspirin increases the bleeding HR to 3.70 (2.89–4.76).Citation106 The bleeding risk, however, is grossly time independent. This concept was supported by an observational Finnish study which highlighted the importance of maintaining DAT in patients on long-term anticoagulation therapy in the first 4 weeks following PCI.Citation107 Strategies to minimize the bleeding risk in this patient population include maintaining the international normalized ratio levels at the lowest possible therapeutic levelCitation108,Citation109 and minimizing the duration of triple therapy with the preferential use of bare metal stents (BMS).Citation110 The use of a PPI (which will be expanded on later in this article) appears appropriate for moderate to high bleeding risk subjects that require both concomitant anticoagulation and antiplatelet therapy.
The risk of stent thrombosis in patients who undergo PCI and stent implantation decreases exponentially over time. Rates of endothelialization differ between BMS and DESs, influencing the decision about the duration of DAT. This has recently been a major concern with an increase of stent thrombosis rates seen in DES compared to BMS with an associated suggestion of increased adverse outcomes.Citation111 This has resulted in many interventional cardiologists electing to prolong DAT in subjects following DES compared to BMS insertion. Definitive studies in this area to guide the clinician are limited; however, recent data suggest that if a patient has been clinically stable on DAT for 12 months following a DES insertion, continuation of DAT for a further 12 months compared to aspirin therapy alone is not associated with any reduction in adverse cardiovascular events.Citation112
Pharmacogenetics and pharmacodynamics
Consistent and effective levels of platelet inhibition are essential for obtaining optimal patient outcomes with anti-platelet therapy in ACS patients. The complex and dynamic nature of platelet functionCitation113 and the variety of targets for platelet inhibition are some of the reasons why no single platelet function test (PFT) is currently in routine clinical use.Citation114 In a recent trial looking at six different PFTs in PCI, only three out of six tests had a modest predictive value for adverse cardiovascular events, and no test predicted bleeding complications.Citation115 The intrinsically constant nature of interindividual allelic variation have led some to propose genetic testing as the new standard of care for individualized antiplatelet therapy.Citation116
Aspirin resistance has been used to describe incomplete platelet response, with prevalence estimates between 5% and 65%.Citation30 Lack of a ‘gold standard definition’, assay variability, and the contribution of composite processes may explain this wide range.Citation117 Two meta-analyses using studies with heterogeneous methods have suggested a nearly four-fold increase in recurrent cardiovascular events in patients poorly responsive to aspirin.Citation118,Citation119 The clinical utility of point of care assays for aspirin response in ACS should be tested in large randomized trials.
A large body of data supports the association of genetic polymorphisms in the hepatic cytochrome 2C19 (CYP2C19) with variable levels of the active metabolite of clopidogrel. This was subsequently shown to result in high levels of residual platelet reactivity and adverse clinical outcomes,Citation52,Citation66,Citation67,Citation71 prompting the FDA to issue a boxed warning about the diminished effectiveness in patients with loss-of-function alleles.Citation120 Other factors, both genetic and nongenetic, most likely contribute to this clinically important phenomenon. Response to the newer P2Y12 receptor antagonists (prasugrel, ticagrelor) does not appear to be influenced by CYP2C19 allelic variation.Citation53,Citation121,Citation122 The future of individualized antiplatelet therapy may involve a combination of genotypic and phenotypic testing, which will assist in guiding treatment algorithms. While the mechanisms relating to clopidogrel resistance (nonresponders) are multifactorial, the definitive treatment to counteract the associated adverse outcomes remains uncertain.Citation123
Drug interactions
NSAIDs are used daily by more than 30 million people worldwide.Citation124 Their use is particularly prevalent amongst the elderly; a group also at increased risk of cardiovascular disease. Ibuprofen, the most widely used NSAID, is believed to interfere with aspirin by binding to COX-1 and attenuating its antiplatelet activity.Citation125 This effect has also been observed with other NSAIDs,Citation126–Citation128 but specifically not with COX-2 selective inhibitors.Citation129 The FDA recommends that aspirin should be taken at least 30 min before or 8 h after nonselective NSAID ingestion to preserve its efficacy.Citation130 It is incumbent amongst all providers of care for ACS patients to realize that the use of all nonaspirin NSAIDs is associated with adverse cardiovascular outcomes.Citation131 At-risk patients, particularly in the post-ACS and PCI setting, should be provided with education about the risks of NSAIDs (especially over the counter use), and alternative modes of analgesia should be provided wherever possible.Citation132
