Abstract
As TNF-α is a major factor in the immune defense against herpes zoster (HZ); an increased incidence and severity of HZ cases were suspected in patients undergoing treatment with TNF antagonists. Several studies and clinical experience provided evidence that the incidence of HZ increases by twofold to threefold in this patient category. The number of severe cases of HZ, with multisegmental, disseminated cutaneous, and/or systemic involvement, is also increased. Concerning psoriasis patients under biologicals, the clinician should be more alert for an eventual HZ event, in particular during the first year of biological treatment, and be aware of the possibility of more severe HZ cases. HZ may also undergo an age-shift toward younger patients. Rapid identification of risk factors for severe HZ, such as severe prodromal pains and/or the presence of satellite lesions, is recommended. The treatment recommendations of HZ in this patient group are identical to the recently published guidelines for the management of HZ. The live attenuated viral vaccine OKA/Merck strain anti-HZ vaccination is recommended before initiating biological treatment in psoriasis patients. The new adjuvanted anti-HZ vaccine will probably also benefit patients while on biological treatment.
Introduction
Psoriasis is an inflammatory, predominantly skin disease, affecting ~1%–5% of the population and has a high impact on the patient’s quality of life.Citation1 Moreover, severe and longstanding cases of psoriasis are often associated with a moderate-to-severe metabolic syndrome, potentially reducing the life expectancy by some years.Citation1
Nowadays, moderate-to-severe plaque and patch-type psoriasis vulgaris can very effectively be treated with first-generation biologicals. They are represented by the TNF-α-antagonists,Citation2 including the receptor antagonist etanercept, a recombinant fusion protein that inhibits soluble and membrane-bound TNF-αCitation3; the monoclonal chimeric antibody infliximabCitation4 that binds membrane-bound and soluble TNF-α; and the human monoclonal antibody adalimumab that blocks TNF-α interactions with the p55 and p75 cell surface TNF receptors. The TNF-α-antagonists achieve PASI75 improvements in a high proportion of patients,Citation5,Citation6 a significant improvement compared to the older psoriasis treatments such as methotrexate, acitretin, and ciclosporin.Citation2
Although the overall safety records of TNF-α-antagonists are outstanding, there is evidence of an increased propensity to infections,Citation7–Citation10 in particular viral infections. Among these, varicella zoster virus (VZV), herpes simplex virus, hepatitis virus infections, and viral infections affecting the ear–nose–throat region are the most common.Citation11–Citation13
The second-generation biological agents for patch and plaque-type psoriasis include ustekinumab, which prevents the actions of IL-12 and IL-23 by binding to their mutual subunit p40Citation14; secukinumab, a human IgG1κ monoclonal antibody that selectively binds and neutralizes IL-17ACitation15,Citation16; ixekizumab, a humanized IgG4 monoclonal antibody that neutralizes IL-17ACitation17; and apremilast, a PDE4 inhibitor.Citation18 The safety assessments of the initial trials demonstrated a similar rate of viral infections compared to the placebo groups. This fact may be due to the relative recent introduction of these agents in clinical practice. Another hypothesis is that the targeted cytokine pathways are less important for antiviral host defense mechanisms.
The cutaneous eruption-termed herpes zoster (HZ) is a self-limiting, dermatomally localized, papulo-vesicular–pustular, and crusted eruption caused due to the reactivation of the VZV that remained dormant in the dorsal root ganglia after the primary contact with VZV in the form of chickenpox during childhood.Citation19–Citation21 Approximately 1 million new cases of HZ are diagnosed each year in the USA.Citation22 Every year, 96 HZ-related deaths are reported, all diagnosed in elderly and/or immunocompromised patients.Citation22 The incidence of HZ is rising with increasing age, and in patients older than 60 years, there are approximately ten cases of HZ per 1,000 US population per year.Citation22 In patients aged between 35 years and 44 years, ~194 women and 261 men experience HZ per 100,000 population, with these values increasing to 1,624 women and 1,112 men in patients older than 75 years.
