Abstract
Acrodermatitis continua of Hallopeau (ACH) is a rare, sterile pustular eruption of one or more digits. The condition presents with tender pustules and underlying erythema on the tip of a digit, more frequently arising on a finger than a toe. As far as classification, ACH is considered a localized form of pustular psoriasis. The eruption typically occurs after local trauma or infection, but such a history is not always present and various other etiologies have been described including infectious, neural, inflammatory, and genetic causes. The natural progression of ACH is chronic and progressive, often resulting in irreversible complications such as onychodystrophy that can result in anonychia, as well as osteitis that can result in osteolysis of the distal phalanges. Because of the rarity of ACH, there have been no randomized controlled studies to evaluate therapies, resulting in an absence of standardized treatment guidelines. In clinical practice, a wide variety of treatments have been attempted, with outcomes ranging from recalcitrance to complete resolution. In recent years, the introduction of biologics has provided a new class of therapy that has revolutionized the treatment of ACH. Specifically, rapid and sustained responses have been reported with the use of anti-tumor necrosis factor agents like infliximab, adalimumab, and etanercept; IL-17 inhibitors like secukinumab; IL-12/23 inhibitors like ustekinumab; and IL-1 inhibitors like anakinra. Nevertheless, there remains a considerable need for more research into treatment for the benefit of individual patients with ACH as well as for the clinical knowledge gained by such efforts. The purpose of this review is to provide a comprehensive overview of the key features of ACH as well as a discussion of clinical management strategies for this unique and debilitating condition.
Introduction
Acrodermatitis continua of Hallopeau (ACH) is a rare, chronic, sterile pustular eruption of one or more digits. This entity has also been called pustular acrodermatitis, acrodermatitis continua suppurativa, dermatitis perstans, acropustulosis, acrodermatitis perstans, and dermatitis repens.Citation1–Citation4
ACH presents with tender, sterile pustules and underlying erythema on the tip of a digit, more frequently arising on a finger than a toe.Citation1,Citation5 The eruption typically occurs after local trauma or infection,Citation6–Citation8 however, such a history is not always presentCitation4 and neural and inflammatory etiologies have also been described.Citation5 ACH always involves the nail apparatus; if there is no nail involvement, then alternative diagnoses such as palmoplantar pustulosis (PPP) should be considered.Citation6 Over time, ACH becomes chronic and can exhibit proximal progression, extending to the dorsal aspect of the hand or the foot.Citation7 Without successful treatment, ACH can lead to several complications, including onychodystrophy that can result in anonychia as well as osteitis that can result in osteolysis of the distal phalanges.Citation4,Citation5,Citation7–Citation9 In these ways, ACH can cause profound disability and have a tremendously negative impact on quality of life in these patients.
As far as classification, ACH is considered a localized form of pustular psoriasis.Citation2,Citation5,Citation10 It appears to be most common in middle-aged females, but due to the overall rarity of ACH, epidemiological information is limited to anecdotal case reports.Citation6,Citation9,Citation11,Citation12 Relatedly, there are no standardized guidelines for treating ACH, but rather various case reports with a wide range of therapeutic approaches and outcomes.
The purpose of this review is to provide a comprehensive overview of the key features of ACH as well as a discussion of clinical management strategies for this unique and debilitating condition.
Methods
This review is based on a literature search in MEDLINE and Embase databases. The search was performed in February 2019. The following keywords were used: “Acrodermatitis continua of hallopeau,” “acrodermatitis continua,” “dermatitis repens,” “acrodermatitis perstans,” “Hallopeau*,” AND “psoria*.” Only articles written in English were included, and no restrictions were set on study type. No institutional review board approval was required for this review article.