Given their broad indications and frequent coprescription, the purported interaction between PPIs and clopidogrel has generated unprecedented attention in both the lay and research community. Concomitant use of PPIs may competitively inhibit activation of clopidogrel by CYP2C19, thereby reducing its antiplatelet activity in the same way allelic variations have been reported.Citation133 The evidence supporting this theory, however, has been conflicting and can be categorized into studies looking at pharmacodynamic and platelet function studies and those looking at clinical effect. In one study of patients who were given a high maintenance dose of clopidogrel, both omeprazole and pantoprazole were associated with reduced platelet inhibition as assessed by vasodilator-stimulated phosphoroprotein (44% vs 23%; P = 0.04). Two other randomized trials utilizing ex vivo assays demonstrated the same attenuation with omeprazole;Citation77,Citation78 however, other PPIs did not seem to have the same impact in two further studies.Citation78,Citation80 In terms of clinical effect of interaction, large observational studies of differing size, populations, and methodologies have looked at whether patients prescribed a PPI have had increased CV events, with some reporting small but significant associations yet others reporting no difference. Whether the differences in results reflect a number of confounding factors or true clinical interaction is impossible to determine retrospectively. One of the largest observational studies randomized 13,608 patients to clopidogrel or prasugrel and found no difference between CV events in those patients on PPI compared to those who were not (regardless of which PPI was used) in either treatment arm (clopidogrel HR = 0.94; 95% CI: 0.80–1.11 and prasugrel HR = 1.00, 95% CI: 0.84–1.20).Citation79 The only RCT published to date, as alluded to above, involved 3761 patients who were randomized to clopidogrel and omeprazole combination or clopidogrel alone.Citation100 All patients were also on aspirin, and no difference in composite CV event rate was observed between the arms (MI, stroke, CABG, and PCI CV death). The conclusions of the study, which appear to refute any potential interaction, have been somewhat controversial given the trial was stopped before full enrollment and the low event rate resulting in broad confidence intervals.
In summary, despite being theoretically plausible and biologically measurable with platelet function testing, the clinical effect of a PPI–clopidogrel interaction is inconsistently demonstrated, of small magnitude when observed, and the only randomized trial refutes its existence. In spite of this and a recent consensus document from the ACC/AHA/AGA which reports an inability to exclude an interaction on current evidence, the FDA remains reticent to remove its black box warning on clopidogrel and has reissued its warning.Citation134 The risk of GIB remains an important clinical problem for patients requiring DAT. The consensus statement suggests that the risk reduction with PPIs is substantial in patients with risk factors for GIB (prior bleed, advancing age, concomitant anticoagulation/steroid/NSAID, or H. pylori infection) and thus will outweigh any potential reduction in the CV efficacy of antiplatelet treatment because of a drug–drug interaction. In patients without GIB risk factors, there appears to be little incremental absolute benefit in adding PPI.
Discussion
The growing body of evidence highlighting the central role of platelets to the development of ACS ensures that antiplatelet therapy will continue to be the cornerstone of management for the foreseeable future. The relentless search for increasingly aggressive antithrombotic activity has resulted in increased efficacy, but it has come at the expense of increased rates of bleeding. While the quest continues, the current absence of a one-size-fits-all antiplatelet ‘panacea’ mandates an individualized approach to therapy. Evidence-based algorithms will incorporate evolving trial data assessing duration, timing, and dose of the current agents as well as the impact of new agents on thrombosis and bleeding balance. The not-so-distant future of antiplatelet therapy is destined to account for clinical phenotypes and utilize pharmacogenomics in combination with platelet function testing to individualize therapy. Regular medication reviews and education to both patients and other health care professionals will be critical in ensuring the optimal implementation and utilization of such algorithms.
