Abstract
Psoriasis is associated with several comorbidities ranging from cardiovascular comorbidity and mental disorders to other immune mediated inflammatory diseases. However, most of these co-morbidities are often overlooked or diagnosed late. Furthermore, evidence suggests that comorbidities are undertreated. Here, we provide an overview of comorbidities in psoriasis and present a simple rundown of considerations of relevance to the clinician. We hope that this review may raise clinicians’ awareness of comorbidities in psoriasis and provide simple guidance regarding screening tools and treatment decisions in psoriasis with comorbidities.
Introduction
Among dermatologists and other healthcare professionals (HCPs) involved in the treatment and care of psoriasis patients, awareness is growing that psoriasis is not solely a skin disease. A large body of research has been devoted to the study of psoriasis and its comorbidities. However, it remains to be demonstrated whether this translates into an increased awareness and effort to treat these comorbidities in routine clinical practice. The clinician has an impressive armamentarium for examining comorbidities associated with psoriasis, but how the information obtained when deploying this diagnostic arsenal is brought to best clinical use remains to be established; When should screening and examinations for comorbidities be initiated, and in which instances should a change of treatment be considered of skin and joint symptoms due to comorbidities?
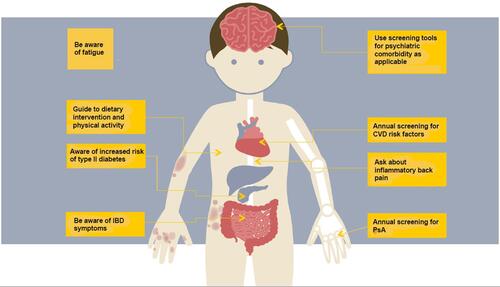
We therefore attempted to provide an overview of considerations for the physician when treating patients with psoriasis in order to enhance patient-centric care in those who suffer from multiple concomitant conditions. See and for comprised overview of comorbidities and relevant considerations.
Table 1 Considerations for the Clinician Regarding Psoriasis Comorbidities
Cardiovascular Comorbidity
Psoriasis, particularly severe psoriasis,Citation1 is associated with an increased risk of cardiovascular disease (CVD); acute myocardial infarction,Citation1,Citation2,Citation3 abdominal aortic aneurysm,Citation4 stroke,Citation5–Citation7 aortic valve stenosis,Citation8 atrial fibrillation,Citation6 and coronary artery disease.Citation9,Citation159 The increased incidence of traditional cardiovascular risk factors in psoriasis patients with psoriasis, e.g., hypercholesterolemia, metabolic syndrome and obesity, directly contributes to the overall increased risk of CVD in patients with psoriasis.Citation10,Citation13–Citation15,Citation19 Metabolic syndrome represents a group of cardiovascular risk factors; including hypertension, dyslipidemia, obesity and insulin resistance.Citation13,Citation16 The syndrome has been associated with an increased risk of CVD beyond the sum of the singular risk factors, and studies have shown an association between metabolic syndrome and psoriasis.Citation13
The main cause of increased CVD risk in psoriasis patients is still being studied: Mechanistic studies indicate that chronic inflammatory processes drives premature atherosclerosis through shared immunopathogenic mechanisms in psoriasis patients.Citation17 Evidence of systemic inflammation in psoriasisCitation18,Citation21 and an association of psoriasis with premature coronary artery disease has been reported.Citation9,Citation159,Citation160 However, other studies rebut these chronic inflammatory theories, and a final link between psoriasis and CVD has yet to be established.Citation22 Thus, it remains elusive to which extent the increased risk of CVD may be due to an increased prevalence of CV risk factors or whether it is caused by psoriasis itself due to, for example, chronic low-grade inflammation.