Results
Pathophysiological mechanisms
As mentioned, various etiologies have been described for ACH, including traumatic, infectious, neural, and inflammatory causes.Citation5 In the majority of case reports, the patient recalls a minor trauma or infection preceding symptoms in the same location; however, many patients deny such precipitating factors.Citation9,Citation13
In recent years, several studies have suggested genetic causes of ACH. For example, two papers published in 2011 reported mutations in the IL36RN gene among patients with pustular psoriasis.Citation14,Citation15 IL36RN is amply expressed in the skin and belongs to the IL-1 cytokine family that is involved in the innate immune system. Several IL36 isoforms (IL-36α, IL-36β, and IL-36γ) induce cytokines that contribute to a pro-inflammatory cascade by binding to the IL-1 RL2 receptor and initiating the recruitment and localization of T cells, neutrophils, and dendritic cells to the skin.Citation10,Citation14–Citation16 The IL36RN gene encodes the IL36 receptor agonist (IL-36Ra), which normally acts to inhibit such pro-inflammatory signaling. Mutations in IL36RN can thereby lead to unregulated secretion of inflammatory cytokines,Citation14,Citation15,Citation17 and such mutations have been found in several cases of familial generalized pustular psoriasis (GPP). This inherited form is now known as DITRA, ie, “Deficiency of Interleukin-Thirty six Receptor Antagonist.” The authors of these papers suggest that such genetic mutations may also be related to other forms of pustular psoriasis such as ACH.Citation10,Citation15,Citation18
In 2013, a team of researchers identified a homozygous missense mutation in IL36RN in a male patient with ACH as well as his female sibling with GPP, confirming that these two diseases are likely related.Citation10 Genetic linkages are also seen within single patients; for example, a 2019 study identified nine patients with both ACH and GPP and four patients with both ACH and PPP.Citation19 Such reports are further supported by additional studies confirming the genotype–phenotype correlation between IL36RN mutations and GPP, PPP, and ACH.Citation17,Citation20–Citation22 As of May 2017, a total of 16 IL36RN mutations had been discovered, and these disease alleles are now believed to account for approximately 20% of ACH cases.Citation6
While IL36RN mutations are the most frequent genetic abnormality in pustular forms of psoriasis,Citation19 CARD14 and AP1S3 have also been identified as causative or susceptibility genes in ACH.Citation20,Citation23,Citation24 First, CARD14 encodes proteins produced by keratinocytes, and gain-of-function mutations lead to increased activation of nuclear factor-kappa B signaling and production of tumor necrosis factor-alpha (TNF-α),Citation8 which has been identified in association with ACH as well as GPP, PPP, and psoriasis vulgaris (PV).Citation25–Citation28 Second, AP1S3 plays a role in vesicular trafficking within keratinocytes, and loss-of-function mutations cause abnormal autophagy, increased activation of TNF-α, and overexpression IL36α.Citation8 Variants in AP1S3 have been described to contribute to ACH as well as GPP and PPP.Citation23,Citation29 Interestingly, preliminary research suggests that patients with ACH are more likely to carry two distinct mutations (ie, IL36RN and AP1S3 or IL36RN and CARD14) than patients affected by other forms of pustular psoriasis,Citation19,Citation26,Citation29,Citation30 but there is a need for more investigation in this area.
In summary, current evidence demonstrates that ACH is associated with a variety of genetic mutations in the genes IL36RN, CARD14, and AP1S3. Mutations in these same genes are associated with phenotypically related pustular skin conditions such as GPP and PPP.
Clinical features
Symptoms of ACH typically begin with the tip of one digit turning erythematous and developing painful pustules that migrate under the nail bed and matrix, leading to onychodystrophy.Citation1,Citation3 In the acute phase, the pustules rupture and coalesce to form “a lake of pus that carries the nail away,” as this condition is classically described.Citation1,Citation5 The resulting anonychia leaves behind an erythematous, shiny, and smooth distal digit where pustules often reform.Citation18,Citation31 As the disease progresses, the affected digits can become hyperkeratotic with psoriasiform scaling and continuing pustulation.Citation5 In longstanding cases, ACH can progress to involve additional digits or extend proximally to the dorsal hand or foot; however, the disease typically remains localized to the digit(s) for months or years before such proximal progression.Citation5,Citation32 These clinical findings are seen in , which illustrate a classic case of chronic ACH.
Figure 1 Right foot of a 64-year-old patient who first presented with asymmetric dactylitis characterized by erythema and tenderness of the first, third, and fourth distal interphalangeal joints with associated nail deformities. Written informed consent was obtained to take and publish the photographs above. Patient reported a history of possible osteomyelitis diagnosed by primary care provider, but this diagnosis was not supported by imaging, and symptoms progressed despite antibiotic treatment. Initially treated with topical steroids, resulting in preliminary improvement of skin changes. Approximately, 6 months after skin changes began, patient was found to have the findings documented in these figures. The clinical history and presentation of pustules, scale, erythema, and nail dystrophy of the first, third, and fourth digits were consistent with chronic Acrodermatitis continua of Hallopeau.