Disclosure
None of the authors have conflicts of interest to declare in relation to this paper.
References
- ClemetsonKJClemetsonJMPlatelet collagen receptorsThromb Haemost200186118919711487007
- KamathSBlannADLipGYPlatelet activation: assessment and quantificationEur Heart J200122171561157111492985
- PatronoCGarcía-RodríguezLALandolfiRBaigentCLow-dose aspirin for the prevention of atherothrombosisN Engl J Med2005353222373238316319386
- GurbelPABlidenKPDiChiaraJEvaluation of dose-related effects of aspirin on platelet function: results from the Aspirin-Induced Platelet Effect (ASPECT) studyCirculation2007115253156316417562955
- GurbelPATantryUSCombination antithrombotic therapiesCirculation2010121456958320124137
- PatronoCCollerBFitzGeraldGAHirshJRothGPlatelet-active drugs: the relationships among dose, effectiveness, and side effects: the Seventh ACCP Conference on Antithrombotic and Thrombolytic TherapyChest20041263 Suppl234S264S15383474
- JenkinsCCostelloJHodgeLSystematic review of prevalence of aspirin induced asthma and its implications for clinical practiceBMJ2004328743743414976098
- GrattanCEAspirin sensitivity and urticariaClin Exp Dermatol200328212312712653694
- Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative GroupLancet1988286073493602899772
- Antithrombotic Trialists’ CollaborationCollaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patientsBMJ20023247329718611786451
- Antiplatelet Trialists’ CollaborationCollaborative overview of randomised trials of antiplatelet therapy – I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patientsBMJ19943086921811068298418
- BenoitPDognéJMPlatelet ADP receptors and their antagonistsMini Rev Med Chem20033214514812570847
- HollopeterGJantzenHMVincentDIdentification of the platelet ADP receptor targeted by antithrombotic drugsNature2001409681720220711196645
- LeonMBBaimDSPopmaJJA clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study InvestigatorsN Engl J Med199833923166516719834303
- SchömigANeumannFJKastratiAA randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stentsN Engl J Med199633417108410898598866
- GurbelPATantryUSDelivery of glycoprotein IIb/IIIa inhibitor therapy for percutaneous coronary intervention: why not take the intracoronary highway?Circulation2010121673974120124130
- LevineGNBergerPBCohenDJNewer pharmacotherapy in patients undergoing percutaneous coronary interventions: a guide for pharmacists and other health care professionalsPharmacotherapy200626111537155617064198
- SharisPJCannonCPLoscalzoJThe antiplatelet effects of ticlopidine and clopidogrelAnn Intern Med199812953944059735068
- Paradiso-HardyFLAngeloCMLanctôtKLCohenEAHematologic dyscrasia associated with ticlopidine therapy: evidence for causalityCMAJ2000163111441144811192649
- BhattDLBertrandMEBergerPBMeta-analysis of randomized and registry comparisons of ticlopidine with clopidogrel after stentingJ Am Coll Cardiol200239191411755280
- SmallDSFaridNALiYGEffect of ranitidine on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrelCurr Med Res Opin20082482251225718786302