Further to this, it has been shown that despite an increased risk of coronary calcification in patients with psoriasis, these patients do not have a poorer prognosis than similar patients without psoriasis. Thus, after adjusting for other predictors, no increased risk of CV events and death was demonstrated in patients with psoriasis.Citation9
Nonetheless, despite a vast body of literature showing an overall increased risk of CVD in patients with psoriasis, it has been found, that patients with psoriasis and CVD risk factors receive less cardio-protective medical therapy than controls without psoriasis, or no such treatment at all.Citation23 Danish guidelines recommend that patients with psoriasis are treated according to the general guidelines for CVD risk factors, as a minimum.Citation24 It has been suggested that CVD risk factor scores should be multiplied by 1.5 for patients with severe psoriasis.Citation11
The European Academy of Dermatology and Venerology’s (EADV) guidelines recommend screening patients with psoriasis upon systemic treatment and local treatment for CVD risk factors every six and twelve months, respectively.Citation25
The European Society of Cardiology recommends CVD screening as a minimum and that the severity of the skin disease is taken into account if it is uncertain whether treatment for CVD risk factors should be initiated.Citation26
Furthermore, the British guideline recommends CVD risk assessment in adults with severe psoriasis at presentation and further cardiovascular assessment every five years.Citation27
Even greater attention to co-morbidities may be warranted in young patients with psoriasis and patients with psoriasis with severe skin affection since they carry the largest risk of CVD.Citation3
Adequate treatment of psoriasis activity has been suggested to reduce the risk of CVD. More specifically, biologic therapy, including TNF-alfa inhibitors, has been associated with, eg a reduced risk of major cardiac events, reduced coronary artery disease progression, and reduced vascular inflammation.Citation12,Citation28–Citation32 Other studies have found no reduction in risk of CVD, vascular inflammation or cardiovascular events.Citation33–Citation37
Lastly, studies have shown that dietary intervention and physical exercise may have a positive effect on the cardiovascular risk profile and the severity of psoriasis.Citation38,Citation39 Improvement of the cardiovascular risk profile by dietary means is clinically relevant. In short, it is recommended to eat higher volumes of fruit, vegetables, fish, wholegrain and polyunsaturated fats (fish, nuts etc.). Simultaneously, consuming only limited amounts of red and processed meat and refined carbohydrates is recommended.Citation26
Psoriatic Arthritis
Psoriatic arthritis (PsA) is an inflammatory, musculoskeletal, heterogenic disease that may include arthritis, enthesitis, dactylitis, axial disease and skin/nail disease.Citation40 PsA has a well-established association with psoriasis.Citation14,Citation42–Citation47 Approximately 70–80% of patients develop psoriasis before PsA. Simultaneous debut of psoriasis and PsA is seen in 10–15%, whereas PsA develops first in 10–15%.Citation48–Citation50
Importantly, a diagnostic delay of as little as 6 months is associated with peripheral joint damage and worse long-term functional outcome, and earlier treatment initiation is associated with a better clinical outcome.Citation51,Citation52 Inhibition of structural damage progression has been shown for several biologics and underpins the need to initiate effective disease-modifying therapy in patients with active PsA.Citation53 Several effective treatments with proven efficacy in both psoriasis and PsA are available.Citation77
The prevalence of PsA among patients with psoriasis depends on many factors, including geography, ethnicity and the applied diagnostic criteria. However, the prevalence of PsA is generally estimated to fall in the 20–30% range.Citation14,Citation42–Citation44,Citation56–Citation59
Like psoriasis, PsA heavily affects patients’ quality of life.Citation40,Citation45,Citation60 Despite the relatively extensive prevalence of PsA and its negative effect on quality of life, PsA remains difficult to diagnose and is therefore potentially undertreated.Citation44,Citation45,Citation61,Citation62,Citation63,Citation64
PsA is typically diagnosed clinically according to “CASPAR” (Classification for Psoriatic Arthritis) criteria.Citation64 These criteria include both clinical and paraclinical parameters. To diagnose a patient with PsA, the patient must have inflammatory joint disease and score at least three points on other parameters.Citation64,Citation65 Despite widespread use of these criteria, PsA remains difficult to diagnose and differentiate from osteoarthritis.Citation66
Even though PsA is a clinical diagnosis, numerous strides have been made to establish a single biomarker that may independently assist the physician in easily diagnosing PsA. Multiple candidate biomarkers have been suggested and investigated, but unfortunately none have so far proven successful in diagnosing PsA.Citation162,Citation163 Aside from biomarkers and the clinician’s assessments - screening questionnaires, ultrasound, x-ray and/or magnetic resonance imaging (MRI) may be used in the diagnostic process.Citation46,Citation62,Citation68,Citation71,Citation72,Citation163
A PsA screening questionnaire appears to be a feasible tool in the busy daily clinical practice of many dermatologists.Citation54 As to the choice of questionnaire, several validated questionnaires have shown good diagnostic performance.Citation54 These include for example Psoriasis Epidemiology Screening Tool (PEST),Citation71 The 4-item Psoriatic Arthritis Uncluttered Screening Evaluation (PURE-4),Citation72 Early Arthritis for Psoriatic Patients (EARP),Citation69 and Toronto Psoriatic Arthritis Screening (ToPAS).Citation73 However, the number of items, domains included, and how questions are presented varies between these questionnaires. A specific screening questionnaire can currently not be recommended unambiguously as the most useful for clinical practice in favor of the other listed above.