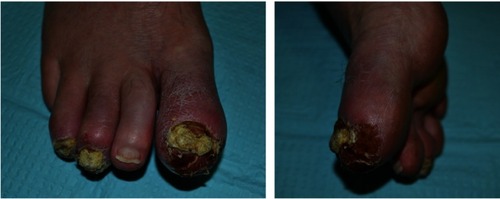
Rarely, the disease can cause osteitis of the underlying phalanges, resulting in osteolysis in severe cases.Citation3,Citation5,Citation31,Citation33,Citation34 Very rarely, ACH has been reported to result in acquired syndactyly, likely due to ACH lesions causing loss of interdigital epidermis and allowing for healing with fusion of adjacent tissues.Citation4,Citation35
There are several aspects of ACH’s clinical features that support its classification as a variant of pustular psoriasis. First, the previously described genetic mutations common to patients with ACH, GPP, and PPP suggest these conditions are on the same autoimmune inflammatory spectrum. Second, several authors have reported the development of joint pain and arthritis in patients with ACH.Citation3,Citation33,Citation34 Soft tissue and bone involvement, often revealed on X-ray imaging, further supports the association of ACH with psoriasis and associated arthritis. Third, there have been instances of geographic tongue in patients with ACH, which is a finding consistent with other forms of pustular psoriasis and other inflammatory conditions.Citation36 Lastly, ACH can progress to GPP, particularly the life-threatening von Zumbusch type.Citation4,Citation5,Citation32,Citation37,Citation38 Such patterns of progression highlight the critical importance of recognizing ACH as a localized form of pustular psoriasis that is unique, can rapidly evolve, and must be aggressively treated to prevent complications.
Differential diagnosis
ACH is commonly misdiagnosed. Based on clinical presentation, its purulence can imitate bacterial, fungal, or viral paronychia.Citation11 Depending on the patient’s age and comorbidities, secondarily infected contact dermatitis, dyshidrotic eczema, or a paraneoplastic process are reasonable possibilities.Citation4 Furthermore, other infections of the digits such as herpetic whitlow can present with vesiculopustules while dermatophytosis can present with scaly erythema, both resembling ACH. Autoimmune conditions such as pemphigus vulgaris can imitate ACH by presenting with inflammation and erosions of the digits. In a review by Seghal et al, the authors warn that late clinical manifestations of ACH may necessitate evaluations for a number of conditions including squamous cell carcinoma, melanoma, pyogenic granuloma, Reiter’s disease, subungual fibroma, glomus tumor, blastomycosis, and onychomycosis.Citation5
The most important condition to compare and contrast with ACH is PPP. Fortunately, several key features help distinguish these related conditions. First, ACH often follows trauma, as previously described, whereas PPP rarely involves a history of injury. Second, suppurative nail involvement is an early and defining feature of ACH whereas PPP does not always affect the nails and is usually non-suppurative.Citation6 Third, ACH most commonly remains unilateral and localized to a limited number of digits and with an irregular distribution for many years, while PPP is most commonly bilateral and symmetrical.Citation2 Lastly, features such as soft tissue sclerosis and osteolysis as described in ACH are not reported in PPP.Citation39
Overall, the similarities between ACH with many other conditions, especially PPP, necessitate a careful consideration of a broad differential diagnosis and a thorough clinical workup, as appropriate.
Histopathology
While the diagnosis of ACH is primarily clinical, based on the history and clinical findings described, skin and nail biopsies with staining and cultures can be helpful to exclude other conditions in the differential.Citation12 ACH appears histologically very similar to pustular psoriasis, with subcorneal neutrophilic pustules, spongiform pustules, and moderate lymphohistiocytic infiltrate.Citation1,Citation4,Citation5 Chronic ACH lesions usually show severe atrophy of the papillary dermis and thinning of the epidermis.Citation5 The nail matrix is always involved and typically exhibits moderate acanthosis, lymphocytic and neutrophilic exocytosis, and spongiosis.Citation40 Lastly, bacterial cultures are routinely sterile but can yield predominant growth of commensals, which is interpreted as non-contributory to the pathogenesis of this non-infectious inflammatory disease.Citation6
Management
ACH is characteristically chronic and recalcitrant in nature, and spontaneous improvement has rarely been observed.Citation2,Citation5,Citation12,Citation32 Furthermore, the condition is so rare that there have been no controlled studies to evaluate therapies, resulting in an absence of standardized treatment guidelines.Citation12 As a result, many therapeutic options have been attempted, with equivocal results as described in case reports.Citation5
Historically, the most common therapies for ACH have been similar to treatments for other forms of psoriasis, especially PPP,Citation1,Citation5,Citation12,Citation31 including topical and oral corticosteroids,Citation5,Citation41 topical vitamin D analogs,Citation42–Citation45 topical fluorouracil,Citation46,Citation47 topical calcineurin inhibitors,Citation48 tar,Citation5 topical and systemic retinoids,Citation39,Citation49,Citation50 cyclosporine,Citation51–Citation54 methotrexate,Citation48,Citation55,Citation56 and phototherapy or photochemotherapy.Citation5 In addition, granulocyte and monocyte adsorption apheresis has been attempted in ACH, with promising results in a small number of patients and far fewer side effects than some of the more traditional therapies.Citation57–Citation60