- QuinnMJFitzgeraldDJTiclopidine and clopidogrelCirculation1999100151667167210517740
- SimonTVerstuyftCMary-KrauseMGenetic determinants of response to clopidogrel and cardiovascular eventsN Engl J Med2009360436337519106083
- ShuldinerARO’ConnellJRBlidenKPAssociation of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapyJAMA2009302884985719706858
- GiustiBGoriAMMarcucciRRelation of cytochrome P450 2C19 loss-of-function polymorphism to occurrence of drug-eluting coronary stent thrombosisAm J Cardiol2009103680681119268736
- MegaJLCloseSLWiviottSDCytochrome p-450 polymorphisms and response to clopidogrelN Engl J Med2009360435436219106084
- SibbingDStegherrJLatzWCytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary interventionEur Heart J200930891692219193675
- GurbelPABlidenKPHiattBLO’ConnorCMClopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivityCirculation2003107232908291312796140
- HochholzerWTrenkDFrundiDTime dependence of platelet inhibition after a 600-mg loading dose of clopidogrel in a large, unselected cohort of candidates for percutaneous coronary interventionCirculation2005111202560256415809367
- MareeAOFitzgeraldDJVariable platelet response to aspirin and clopidogrel in atherothrombotic diseaseCirculation2007115162196220717452618
- YusufSZhaoFMehtaSRChrolaviciusSTognoniGFoxKKEffects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevationN Engl J Med2001345749450211519503
- MehtaSRYusufSPetersRJEffects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE studyLancet2001358928152753311520521
- ChenZMJiangLXChenYPAddition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trialLancet200536694971607162116271642
- SabatineMSCannonCPGibsonCMAddition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevationN Engl J Med2005352121179118915758000
- BertrandMERupprechtHJUrbanPGershlickAHDouble-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: the clopidogrel aspirin stent international cooperative study (CLASSICS)Circulation2000102662462910931801
- BennettCLConnorsJMCarwileJMThrombotic thrombocytopenic purpura associated with clopidogrelN Engl J Med2000342241773177710852999
- AlmsherqiZAMcLachlanCSSharefSMNon-bleeding side effects of clopidogrel: have large multi-center clinical trials underestimated their incidence?Int J Cardiol2007117341541716919820
- FischerTCWormMGronebergDAClopidogrel-associated angioedemaAm J Med20031141777812543298
- KhambekarSKKovacJGershlickAHClopidogrel induced urticarial rash in a patient with left main stem percutaneous coronary intervention: management issuesHeart2004903e1414966075
- HagiharaKKazuiMKuriharaABiotransformation of prasugrel, a novel thienopyridine antiplatelet agent, to the pharmacologically active metaboliteDrug Metab Dispos201038689890420228231
- SmallDSFaridNAPayneCDEffects of the proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrelJ Clin Pharmacol200848447548418303127
- US Food and Drug AdministrationCardiovascular and Renal Drug Advisory Committee Briefing Document on Prasugrel for ACSRockville (MD)FDA2009 Available from: http://www.fda.gov/ohrms/dockets/ac/09/briefing/2009-4412b1-01-FDA.pdf. Accessed 2010 May 22.