Severe and extensive cutaneous HZ, multidermatomal HZ, and even systemic dissemination of VZV are the major complications of HZ. Especially, the immunocompromised population, including HIV patients, organ and bone marrow transplant recipients, and patients under immunosuppressive medication, is at risk for these complications.Citation19–Citation21
Postherpetic neuralgia (PHN) is the most feared complication following the resolution of the cutaneous lesions of HZ. The precise pathomechanisms are still not totally elucidated. The incidence of PHN also rises with increasing age. The risk of PHN is increased with, among the most important factors, age, severe cutaneous HZ, female sex, and/or severe prodromal pains.Citation19–Citation21 The term zoster-associated pain is used to describe the entire pain spectrum of HZ, including the prodromal and concomitant pains as well as the PHN.
TNF-α is a major factor in the host immune response against HZ.Citation23,Citation24 Consequently, TNF-α antagonists could increase the risk and the severity of HZ. Indeed, since the earliest use of TNF-α antagonists, severe cases of HZ have continuously been reported.Citation25 In contrast, for secukinumab, ixekizumab, and apremilast, not directly interfering with the TNF-α pathway, no reports have been published, until today, concerning severe HZ eruptions during treatment.
From the clinician’s point of view, five major questions arise in relationship to the management of HZ in psoriasis patients under biological agents:
Is the incidence of HZ increased in psoriasis patients receiving biological agents?
Is the severity of HZ increased in psoriasis patients under biological agents?
Are the incidence and severity of PHN increased in psoriasis patients under biological agents?
Is anti-HZ vaccination indicated in patients before or while on biological treatment?
What are the recommendations in terms of management of HZ in patients with psoriasis receiving biologicals?
Is the incidence of HZ increased in psoriasis patients receiving biological agents?
This question is most frequently addressed by studying the crude incidence rates (CIRs) calculated by drug exposure and Cox proportional hazard models evaluating the adjusted association between a biological agent and the HZ event.
In general, these studies demonstrated that the exposition to biologicals increases the risk of HZ twofold to threefold, independent of whether these studies were dealing with inflammatory joint diseases,Citation11,Citation26–Citation35 inflammatory intestinal bowel diseases,Citation36,Citation37 or inflammatory skin diseases.Citation12,Citation30,Citation38–Citation40 Whether there is a specific drug-associated risk remains still debated. An overview, including several cohort studies, randomized controlled trials, and case reports, suggested that infliximab conferred an increased risk of HZ, whereas adalimumab, etanercept, and ustekinumab did not. However, data still remain controversial. In sum, no specific biological agent seems to exhibit a particular HZ risk compared to another.Citation12 Interestingly, the patients seem to be more at risk of developing HZ during the first months of treatmentCitation41 and the time to HZ seems to be shortened in patients treated with TNF-α antagonists.Citation34
However, incidence data should be interpreted with caution for various reasons: the incidence of HZ increases progressively with age, a parameter often neglected in studies, and the study populations are extremely heterogeneous in many respects (previous exposure to one or more disease-modifying antirheumatic drugs and duration of exposure, previous exposure to other biological agents and duration of exposure, other concomitant immunosuppression, other concomitant medication, combination therapies, etc).
Psoriasis is probably not different in terms of risk for HZ during biological treatment compared to the other inflammatory rheumatologic or gastrointestinal diseases. However, only a small number of studies specifically addressed this issue of psoriasis patients.Citation12,Citation38,Citation39