In most reported cases, a combination of these modalities is used (ie, systemic oral medication plus a topical applied to the affected area), with clinical responses ranging from recalcitrance to complete resolution.Citation5 Nail lesions are typically more difficult to manage than skin,Citation61 and recurrence is common with decrease in dosing or cessation of treatment.Citation5 Furthermore, the potential toxicities associated with several of these systemic therapies can prevent or limit the duration of treatment in certain individuals, especially children and elderly patients with ACH.Citation5,Citation62–Citation64
In recent years, the introduction of biologics has provided a new class of therapy that has revolutionized the treatment of ACH.Citation5 Specifically, rapid and sustained responses have been reported with the use of anti-TNF agents like infliximab,Citation55,Citation65,Citation66 adalimumab,Citation67–Citation71 and etanercept,Citation72–Citation77 IL-17 inhibitors like secukinumab,Citation78–Citation80 IL-12/23 inhibitors like ustekinumab,Citation81,Citation82 and IL-1 inhibitors like anakinra.Citation83 Furthermore, the identification of IL36RN mutations as a genetic contributor to ACH has informed the development of biologics that specifically block IL-36 signaling.Citation19 A 2017 paper described a high affinity, anti-human IL-36 receptor antagonist antibody that was initially tested in vitro and shown to directly inhibit IL-36-R-mediated signaling and inflammatory cytokine production in primary human keratinocytes and dermal fibroblases.Citation84 Following this work, a Phase I proof-of-concept study among seven patients with GPP treated with this anti-IL-36 monoclonal antibody showed significant improvement in symptoms.Citation85 Interestingly, the anti-IL-36 treatment was equally effective regardless of the absence or presence of an IL36RN mutation, suggesting the IL-36 pathway can be pathogenic regardless of a patient’s genetic background.Citation8,Citation84,Citation85
The use of both existing and emerging biologic agents to treat ACH, especially cases that are refractory to traditional treatments, is an area of ongoing research and development. No single agent has yet been identified as superior for ACH, but several have demonstrated efficacy, representing a promising new chapter for managing this chronic and often debilitating disease.
Discussion
These results demonstrate that ACH is a rare condition that is genetically and phenotypically related to other forms of pustular psoriasis but distinct in several dimensions that influence clinical outcomes. The relatively sparse number of case reports and consequent lack of epidemiological or controlled studies can make this condition particularly difficult for clinicians to manage. Fortunately, biologic agents offer a new treatment option with overwhelmingly positive results, especially in recalcitrant and refractory cases.Citation5
Nevertheless, there remains a need for more targeted research on the causes and management of ACH. First, the knowledge gained by identifying genetic mutations as a cause of ACH needs to be harnessed to develop more targeted treatment options. For example, IL36RN mutations result in defects in IL-1 signaling, as discussed. This elucidates why agents like anakinra (an IL-1 antagonist) have been reported as successful in treating ACH, but optimal treatment may require developing agents that specifically target IL-36 or common pathways between IL-1 and IL-36.Citation8,Citation10 As such research moves forward, it will be critical to recruit patients with ACH who have different genetic backgrounds to determine the efficacy of these new agents across the spectrum of genetic profiles, including patients with no identified mutations.
Second, genetic studies have revealed strong associations between IL36RN mutations but have also uncovered variants in other genes among patients with ACH, such as CARD14Citation21 and AP1S3.Citation20 These findings suggest a more complicated genetic picture to this disease beyond IL36RN, and there is a need for additional studies to more fully characterize its genetic underpinnings.
Third and perhaps most importantly, the rarity of ACH has thus far prevented rigorous research to establish evidence-based treatment guidelines. Given the pain and disfigurement caused by ACH, the potential for serious and irreversible complications, and the association of this rare variant with life-threatening forms of pustular psoriasis, there remains considerable need for more research into treatment for the benefit of individual patients with ACH as well as for the clinical knowledge gained by such efforts.
Disclosure
Tina Bhutani has received research funding from the National Psoriasis Foundation and has served as a research investigator and a consultant for Eli Lilly, Janssen, Merck, Celgene and Regeneron. Wilson Liao is funded in part by grants from the National Institutes of Health (R01AR065174, U01AI119125) and has served as a research investigator for Abbvie, Amgen, Janssen, Novartis, Pfizer and Regenero. The authors report no other conflicts of interest in this work.