- JakubowskiJAWintersKJNaganumaHWallentinLPrasugrel: a novel thienopyridine antiplatelet agent. A review of preclinical and clinical studies and the mechanistic basis for its distinct antiplatelet profileCardiovasc Drug Rev200725435737418078435
- WiviottSDTrenkDFrelingerALPrasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the prasugrel in comparison to clopidogrel for inhibition of platelet activation and aggregation-thrombolysis in myocardial infarction 44 trialCirculation2007116252923293218056526
- MatsushimaNJakubowskiJAAsaiFPlatelet inhibitory activity and pharmacokinetics of prasugrel (CS-747) a novel thienopyridine P2Y12 inhibitor: a multiple-dose study in healthy humansPlatelets200617421822616769599
- NorgardNBAbu-FadelMComparison of prasugrel and clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary interventionVasc Health Risk Manag2009587388219898643
- WiviottSDBraunwaldEMcCabeCHPrasugrel versus clopidogrel in patients with acute coronary syndromesN Engl J Med2007357202001201517982182
- SerebruanyVLThe FDA prasugrel review: adjudication of myocardial infarction controversyCardiology2009114212612919506374
- SerebruanyVLBleeding risks with prasugrel in the TRITON trial: good news … bad newsCardiology2008111426526718434736
- AngiolilloDJGuzmanLAClinical overview of promising non-thienopyridine antiplatelet agentsAm Heart J20081562 SupplS23S2818657683
- WangKZhouXZhouZBlockade of the platelet P2Y12 receptor by AR-C69931MX sustains coronary artery recanalization and improves the myocardial tissue perfusion in a canine thrombosis modelArterioscler Thromb Vasc Biol200323235736212588784
- HustedSvan GiezenJJTicagrelor: the first reversibly binding oral P2Y12 receptor antagonistCardiovasc Ther200927425927419604248
- GurbelPABlidenKPButlerKRandomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET studyCirculation2009120252577258519923168
- HustedSEmanuelssonHHeptinstallSSandsetPMWickensMPetersGPharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirinEur Heart J20062791038104716476694
- CannonCPHustedSHarringtonRASafety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trialJ Am Coll Cardiol200750191844185117980250
- StoreyRFBlidenKPPatilSBIncidence of dyspnea and assessment of cardiac and pulmonary function in patients with stable coronary artery disease receiving ticagrelor, clopidogrel, or placebo in the ONSET/OFFSET studyJ Am Coll Cardiol201056318519320620737
- CannonCPHarringtonRAJamesSComparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind studyLancet2010375971128329320079528
- StoneGWTicagrelor in ACS: redefining a new standard of care?Lancet2010375971126326520079529
- HughesSFirst “comparison” of prasugrel and ticagrelor2010916 Available from: http://www.theheart.org/article/1122713.do. Accessed 2010 Oct 20
- ChandlerABEarhartADSpeichHERegulation of CD40L (CD154) and CD62P (p-selectin) surface expression upon GPIIb-IIIa blockade of platelets from stable coronary artery disease patientsThromb Res20101251445219487018
- AlexanderKPChenAYRoeMTExcess dosing of antiplatelet and antithrombin agents in the treatment of non-ST-segment elevation acute coronary syndromesJAMA2005294243108311616380591
- MerliniPARossiMMenozziAThrombocytopenia caused by abciximab or tirofiban and its association with clinical outcome in patients undergoing coronary stentingCirculation2004109182203220615117843
- AndersonJLAdamsCDAntmanEMACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to revise the 2002 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency MedicineJ Am Coll Cardiol2007507e1e15717692738
- AntmanEMHandMArmstrongPW2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelinesJ Am Coll Cardiol200851221024718191746
- SmithSCJrFeldmanTEHirshfeldJWJrACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention-summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (ACC/AHA/SCAI writing committee to update the 2001 guidelines for percutaneous coronary intervention)J Am Coll Cardiol200647121623516386696
- GiuglianoRPWhiteJABodeCEarly versus delayed, provisional eptifibatide in acute coronary syndromesN Engl J Med2009360212176219019332455