An Israelian study evaluated the incidence of HZ among psoriasis patients treated with phototherapy, traditional systemic medications, and biological drugs. The incidence rates of HZ were calculated for each medication, and hazard ratios were adjusted for age, sex, and health care utilization burden. This study, which included a total of 22,330 psoriasis patients accounting for 215,656 patient years (py), revealed 1,321 cases of HZ. The CIRs were the following: 6.0 for ultraviolet B phototherapy (95% confidence interval [CI]: 0–12.8), 10.1 for psoralen and ultraviolet A (95% CI: 1.3–19), 5.4 for acitretin (95% CI: 2.2–8.7), 17 for methotrexate (95% CI: 10.6–23.4), 13.9 for etanercept (95% CI: 0.3–27.4), 19.3 for infliximab (95% CI: 0–45.8), and 4.6 for controls (95% CI: 4.3–5) per 1,000 py. No case of HZ was observed among patients treated with alefacept, efalizumab, or adalimumab. A multivariate analysis demonstrated that age, female sex, health care utilization pattern, and corticosteroid treatment were all associated with the time to HZ. Only the association of HZ with infliximab treatment approached statistical significance (hazard ratio: 1.77, 95% CI: 0.92–3.43).Citation38 The authors concluded that some biological drugs were associated with a higher incidence of HZ compared with controls, although not statistically significant.Citation38 Another study included a total of 1,220 eligible patients on biologicals, representing 4,206 py. The CIR per 1,000 py was 5.2 (95% CI: 3–7.4). Eleven HZ cases occurred during adalimumab treatment (CIR 7.1 per 1,000 py, 95% CI: 2.9–11.3), four during etanercept treatment (CIR: 5.1 per 1,000 py, 95% CI: 0.1–10), four during infliximab treatment (CIR: 2.4 per 1,000 py, 95% CI: 0–4.7), two during ustekinumab treatment (CIR: 53.5 per 1,000 py, 95% CI: 0–125.6), and one during rituximab treatment (CIR: 5.2 per 1,000 py, 95% CI: 0–17.6). The incidence was higher for patients older than 60 years in this study, compared to that of the general population, although not statistically significant. Fourteen cases of HZ were observed in patients suffering from chronic inflammatory joint disease (14/737=1.89%), five in those suffering from psoriasis (5/238=2.1%), and three in those suffering from chronic inflammatory intestinal disease (3/360=0.83%).Citation39 Specifically assessing psoriasis patients, three cases of HZ were observed during etanercept treatment in 200 py, three cases of HZ during infliximab treatment in 166 py, zero cases of HZ during adalimumab treatment in 325 py, and two cases of HZ during ustekinumab treatment in 26 py.Citation39 In total, five cases of HZ were observed during a total of 717 py (0.7%) in psoriasis patients.
Consequently, considering that ~1 million patients will experience HZ each year in a total USA population of 320 million,Citation22 representing 0.3125% of the population, it seems that biological treatments for psoriasis increase the risk of HZ by approximately twofold.
Is the severity of HZ increased in psoriasis patients under biological agents?
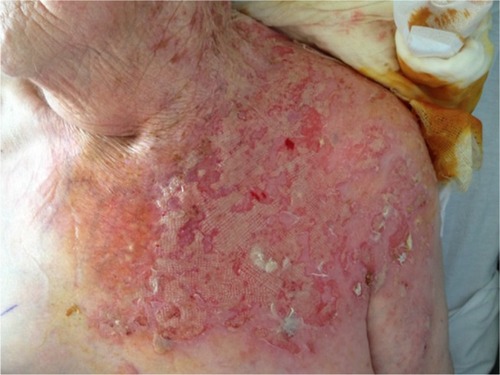
In the majority of psoriasis patients undergoing biological treatments, the course of HZ will not differ from HZ observed in a normal, nonimmunocompromised, age-matched population in terms of severity. However, the risk and incidence of severe HZ clearly increase in this population. Indeed, some of the patients may present very severe HZ in terms of cutaneous extension inside the involved dermatome(s), multidermatomal involvement in adjacent dermatomes and nonadjacent dermatomes, and increased duration of the HZ skin lesions ( and ).Citation26–Citation29,Citation38,Citation39,Citation42,Citation43 In a study that identified 86 cases of HZ among 82 patients under biologicals, multidermatomal HZ was observed in 18.3% of patients, HZ ophthalmicus in 4.9%, and hospitalization due to severe disease in 14.6%. Complications were reported in three patients.Citation28 In another study, bidermatomal and multidermatomal HZ were observed in 45% and 32% of the HZ patients, respectively. Furthermore, two patients presented with a protracted course (>4 weeks) of cutaneous VZV infection.Citation39
Figure 1 Severe and extensive multidermatomal unilateral HZ of the sacral dermatomes occurring during the use of TNF antagonists for psoriasis.
Abbreviation: HZ, herpes zoster.
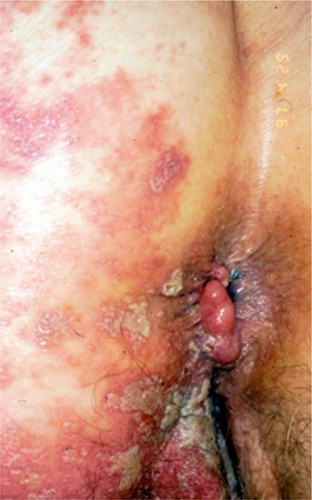
Figure 2 Severe multisegmental HZ in an elderly woman using TNF antagonists for psoriasis.