References
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3(6):389–400. doi:10.2165/00128071-200203060-0000312113648
- Benjegerdes KE, Hyde K, Kivelevitch D, Mansouri B. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131–144. doi:10.2147/PTT.S9895429387600
- Jo SJ, Park JY, Yoon HS, Youn JI. Case of acrodermatitis continua accompanied by psoriatic arthritis. J Dermatol. 2006;33(11):787–791. doi:10.1111/j.1346-8138.2006.00180.x17073995
- Waller JM, Wu JJ, Murase JE, Dyson SW, Kelly KM. Chronically painful right thumb with pustules and onycholysis. Diagnosis: acrodermatitis continua of Hallopeau. Clin Exp Dermatol. 2007;32(5):619–620. doi:10.1111/j.1365-2230.2007.02440.x17501968
- Sehgal VN, Verma P, Sharma S, et al. Acrodermatitis continua of Hallopeau: evolution of treatment options. Int J Dermatol. 2011;50(10):1195–1211. doi:10.1111/j.1365-4632.2011.04993.x21950286
- Navarini AA, Burden AD, Capon F, et al. European consensus statement on phenotypes of pustular psoriasis. J Eur Acad Dermatol Venereol. 2017;31(11):1792–1799. doi:10.1111/jdv.1438628585342
- Hoegler KM, John AM, Handler MZ, Schwartz RA. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32(10):1645–1651. doi:10.1111/jdv.1494929573491
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178(3):614–618. doi:10.1111/bjd.1623229333670
- Yerushalmi J, Grunwald MH, Hallel-Halevy D, Avinoach I, Halevy S. Chronic pustular eruption of the thumbs. Diagnosis: acrodermatitis continua of Hallopeau (ACH). Arch Dermatol. 2000;136(7):925–930.
- Abbas O, Itani S, Ghosn S, et al. Acrodermatitis continua of Hallopeau is a clinical phenotype of DITRA: evidence that it is a variant of pustular psoriasis. Dermatology. 2013;226(1):28–31. doi:10.1159/000346572
- Rosenberg BE, Strober BE. Acrodermatitis continua. Dermatol Online J. 2004;10(3):9.
- Korman AM, Rzepka PV, Sopkovich JA. Acrodermatitis continua of Hallopeau. JAMA Dermatol. 2018;154(11):1346. doi:10.1001/jamadermatol.2017.619530285064
- Naldi L, Gambini D. The clinical spectrum of psoriasis. Clin Dermatol. 2007;25(6):510–518. doi:10.1016/j.clindermatol.2007.08.00318021886
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89(3):432–437. doi:10.1016/j.ajhg.2011.07.02221839423
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365(7):620–628. doi:10.1056/NEJMoa101306821848462
- O’Keefe E, Braverman IM, Cohen I. Annulus migrans. Identical lesions in pustular psoriasis, Reiter’s syndrome, and geographic tongue. Arch Dermatol. 1973;107(2):240–244.4685581
- Tauber M, Bal E, Pei XY, et al. IL36RN mutations affect protein expression and function: a basis for genotype-phenotype correlation in pustular diseases. J Invest Dermatol. 2016;136(9):1811–1819. doi:10.1016/j.jid.2016.04.03827220475
- Haneke E. Nail psoriasis: clinical features, pathogenesis, differential diagnoses, and management. Psoriasis (Auckl). 2017;7:51–63. doi:10.2147/PTT.S12628129387608
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2018;143(3):1021–1026.30036598
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366–1369. doi:10.1038/jid.2012.49023303454
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74(3):187–192. doi:10.1016/j.jdermsci.2014.02.00624656634
- Liang J, Huang P, Li H, et al. Mutations in IL36RN are associated with geographic tongue. Hum Genet. 2017;136(2):241–252. doi:10.1007/s00439-016-1750-y27900482
- Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94(5):790–797. doi:10.1016/j.ajhg.2014.04.00524791904
- Mossner R, Frambach Y, Wilsmann-Theis D, et al. palmoplantar pustular psoriasis is associated with missense variants in CARD14, but not with loss-of-function mutations in IL36RN in European patients. J Invest Dermatol. 2015;135(10):2538–2541. doi:10.1038/jid.2015.18625989471
- Korber A, Mossner R, Renner R, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J Invest Dermatol. 2013;133(11):2634–2637. doi:10.1038/jid.2013.21423648549
- Berki DM, Liu L, Choon SE, et al. Activating CARD14 mutations are associated with generalized pustular psoriasis but rarely account for familial recurrence in psoriasis vulgaris. J Invest Dermatol. 2015;135(12):2964–2970. doi:10.1038/jid.2015.28826203641
- Jordan CT, Cao L, Roberson ED, et al. PSORS2 is due to mutations in CARD14. Am J Hum Genet. 2012;90(5):784–795. doi:10.1016/j.ajhg.2012.03.01222521418
- Sugiura K, Muto M, Akiyama M. CARD14 c.526G>C (p.Asp176His) is a significant risk factor for generalized pustular psoriasis with psoriasis vulgaris in the Japanese cohort. J Invest Dermatol. 2014;134(6):1755–1757. doi:10.1038/jid.2014.4624476623