- EagleKAMontoyeCKRibaALGuideline-based standardized care is associated with substantially lower mortality in medicare patients with acute myocardial infarction: the American College of Cardiology’s Guidelines Applied in Practice (GAP) Projects in MichiganJ Am Coll Cardiol20054671242124816198838
- PetersonEDRoeMTMulgundJAssociation between hospital process performance and outcomes among patients with acute coronary syndromesJAMA2006295161912192016639050
- LaBreshKAEllrodtAGGliklichRLiljestrandJPetoRGet with the guidelines for cardiovascular secondary prevention: pilot resultsArch Intern Med2004164220320914744845
- MehtaRHMontoyeCKGalloglyMImproving quality of care for acute myocardial infarction: the Guidelines Applied in Practice (GAP) initiativeJAMA2002287101269127611886318
- MehtaRHRoeMTChenAYRecent trends in the care of patients with non-ST-segment elevation acute coronary syndromes: insights from the CRUSADE initiativeArch Intern Med2006166182027203417030838
- MehtaRHMontoyeCKFaulJEnhancing quality of care for acute myocardial infarction: shifting the focus of improvement from key indicators to process of care and tool use: the American College of Cardiology Acute Myocardial Infarction Guidelines Applied in Practice Project in Michigan: Flint and Saginaw ExpansionJ Am Coll Cardiol200443122166217315193675
- BahitMCMurphySAGibsonCMCannonCPCritical pathway for acute ST-segment elevation myocardial infarction: estimating its potential impact in the TIMI 9 registryCrit Pathw Cardiol20021210711218340294
- ColemanEASmithJDRahaDMinSJPosthospital medication discrepancies: prevalence and contributing factorsArch Intern Med2005165161842184716157827
- HoPMSpertusJAMasoudiFAImpact of medication therapy discontinuation on mortality after myocardial infarctionArch Intern Med2006166171842184717000940
- HoPMTsaiTTMaddoxTMDelays in filling clopidogrel prescription after hospital discharge and adverse outcomes after drug-eluting stent implantation: implications for transitions of careCirc Cardiovasc Qual Outcomes20103326126620407117
- MelloniCAlexanderKPOuFSPredictors of early discontinuation of evidence-based medicine after acute coronary syndromeAm J Cardiol2009104217518119576342
- Ben-DorITorgusonRScheinowitzMIncidence, correlates, and clinical impact of nuisance bleeding after antiplatelet therapy for patients with drug-eluting stentsAm Heart J2010159587187520435198
- WangTYXiaoLAlexanderKPAntiplatelet therapy use after discharge among acute myocardial infarction patients with in-hospital bleedingCirculation2008118212139214518981304
- TrentmanTLRosenfeldDMDanielsonDRHagstromSGDrug-eluting stents: patient understanding of the risks of premature cessation of antiplatelet drugsJ Cardiothorac Vasc Anesth200822680681018834797
- ChewDPBhattDLLincoffAMDefining the optimal activated clotting time during percutaneous coronary intervention: aggregate results from 6 randomized, controlled trialsCirculation2001103796196611181470
- BrenerSJMoliternoDJLincoffAMSteinhublSRWolskiKETopolEJRelationship between activated clotting time and ischemic or hemorrhagic complications: analysis of 4 recent randomized clinical trials of percutaneous coronary interventionCirculation2004110899499815302778
- NdrepepaGBergerPBMehilliJPeriprocedural bleeding and 1-year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end pointJ Am Coll Cardiol200851769069718279731
- MehranRPocockSJNikolskyEA risk score to predict bleeding in patients with acute coronary syndromesJ Am Coll Cardiol201055232556256620513595
- KinnairdTDStabileEMintzGSIncidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventionsAm J Cardiol200392893093514556868
- MoscucciMFoxKACannonCPPredictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE)Eur Heart J200324201815182314563340
- RaoSVO’GradyKPieperKSImpact of bleeding severity on clinical outcomes among patients with acute coronary syndromesAm J Cardiol20059691200120616253582
- SubherwalSBachRGChenAYBaseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) bleeding scoreCirculation2009119141873188219332461