Abbreviation: HZ, herpes zoster.
It is not determined whether the cases of severe HZ were associated with a specific biologic agent. Currently, clinical experience cannot attribute a specific risk to any particular biological agent. Aside from potential severity, in this patient group, no distinctive clinical features of HZ were noted compared to common HZ.
Are the incidence and severity of PHN increased in psoriasis patients under biological agents?
In the general population older than 50 years, the incidence of PHN is ~2%–9% at 3 months after the resolution of the HZ skin lesions.Citation19–Citation21 Subsequently, the rate of PHN decreases progressively over months. TNF-α has been implicated in the pathogenesis of neuropathic pain. Higher circulating levels of 1β, IL-6, IL-8, IL-10, and TNF-α during HZ were measured compared to controls. Although IL-6 was significantly higher in HZ patients evolving to PHN, there was no clear link between elevated TNF-α levels and PHN.Citation44 The effect of biological treatments on the incidence and severity of PHN remains debated. One study that included 1,220 patients (4,206 py) receiving first-generation biologicals revealed that PHN, persisting for more than 6 months, in 20% of the HZ patients,Citation39 was significantly higher compared to the general population. In contrast, in another patient group, only 2.4% of the patients under biologicals experienced PHN.Citation28 Another study that included 206 patients presenting HZ while on TNF-α inhibitors only diagnosed PHN in <1% of the patients.Citation45
Hence, the precise impact of PHN on the first-generation biological treatments for psoriasis remains nonelucidated. No data are available for the second-generation biologicals.
Is anti-HZ vaccination indicated in patients before or while on biological treatment?
Owing to the increased incidence and risk of severe HZ, vaccination against HZ seems a reasonable medical attitude. Vaccination against HZ aims to boost the VZV-specific cell-mediated immunity. VZV-specific cell-mediated immunity is acquired during varicella and subsequently progressively wanes over the years until a threshold value is reached that allows VZV reactivation in the dorsal root ganglia, finally leading to HZ.
The current HZ vaccine is a more concentrated (~14-fold) form of the varicella vaccination OKA/Merck strain (Zostavax). This vaccine is a live attenuated viral vaccine (LAVV). Although highly efficacious with a 66.5% reduction in PHN (P<0.001) and a 51.3% (P<0.001) reduction in the incidence of HZ,Citation46 it is theoretically contraindicated to administer this type of vaccine during the use of TNF antagonists and other biological agents. However, in a retrospective cohort including 463,541 Medicare beneficiaries older than 60 years with rheumatoid arthritis, psoriasis, psoriatic arthritis, ankylosing spondylitis, or chronic inflammatory bowel diseases, the overall CIR of HZ was 7.8 cases per 1,000 py in the group having received the HZ vaccine and the rate among the unvaccinated was 11.6 cases per 1,000 py. Among the 633 patients exposed to biologicals at the time of vaccination, no single case of HZ occurred.Citation47 Despite these reassuring results, it remains cautious to refrain from vaccinating with an LAVV system while on biologicals.
Recently, the efficacy and safety of a new adjuvanted HZ subunit vaccine containing the VZV glycoprotein E and the AS01B adjuvant system have been evaluated in a randomized, placebo-controlled, multicenter, Phase III study that included 15,411 adults older than 50 years of age and stratified according to age groups (50–59 years, 60–69 years, and older than 70 years).Citation48 During a mean follow-up period of 3.2 years, HZ was confirmed in six participants among the 7,698 vaccinated volunteers (CI: 0.3 per 1,000 py) and in 210 participants among the 7,713 volunteers comprising the placebo group (CI: 9.1 per 1,000 py). The overall vaccine efficacy against HZ was 97.2% (95% CI: 93.7–99; P<0.001). The efficacy of the vaccine in adults older than 70 years was similar to that observed in the younger age groups.Citation48 The major advantage of this type of vaccine is that patients receiving biologicals are allowed to be vaccinated.