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and up-regulating IL-36 production. J Invest Dermatol. 2016;136(11):2251–2259. doi:10.1016/j.jid.2016.06.61827388993
- Mossner R, Wilsmann-Theis D, Oji V, et al. The genetic basis for most patients with pustular skin disease remains elusive. Br J Dermatol. 2018;178(3):740–748. doi:10.1111/bjd.1586728887889
- Alorainy M, Alshaya M, Al-Rikabi A, Ayesh M, Alsaif F. Acrodermatitis continua of Hallopeau with bone resorption in an 8-year-old patient: a case report. Case Rep Dermatol. 2017;9(3):259–264. doi:10.1159/00048537029422844
- Kim KH, Kim HL, Suh HY, et al. A case of acrodermatitis continua accompanying with osteolysis and atrophy of the distal phalanx that evoluted into generalized pustular psoriasis. Ann Dermatol. 2016;28(6):794–795. doi:10.5021/ad.2016.28.6.79427904292
- Okuno H, Ogura K, Okuyama R, Itoi E. Two cases of acrodermatitis continua of Hallopeau associated with generalized arthritis. Acta Dermatovenerol Croat. 2013;21(4):265–267.24476617
- Mueller RB, Ogilvie A, Schwarz S, Kern P, Schett G, Sticherling M. Adalimumab treatment of a patient with psoriasis suppurativa Hallopeau associated osteoarthropathy. Clin Exp Rheumatol. 2009;27:887.19917179
- Kirkup ME, Lovell CR. Acquired syndactyly secondary to acrodermatitis continua of Hallopeau. Br J Dermatol. 2005;152:1083–1084. doi:10.1111/j.1365-2133.2005.06580.x15888186
- Sadlier M. Acrodermatitis continua of Hallopeau and geographic tongue are variants of pustular psoriasis. JAAD Case Rep. 2018;4(3):277. doi:10.1016/j.jdcr.2017.08.02229687071
- Baker H, Ryan TJ. Generalized pustular psoriasis. A clinical and epidemiological study of 104 cases. Br J Dermatol. 1968;80(12):771–793. doi:10.1111/j.1365-2133.1968.tb11947.x4236712
- Ranugha PS, Kumari R, Thappa DM. Acrodermatitis continua of hallopeau evolving into generalised pustular psoriasis. Indian J Dermatol. 2013;58(2):161. doi:10.4103/0019-5154.108096
- Pearson LH, Allen BS, Smith JG Jr. Acrodermatitis continua of Hallopeau: treatment with etretinate and review of relapsing pustular eruptions of the hands and feet. J Am Acad Dermatol. 1984;11(4 Pt 2):755–762.6491002
- Sehgal VN, Sharma S. Significance of Gram’s stain smear, potassium hydroxide mount, culture, and microscopic pathology in the diagnosis of acrodermatitis continua of Hallopeau. Skinmed. 2011;9(4):260–261.21980714
- Sotiriadis D, Patsatsi A, Sotiriou E, Papagaryfallou I, Chrysomallis F. Acrodermatitis continua of Hallopeau on toes successfully treated with a two-compound product containing calcipotriol and betamethasone dipropionate. J Dermatolog Treat. 2007;18(5):315–318. doi:10.1080/0954663070136711817852633
- Piraccini BM, Tosti A, Iorizzo M, Misciali C. Pustular psoriasis of the nails: treatment and long-term follow-up of 46 patients. Br J Dermatol. 2001;144(5):1000–1005. doi:10.1046/j.1365-2133.2001.04189.x11359388
- Mozzanica N, Cattaneo A. The clinical effect of topical calcipotriol in acrodermatitis continua of hallopeau. Br J Dermatol. 1998;138(3):556. doi:10.1046/j.1365-2133.1998.02034.x
- Emtestam L, Weden U. Successful treatment for acrodermatitis continua of Hallopeau using topical calcipotriol. Br J Dermatol. 1996;135(4):644–646.8915165
- Brill TJ, Elshorst-Schmidt T, Valesky EM, Kaufmann R, Thaci D. Successful treatment of acrodermatitis continua of Hallopeau with sequential combination of calcipotriol and tacrolimus ointments. Dermatology. 2005;211(4):351–355. doi:10.1159/00008850716286746
- Fredriksson T. Topically applied fluorouracil in the treatment of psoriatic nails. Arch Dermatol. 1974;110(5):735–736.4418256
- Tsuji T, Sugai T. Topically administered fluorouracil in psoriasis. Arch Dermatol. 1972;105(2):208–212.5060863
- Wilsmann-Theis D, Hagemann T, Dederer H, Wenzel J, Bieber T, Novak N. Successful treatment of acrodermatitis continua suppurativa with topical tacrolimus 0.1% ointment. Br J Dermatol. 2004;150(6):1194–1197. doi:10.1111/j.1365-2133.2004.05992.x15214910
- van Dooren-Greebe RJ, van de Kerkhof PC, Chang A, Happle R. Acitretin monotherapy in acrodermatitis continua Hallopeau. Acta Derm Venereol. 1989;69(4):344–346.2568060
- Slawsky LD, Libow LF. Successful treatment of acrodermatitis continua of Hallopeau with etretinate. J Am Acad Dermatol. 1990;23(6 Pt 1):1176–1178.2273128
- Zachariae H, Thestrup-Pedersen K. Ciclosporin A in acrodermatitis continua. Dermatologica. 1987;175(1):29–32.