- DelhayeCWakabayashiKMaluendaGBody mass index and bleeding complications after percutaneous coronary intervention: does bivalirudin make a difference?Am Heart J201015961139114620569731
- ScheimanJMNSAIDs, gastrointestinal injury, and cytoprotectionGastroenterol Clin North Am19962522792989229573
- LanasAScheimanJLow-dose aspirin and upper gastrointestinal damage: epidemiology, prevention and treatmentCurr Med Res Opin200723116317317257477
- MaLElliottSNCirinoGBuretAIgnarroLJWallaceJLPlatelets modulate gastric ulcer healing: role of endostatin and vascular endothelial growth factor releaseProc Natl Acad Sci U S A200198116470647511353854
- AbrahamNSGrahamDYNSAIDs and gastrointestinal complications: new clinical challengesExpert Opin Pharmacother20056152681268916316306
- NikolskyEStoneGWKirtaneAJGastrointestinal bleeding in patients with acute coronary syndromes: incidence, predictors, and clinical implications: analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trialJ Am Coll Cardiol200954141293130219778672
- TahaASMcCloskeyCPrasadRBezlyakVFamotidine for the prevention of peptic ulcers and oesophagitis in patients taking low-dose aspirin (FAMOUS): a phase III, randomised, double-blind, placebo-controlled trialLancet2009374968411912519577798
- LanasAGarcía-RodríguezLAArroyoMTEffect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulantsAm J Gastroenterol2007102350751517338735
- RayWAMurrayKTGriffinMROutcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort studyAnn Intern Med2010152633734520231564
- NgFHWongSYLamKFGastrointestinal bleeding in patients receiving a combination of aspirin, clopidogrel, and enoxaparin in acute coronary syndromeAm J Gastroenterol2008103486587118177451
- BhattDLCryerBLContantCFClopidogrel with or without Omeprazole in Coronary Artery DiseaseN Engl J Med2010363201909191720925534
- BhattDLScheimanJAbrahamNSACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus DocumentsJ Am Coll Cardiol200852181502151719017521
- ChaseAJFretzEBWarburtonWPAssociation of the arterial access site at angioplasty with transfusion and mortality: the M.O.R.T.A.L study (Mortality benefit Of Reduced Transfusion after percutaneous coronary intervention via the Arm or Leg)Heart20089481019102518332059
- VorobcsukAKónyiAAradiDTransradial versus transfemoral percutaneous coronary intervention in acute myocardial infarction systematic overview and meta-analysisAm Heart J2009158581482119853703
- BertrandOFLaroseERodés-CabauJIncidence, range, and clinical effect of hemoglobin changes within 24 hours after transradial coronary stentingAm J Cardiol2010106215516120598996
- PancholySPatelTSanghviKThomasMPatelTComparison of door-to-balloon times for primary PCI using transradial versus transfemoral approachCatheter Cardiovasc Interv201075799199520517957
- ArzamendiDLyHQTanguayJFEffect on bleeding, time to revascularization, and one-year clinical outcomes of the radial approach during primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarctionAm J Cardiol2010106214815420598995
- HansenMLSørensenRClausenMTRisk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillationArch Intern Med2010170161433144120837828
- KarjalainenPPPorelaPYlitaloASafety and efficacy of combined antiplatelet-warfarin therapy after coronary stentingEur Heart J200728672673217267456
- SarafoffNNdrepepaGMehilliJAspirin and clopidogrel with or without phenprocoumon after drug eluting coronary stent placement in patients on chronic oral anticoagulationJ Intern Med2008264547248018624903
- KingSB3rdSmithSCJrHirshfeldJWJr2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/ American Heart Association Task Force on practice guidelinesJ Am Coll Cardiol200851217220918191745
- SchömigASarafoffNSeyfarthMTriple antithrombotic management after stent implantation: when and how?Heart200995151280128519605755
- LagerqvistBJamesSKStenestrandULindbäckJNilssonTWallentinLLong-term outcomes with drug-eluting stents versus bare-metal stents in SwedenN Engl J Med2007356101009101917296822