In sum, it is advisable to administer the LAVV-type anti-HZ vaccine before initiating biologicals for psoriasis patients older than 50 years.Citation49–Citation52 For patients already under biologicals, the adjuvanted vaccine type will probably be recommended when commercially available.Citation49
What are the recommendations in terms of management of HZ in patients with psoriasis receiving biologicals?
All the studies demonstrated that all the patients experiencing HZ responded positively to antiviral treatment, even the more severe cases. Resistant VZV strains were never encountered.
The treatment recommendations for HZ are not different from those for immunocompetent patients.Citation49 Oral antiviral medication (acyclovir [ACV] 800 mg, five times per day for 7 days; valaciclovir 2×500 mg, three times per day for 7 days; famciclovir 500 mg, three times per day for 7 days; or brivudin 125 mg/d for 7 days) is recommended for the subgroups of patients listed in . Intravenous administration of ACV (10 mg/kg/8 h for 7 days at least) is suggested for patients with complicated HZ or who are at a high risk of complicated HZ ().Citation49 Antiviral treatment should be initiated as soon as possible (<72 hours after the appearance of skin lesions). For ACV, famciclovir, and valaciclovir, the renal function should be tested before administration and dosages should be reduced in the case of renal insufficiency. The association of fluorouracil and brivudin should absolutely be avoided.
Table 1 Indications of oral antiviral medication
Table 2 Indications of intravenous administration of ACV as suggested for patients with complicated HZ or who are at a high risk of complicated HZ
No specific diagnostic techniques to prove the diagnosis of HZ are indicated for common HZ of the trunk.Citation53 In contrast, HZ of the orofacial and anogenital area should be differentiated from zosteriform herpes simplex virus infections.Citation53
Although it is not possible to predict the severity of HZ in a given patient, the presence of satellite lesions at the beginning of the HZ eruption and the presence of a very painful prodrome are helpful clinical indicators for an increased risk of severe HZ.Citation54
Conclusion and recommendations
Concerning HZ in psoriasis patients receiving biologicals, the following statements are proposed:
Psoriasis does not confer a specific higher risk of HZ compared to the other inflammatory joint and intestinal diseases.
The administration of biologicals in these patient groups increases the incidence of HZ by twofold to threefold.
In patients treated with biologicals, one may expect HZ in younger age groups compared to common HZ.
One should be more vigilant for a possible diagnosis of HZ in the first year after the instauration of a biological therapy.
HZ is usually not different from common HZ in the immunocompetent population.
Very severe and extensive cases of HZ are rare in these patient groups.
In the case of HZ, look for risk factors for severe HZ, especially satellite lesions and severe prodromal pains.
Antiviral treatment and management of HZ in psoriasis patients under biologicals are not different compared to their nonimmuncompromised counterparts.
Intravenous ACV treatment is recommended for severe cases of HZ.
If possible, the HZ LAVV vaccine should be administered before starting biologicals in patients never having experienced HZ previously.
When commercially available, the new subunit HZ vaccine should be provided before or during biological treatments in patients never having experienced HZ previously.
Disclosure
The authors report no conflicts of interest in this work.
References
- de la BrassinneMFaillaVNikkelsAFPsoriasis: state of the art 2013. Part I: clinical, historical, epidemiological and genetic aspects, co-morbidities and pathogenesisActa Clin Belg20136842743224635330
- de la BrassinneMNikkelsAFPsoriasis: state of the art 2013. Part II: therapeuticsActa Clin Belg20136843344124635331
- LeonardiCLPowersJLMathesonRTEtanercept Psoriasis Study GroupEtanercept as monotherapy in patients with psoriasisN Engl J Med2003349212014202214627786