- Harland CC, Kilby PE, Dalziel KL. Acrodermatitis continua responding to cyclosporin therapy. Clin Exp Dermatol. 1992;17(5):376–378.1458653
- Peter RU, Ruzicka T, Donhauser G, Braun-Falco O. Acrodermatitis continua-type of pustular psoriasis responds to low-dose cyclosporine. J Am Acad Dermatol. 1990;23(3 Pt 1):515–516.2212157
- Georgakopoulos JR, Ighani A, Yeung J. Short- and long-term management of an acute pustular psoriasis flare: a case report. J Cutan Med Surg. 2017;21(5):452–456. doi:10.1177/120347541771249928535721
- Rubio C, Martin MA, Arranz Sanchez DM, Vidaurrazaga C, Casado M. Excellent and prolonged response to infliximab in a case of recalcitrant acrodermatitis continua of Hallopeau. J Eur Acad Dermatol Venereol. 2009;23:707–708. doi:10.1111/j.1468-3083.2008.02998.x18785890
- Chowdhury MM, Motley RJ. Treatment of acrodermatitis continua of Hallopeau with oral propylthiouracil and methotrexate. Clin Exp Dermatol. 2001;26(8):657–660.11722449
- Sakanoue M, Takeda K, Kawai K, Kanekura T. Granulocyte and monocyte adsorption apheresis for refractory skin diseases due to activated neutrophils, psoriasis, and associated arthropathy. Ther Apher Dial. 2013;17(5):477–483. doi:10.1111/1744-9987.1211324107275
- Seishima M, Mizutani Y, Shibuya Y, Nagasawa C, Aoki T. Efficacy of granulocyte and monocyte adsorption apheresis for pustular psoriasis. Ther Apher Dial. 2008;12(1):13–18. doi:10.1111/j.1744-9987.2007.00536.x18257807
- Shukuya R, Hasegawa T, Niwa Y, Okuma K, Ikeda S. Granulocyte and monocyte adsorption apheresis for generalized pustular psoriasis. J Dermatol. 2011;38(12):1130–1134. doi:10.1111/j.1346-8138.2011.01279.x22007872
- Kanekura T, Hiraishi K, Kawahara K, Maruyama I, Kanzaki T. Granulocyte and monocyte adsorption apheresis (GCAP) for refractory skin diseases caused by activated neutrophils and psoriatic arthritis: evidence that GCAP removes Mac-1-expressing neutrophils. Ther Apher Dial. 2006;10(3):247–256. doi:10.1111/j.1744-9987.2006.00369.x16817789
- Iijima S, Okazaki Y, Watanabe S, Maruyama Y. Case of acrodermatitis continua of Hallopeau following psoriasis with atypical clinical presentation. J Dermatol. 2014;41(11):1006–1008. doi:10.1111/1346-8138.1263925346303
- Hjorth N, Thomsen K. Parakeratosis pustulosa. Br J Dermatol. 1967;79(10):527–532. doi:10.1111/j.1365-2133.1967.tb11407.x6061563
- Chen P, Li C, Xue R, et al. Efficacy and safety of acitretin monotherapy in children with pustular psoriasis: results from 15 cases and a literature review. J Dermatolog Treat. 2018;29(4):353–363. doi:10.1080/09546634.2017.139579829098909
- Kiszewski AE, De Villa D, Scheibel I, Ricachnevsky N. An infant with acrodermatitis continua of Hallopeau: successful treatment with thalidomide and UVB therapy. Pediatr Dermatol. 2009;26(1):105–106. doi:10.1111/j.1525-1470.2008.00838.x19250426
- Mang R, Ruzicka T, Stege H. Successful treatment of acrodermatitis continua of Hallopeau by the tumour necrosis factor-alpha inhibitor infliximab (Remicade). Br J Dermatol. 2004;150:379–380. England. doi:10.1111/j.1365-2133.2003.05761.x14996123
- Ahmad K, Rogers S. Three years’ experience with infliximab in recalcitrant psoriasis. Clin Exp Dermatol. 2006;31(5):630–633. doi:10.1111/j.1365-2230.2006.02170.x16901301
- Tobin AM, Kirby B. Successful treatment of recalcitrant acrodermatitis continua of Hallopeau with adalimumab and acitretin. Br J Dermatol. 2005;153:445–446. doi:10.1111/j.1365-2133.2005.06759.x16086767
- Ryan C, Collins P, Kirby B, Rogers S. Treatment of acrodermatitis continua of Hallopeau with adalimumab. Br J Dermatol. 2009;160:203–205. doi:10.1111/j.1365-2133.2008.08893.x18945314