It is also relevant for the dermatologist to be aware that particular psoriasis phenotypes have been associated with an increased risk of PsA, these include nail dystrophy, perianal/intergluteal psoriasis and scalp lesions.Citation164 Furthermore, a high number of actively involved joints, either tender or swollen (defined as five or more); radiographic damage (joint destruction), in particular if there is also inflammation; elevated acute phase reactants; and extra-articular manifestations, in particular dactylitis reportedly predict a more aggressive disease progression.Citation76
Successful/efficient treatment of patients with coexisting psoriasis and PsA requires a therapeutic approach with proven efficacy in both skin and joints. Methotrexate may be effective for psoriasis and peripheral PsA, and several biologics have been approved for both psoriasis and PsA, whereas others have not been approved or have failed to show efficacy in either skin or joints.Citation77 Combination therapy or combing systemic and adjunctive local therapy or, for example, non-steroid anti-inflammatory drugs (NSAIDS) is often required. Specific treatment recommendations may be found in the following references and must be aligned with national healthcare system-specific regulations.Citation77–Citation79,Citation161
Thus, many well-tolerated and efficacious therapies are available. Priority should therefore be given to identifying PsA patients so that they may start relevant treatment, which decreases the risk of structural skeletal damage and potentially lowers the risk of long-term centralised pain syndromes.Citation45,Citation165
Other Immune-Mediated Diseases
Psoriasis is associated with an increased prevalence of other immune-mediated disease (IMID)s.Citation81,Citation82 The mechanisms of this association are not fully understood, but many factors, for example, genetic and environmental factors, have been suggested.Citation82,Citation83 Among other immune-mediated diseases associated with psoriasis are inflammatory bowel disease (IBD), rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome and systemic sclerosis.Citation14,Citation81,Citation82,Citation84–Citation87 We discuss only a selection of these below.
IBD, especially Crohn's disease (CD) and ulcerative colitis (UC), have been associated with psoriasis in several studies.Citation81,Citation90–Citation94,Citation102 Psoriasis patients have been estimated to have a higher risk of CD than the general population, and the same applies to a lesser extent to UC.Citation14,Citation91–Citation94 This risk is particularly pronounced in patients with severe psoriasis.Citation91 Furthermore, IBD is typically diagnosed before psoriasis.Citation95
Coexistence of IBD and psoriasis may require a combined effort by gastroenterologists and dermatologists as treatment modifications may often be necessary. Tumour necrosis factor inhibitor (TNFi) together with ustekinumab have been approved for both IBD and psoriasis and may be a good treatment option. However, up to 5% of patients treated with TNFi may develop paradoxical TNFi-induced psoriasis.Citation96–Citation98 Other treatment modalities used in IBD may not be effective in psoriasis, for example, vedolizumab and azathioprine. The widely used anti-interleukin (IL)-17 drugs for psoriasis are not recommended for patients with IBD.Citation77,Citation94,Citation99–Citation101
Mental Disorders
Mental disorders are well-known comorbidities to psoriasis, especially depression and anxiety.Citation14,Citation103–Citation109 The prevalence of mental disorders in patients with psoriasis is not easily estimated and has been estimated at 10–60%.Citation14,Citation110–Citation112
Intuitively, being diagnosed with psoriasis may have a negative effect on a patient’s mental state.Citation106,Citation113–Citation115 Additionally, an independent, increased risk of mental illness in patients with psoriasis has been shown.Citation105–Citation108,Citation115,Citation116 The negative effect on quality of life paired with the independent, elevated risk of mental disorders has potential to create a vicious cycle.Citation108,Citation117 However, of notice, the psychological impact does not always correlate with the clinical severity of the ongoing skin disease.Citation14 Moreover, psoriasis patients have an elevated risk of suicidal ideation and behaviour (SIB), including a potentially increased risk of completing suicide.Citation118–Citation122
Despite the well-established association between mental disorders and psoriasis, and the fact that early diagnosis of mental illness has proven important, mental disorders in patients with psoriasis are underrecognised and undertreated.Citation108,Citation109,Citation123,Citation124 Screening for mental health disorders, especially in patients with psychiatric risk factors, and consideration of psychotherapy of patients with psoriasis are thus imperative.Citation105,Citation111,Citation117
Multiple depression and mental health disorder questionnaires exist to aid the diagnosis of mental health disorders. Some well-known questionnaires are: the Beck Depression Inventory and the Spielberger State-Trait Anxiety Scale I–II.Citation118,Citation166,Citation167 To our knowledge, no specific questionnaires have proven superior.