- ReichKNestleFOPappKEXPRESS Study InvestigatorsInfliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trialLancet200536694941367137416226614
- GordonKBLangleyRGLeonardiCClinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension studyJ Am Acad Dermatol200655459860617010738
- KimballABBensimonAGGuerinAEfficacy and safety of adalimumab among patients with moderate to severe psoriasis with co-morbidities: subanalysis of results from a randomized, double-blind, placebo-controlled, phase III trialAm J Clin Dermatol2011121515221110526
- IsaacsDInfectious risks associated with biologicsAdv Exp Med Biol201376415115823654064
- PereiraRLagoPFariaRTorresTSafety of anti-TNF therapies in immune-mediated inflammatory diseases: focus on infections and malignancyDrug Dev Res201576841942726482111
- MurdacaGSpanòFContatoreMInfection risk associated with anti-TNF-α agents: a reviewExpert Opin Drug Saf201514457158225630559
- GohLJewellTLaversuchCSamantaAA systematic review of the influence of anti-TNF on infection rates in patients with rheumatoid arthritisRev Bras Reumatol20135350151524477729
- CheHLukasCMorelJCombeBRisk of herpes/herpes zoster during anti-tumor necrosis factor therapy in patients with rheumatoid arthritis. Systematic review and meta-analysisJoint Bone Spine201481321522123932722
- AdelzadehLJourabchiNWuJJThe risk of herpes zoster during biological therapy for psoriasis and other inflammatory conditionsJ Eur Acad Dermatol Venereol201428784685225081573
- CacciapagliaFZuccaroCIannoneFVaricella-zoster virus infection in rheumatoid arthritis patients in the anti-tumour necrosis factor eraClin Exp Rheumatol201533691792326394271
- GriffithsCEMStroberBEvan de KerkhofPACCEPT Study GroupComparison of ustekinumab and etanercept for moderate-to-severe psoriasisN Engl J Med2010362211812820071701
- LangleyRGElewskiBELebwohlMERASURE Study GroupFIXTURE Study GroupSecukinumab in plaque psoriasis – results of two phase 3 trialsN Engl J Med2014714326338
- ThaçiDBlauveltAReichKSecukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trialJ Am Acad Dermatol201573340040926092291
- GordonKBBlauveltAPappKAUNCOVER-1 Study GroupUNCOVER-2 Study GroupUNCOVER-3 Study GroupPhase 3 trials of ixekizumab in moderate-to-severe plaque psoriasisN Engl J Med2016375434535627299809
- BissonnetteRPariserDMWaselNRApremilast, an oral phosphodiesterase-4 inhibitor, in the treatment of palmoplantar psoriasis: results of a pooled analysis from phase II PSOR-005 and phase III efficacy and safety trial evaluating the effects of apremilast in psoriasis (ESTEEM) clinical trials in patients with moderate to severe psoriasisJ Am Acad Dermatol20167519910527021239
- ArvinAMVaricella-zoster virusClin Microbiol Rev1996933613818809466
- GershonAAGershonMDPathogenesis and current approaches to control of varicella-zoster virus infectionsClin Microbiol Rev201326472874324092852
- GildenDNagelMACohrsRJVaricella-zosterHandb Clin Neurol201412326528325015490
- CDC [webpage on the Internet]Shingles (Herpes Zoster) Available from: www.cdc.gov/shinglesAccessed August 23, 2016
- NikkelsAFSadzot-DelvauxCPiérardGEAbsence of ICAM-1 expression in varicella zoster virus infected keratinocytes during herpes zoster. Another immune evasion strategy?Am J Dermatopathol200426273214726820
- ArvinAMMoffatJFSommerMVaricella-zoster virus T cell tropism and the pathogenesis of skin infectionCurr Top Microbiol Immunol201034218920920397071
- TreschSTruebRMKamarachevJFrenchLEHofbauerGFDisseminated herpes zoster mimicking rheumatoid vasculitis in a rheumatoid arthritis patient on etanerceptDermatology2009219434734919648728
- WendlingDStreitGToussirotEPratiCHerpes zoster in patients taking TNFalpha antagonists for chronic inflammatory joint diseaseJoint Bone Spine200875554054318674945
- McDonaldJRZeringueALCaplanLHerpes zoster risk factors in a national cohort of veterans with rheumatoid arthritisClin Infect Dis200948101364137119368499
- StrangfeldAListingJHerzerPRisk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agentsJAMA2009301773774419224750
- García-DovalIPérez-ZafrillaBDescalzoMABIOBADASER 2.0 Study GroupIncidence and risk of hospitalisation due to shingles and chickenpox in patients with rheumatic diseases treated with TNF antagonistsAnn Rheum Dis201069101751175520551153