- Lefkir S, Slimani S, Brahimi N, Ladjouze-Rezig A. Successful treatment of Acrodermatitis continua of Hallopeau associated with psoriatic arthritis with adalimumab. Eur J Rheumatol. 2015;2(2):78–79. doi:10.5152/eurjrheum.2015.007227708932
- Di Costanzo L, Napolitano M, Patruno C, Cantelli M, Balato N. Acrodermatitis continua of Hallopeau (ACH): two cases successfully treated with adalimumab. J Dermatolog Treat. 2014;25(6):489–494. doi:10.3109/09546634.2013.84825924215490
- Sopkovich JA, Anetakis Poulos G, Wong HK. Acrodermatitis continua of hallopeau successfully treated with adalimumab. J Clin Aesthet Dermatol. 2012;5(2):60–62.22468174
- Thielen AM, Barde C, Marazza G, Saurat JH. Long-term control with etanercept (Enbrel) of a severe acrodermatitis continua of Hallopeau refractory to infliximab (Remicade). Dermatology. 2008;217(2):137–139. doi:10.1159/00013461318503258
- Bonish B, Rashid RM, Swan J. Etanercept responsive acrodermatitis continua of Hallopeau: is a pattern developing? J Drugs Dermatol. 2006;5(9):903–904.17039659
- Nikkels AF, Pierard GE. Etanercept and recalcitrant acrodermatitis continua of Hallopeau. J Drugs Dermatol. 2006;5(8):705–706.
- Kazinski K, Joyce KM, Hodson D. The successful use of etanercept in combination therapy for treatment of acrodermatitis continua of hallopeau. J Drugs Dermatol. 2005;4(3):360–364.15898294
- Puig L, Barco D, Vilarrasa E, Alomar A. Treatment of acrodermatitis continua of Hallopeau with TNF-blocking agents: case report and review. Dermatology. 2010;220(2):154–158. doi:10.1159/00027741520110631
- Silpa-archa N, Wongpraparut C. A recalcitrant acrodermatitis continua of Hallopeau successfully treated with etanercept. J Med Assoc Thai. 2011;94(9):1154–1157.21970208
- Balestri R, Rech G, Tasin L, Rizzoli L, Girardelli CR. Acrodermatitis continua of Hallopeau successfully treated with secukinumab. J Dermatolog Treat. 2018;29:1–7.
- Baron JA. Acrodermatitis of Hallopeau and erosive oral mucositis successfully treated with secukinumab. JAAD Case Rep. 2017;3(3):215–218. doi:10.1016/j.jdcr.2017.02.01628443313
- Herrero-Moyano M, Capusan TM, Martinez-Mera C, Llamas-Velasco M, Dauden E. Recalcitrant annular pustular psoriasis associated with psoriatic arthritis successfully treated with secukinumab. JAAD Case Rep. 2018;4(8):842–844. doi:10.1016/j.jdcr.2018.03.01630246137
- Adisen E, Ozer I, Temel B, Gurer MA. Ustekinumab for the treatment of acrodermatitis continua of Hallopeau refractory to anti-TNF agents. Dermatol Ther. 2017;30:2. doi:10.1111/dth.12460
- Saunier J, Debarbieux S, Jullien D, Garnier L, Dalle S, Thomas L. Acrodermatitis continua of Hallopeau treated successfully with ustekinumab and acitretin after failure of tumour necrosis factor blockade and anakinra. Dermatology. 2015;230(2):97–100. doi:10.1159/00036769025471551
- Lutz V, Lipsker D. Acitretin- and tumor necrosis factor inhibitor-resistant acrodermatitis continua of hallopeau responsive to the interleukin 1 receptor antagonist anakinra. Arch Dermatol. 2012;148(3):297–299. doi:10.1001/archdermatol.2011.247322431771
- Ganesan R, Raymond EL, Mennerich D, et al. Generation and functional characterization of anti-human and anti-mouse IL-36R antagonist monoclonal antibodies. MAbs. 2017;9(7):1143–1154. doi:10.1080/19420862.2017.135385328726542
- Bachelez H, Choon SE, Marrakchi S, et al. Inhibition of the interleukin-36 pathway for the treatment of generalized pustular psoriasis. N Engl J Med. 2019;380(10):981–983. doi:10.1056/NEJMc181131730855749