- ParkSJParkDWKimYHDuration of dual antiplatelet therapy after implantation of drug-eluting stentsN Engl J Med2010362151374138220231231
- AngiolilloDJSuryadevaraSCapranzanoPZenniMZGuzmanLABassTAAntiplatelet drug response variability and the role of platelet function testing: a practical guide for interventional cardiologistsCatheter Cardiovasc Interv200973111419089929
- SharmaRKReddyHKSinghVNSharmaRVoelkerDJBhattGAspirin and clopidogrel hyporesponsiveness and nonresponsiveness in patients with coronary artery stentingVasc Health Risk Manag2009596597219997577
- BreetNJvan WerkumJWBoumanHJComparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantationJAMA2010303875476220179285
- DamaniSBTopolEJThe case for routine genotyping in dual-antiplatelet therapyJ Am Coll Cardiol201056210911120471193
- Ben-DorIKleimanNSLevEAssessment, mechanisms, and clinical implication of variability in platelet response to aspirin and clopidogrel therapyAm J Cardiol2009104222723319576352
- SnoepJDHovensMMEikenboomJCvan der BomJGHuismanMVAssociation of laboratory-defined aspirin resistance with a higher risk of recurrent cardiovascular events: a systematic review and meta-analysisArch Intern Med2007167151593159917698681
- KrasopoulosGBristerSJBeattieWSBuchananMRAspirin “resistance” and risk of cardiovascular morbidity: systematic review and meta-analysisBMJ2008336763719519818202034
- US Food and Drug AdministrationFDA Drug Safety Communication: Reduced Effectiveness of Plavix (Clopidogrel) in Patients Who Are Poor Metabolizers of the Drug Updated 2010 Mar 25Rockville, MDFDA2010 Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203888.htm. Accessed 2010 Oct 20
- MegaJLCloseSLWiviottSDCytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomesCirculation2009119192553256019414633
- GurbelPABlidenKPAntoninoMJThe effect of elinogrel on high platelet reactivity during dual antiplatelet therapy and the relation to CYP2C19*2 genotype: first experience in patientsJ Thromb Haemost201081435319817997
- SofiFMarcucciRGoriAMGiustiBAbbateRGensiniGFClopidogrel non-responsiveness and risk of cardiovascular morbidity. An updated meta-analysisThromb Haemost2010103484184820135063
- FriedewaldVERamCVWessonDEWhiteWBWilliamsGWRobertsWCThe editor’s roundtable: effect of nonsteroidal anti-inflammatory drugs on blood pressureAm J Cardiol2010105121759176720538127
- Catella-LawsonFReillyMPKapoorSCCyclooxygenase inhibitors and the antiplatelet effects of aspirinN Engl J Med2001345251809181711752357
- FitzGeraldGAParsing an enigma: the pharmacodynamics of aspirin resistanceLancet2003361935754254412598136
- RayWASteinCMHallKDaughertyJRGriffinMRNon-steroidal anti-inflammatory drugs and risk of serious coronary heart disease: an observational cohort studyLancet2002359930111812311809254
- CaponeMLSciulliMGTacconelliSPharmacodynamic interaction of naproxen with low-dose aspirin in healthy subjectsJ Am Coll Cardiol20054581295130115837265
- WilnerKDRushingMWaldenCCelecoxib does not affect the antiplatelet activity of aspirin in healthy volunteersJ Clin Pharmacol20024291027103012211219
- US Food and Drug AdministrationInformation for Healthcare Professionals: Concomitant Use of Ibuprofen and Aspirin Updated 2009 Sep 29Rockville, MDFDA2006 Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm125222.htm. Accessed Jun 20 2010.
- US Food and Drug AdministrationAnalysis and recommendations for agency action regarding nonsteroidal antiinflammatory drugs and cardiovascular riskJ Pain Palliat Care Pharmacother2005194839716431839
- ScheimanJMFendrickAMSumming the risk of NSAID therapyLancet200736995731580158117499583
- LiXQAnderssonTBAhlströmMWeidolfLComparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activitiesDrug Metab Dispos200432882182715258107
- US Food and Drug AdministrationFDA Reminder to Avoid Concomitant Use of Plavix (Clopidogrel) and Omeprazole Updated 2010 Oct 27Rockville, MDFDA2010 Available from: http://www.fda.gov/Drugs/DrugSafety/ucm231161.htm. Accessed Oct 28 2010.
- SchaferAIAntiplatelet therapyAm J Med199610121992098757361