- SeracGTubachFMarietteXRisk of herpes zoster in patients receiving anti-TNF-α in the prospective French RATIO registryJ Invest Dermatol201213272672922113472
- CurtisJRXieFYunHBernatskySWinthropKLReal-world comparative risks of herpes virus infections in tofacitinib and biologic-treated patients with rheumatoid arthritisAnn Rheum Dis Epub2016425
- YunHXieFDelzellERisks of herpes zoster in patients with rheumatoid arthritis according to biologic disease-modifying therapyArthritis Care Res (Hoboken)201567573173625201241
- ZismanDBittermanHShalomGPsoriatic arthritis treatment and the risk of herpes zosterAnn Rheum Dis201675113113525261573
- SeganJStaplesMPMarchLLassereMChakravartyEFBuchbinderRRisk factors for herpes zoster in rheumatoid arthritis patients: the role of tumour necrosis factor-α inhibitorsIntern Med J201545331031825565419
- WinthropKLBaddleyJWChenLAssociation between the initiation of anti-tumor necrosis factor therapy and the risk of herpes zosterJAMA2013309988789523462785
- LongMDMartinCSandlerRSKappelmanMDIncreased risk of herpes zoster among 108604 patients with inflammatory bowel diseaseAliment Pharmacol Ther201337442042923240738
- FordACPeyrin-BirouletLOpportunistic infections with anti-tumor necrosis factor-α therapy in inflammatory bowel disease: meta-analysis of randomized controlled trialsAm J Gastroenterol201310812681276
- DreiherJKreschFSComaneshterDCohenADRisk of herpes zoster in patients with psoriasis treated with biologicsJ Eur Acad Dermatol Venereol2012261127113221923837
- FaillaVJacquesJCastronovoCNikkelsAFHerpes zoster in patients treated with biologicalsDermatology2012224325125622677775
- Di CostanzoLAyalaFMegnaMGaudielloFPatrìABalatoNThe risk of herpes zoster in the anti-TNF-α era: a case report and review of the literatureJ Dermatol Case Rep2013711423580906
- StrangfeldAEveslageMSchneiderMTreatment benefit or survival of the fittest: what drives the time-dependent decrease in serious infection rates under TNF inhibition and what does this imply for the individual patient?Ann Rheum Dis201170111914192021791449
- FaillaVNikkelsAFUstekinumab and herpes zosterDermatology2011222211912221266799
- FaillaVCastronovoCMeexCNikkelsAFProtracted herpes zoster and severe postherpetic neuralgia after inadvertant infliximab administrationEur J Dermatol20112178278321700542
- ZhuSMLiuYMAnEDChenQLInfluence of systemic immune and cytokine responses during the acute phase of zoster on the development of postherpetic neuralgiaJ Zhejiang Univ Sci B200910862563019650202
- JavedSKamiliQUMendozaNTyringSKPossible association of lower rate of postherpetic neuralgia in patients on anti-tumor necrosis factor-αJ Med Virol201183112051205521915882
- OxmanMNLevinMJJohnsonGRShingles Prevention Study GroupA vaccine to prevent herpes zoster and postherpetic neuralgia in older adultsN Engl J Med2005352222271228415930418
- ZhangJXieFDelzellEAssociation between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseasesJAMA20123081434922760290
- LalHCunninghamALGodeauxOZOE-50 StudyEfficacy of an adjuvanted herpes zoster subunit vaccine in older adultsN Engl J Med2015372222087209625916341
- WernerRNNikkelsAFMarinovicBEuropean consensus-based guideline (S2K) on the management of herpes zoster. Part 2: treatmentJ Eur Acad Dermatol VenereolIn press
- Nordgaard-LassenIDahlerupJFBelardEDanish Society for GastroenterologyGuidelines for screening, prophylaxis and critical information prior to initiating anti-TNF-alpha treatmentDan Med J2012597C448022759856
- TranCTDucancelleAMassonCLunel-FabianiFHerpes zoster: risk and prevention during immunomodulating therapyJoint Bone Spine Epub2016527
- NisarMKOstorAJTNF antagonists and shingles: is vaccination advisable?Ann Rheum Dis201372e123456927
- WernerRNNikkelsAFMarinovicBEuropean consensus-based guideline (S2K) on the management of herpes zoster. Part 1: diagnostic meansJ Eur Acad Dermatol VenereolIn press
- El HayderiLBontemsSNikkels-TassoudjiNSatellite lesions accompanying herpes zoster: a new prognostic sign for high-risk zosterBr J Dermatol201517261530153425556958