However, dermatologists should be particularly attentive to females and younger and more severely affected patients with psoriasis since they have been shown to carry an increased risk of mental illnesses (including SIB).Citation105,Citation113,Citation118,Citation122,Citation127
Studies have shown that biological treatment of psoriasis decreases symptoms of depression; however, treatment of psoriasis does not always equal better odds for remission of mental disorders.Citation14,Citation106,Citation109,Citation116,Citation121,Citation128
Other Comorbidities
Fatigue has been associated with multiple inflammatory diseases, including psoriasis.Citation129 Fatigue may interfere profoundly with a patient’s daily life.Citation129 The severity of fatigue correlates with the severity of psoriasis.Citation130 Studies have shown that up to 50% of patients with psoriasis had considerable fatigue.Citation129 Biological drugs used for the treatment of psoriasis consistently reported a small-to-moderate beneficial effect on fatigue independently of the type of drug.Citation131 In addition to optimal treatment of underlying disease activity, exercise programmes and supervised self-management programmes with cognitive-behavioural therapy or mindfulness may be beneficial in coping with fatigue.Citation132–Citation134
Ocular comorbidities, such as uveitis, has been found in a number of psoriasis patients.Citation135–Citation137 Ocular comorbidities are important to diagnosis and treat, and can easily be overlooked.Citation135 The risk is elevated especially in patients with concomitant PsA as studies have shown that patients with PsA and severe psoriasis have a 2.4‐fold higher risk of developing non-infectious uveitis than the general population.Citation138 Clinicians may screen patients with for example the Ocular Manifestations in Psoriasis Screening questionnaire.Citation135 Clinicians may also be aware of clinical characteristics of uveitis, including: eye redness, eye pain, light sensitivity, blurred vision, floaters and decreased vision.Citation139
Epidemiologic studies have indicated that psoriasis is associated with an increased risk of various types of cancer and increased cancer mortality, although results are conflicting and no full consensus on the matter exists.Citation140 Lymphoma has been studied for its possible association with psoriasis. Thus, some studies have found a slightly increased risk of lymphoma and cutaneous lymphoma among patients with psoriasis,Citation141–Citation146 whereas other studies have found that when systemic psoriasis treatment was taken into account, the risk was not significantly higher than in the general background population.Citation147
Some studies report that psoriasis is associated with a lower socioeconomic status in general and some that a correlation exists between the severity of psoriasis and a lower socioeconomic status.Citation148–Citation150 Clinicians should therefore consider adopting a holistic approach to patients with psoriasis to avoid patients establishing a negative spiral by which disease causes further socioeconomic decline.Citation168
Patients with psoriasis have also been shown to have an increased risk of non-alcoholic fatty liver disease (NAFLD), which in part may be attributed to the increased risk of metabolic syndrome and obesity in psoriasis patientsCitation151–Citation154 and the existence of a Hepato-Dermal Axis has been proposed.Citation155
Furthermore, NAFLD has been associated with reduced eGFR in psoriasis patients.Citation156
Treatment-Related Pitfalls
A noteworthy number of psoriasis patients are treated with biologics and other systemic immunomodulators, which may, in some cases, alter the risk of certain illnesses. Such illnesses should not be considered comorbidities to psoriasis and consequently not directly related to psoriasis. However, although not in scope for this review, they remain important considerations for the clinician and are therefore mentioned briefly below: Biologics do not seem to increase the risk of cancer, although some studies have indicated a slightly higher risk for TNF-inhibitors than for newer biologics and methotrexate.Citation157 The risk of non-melanoma skin cancer is elevated for both anti–TNF agents and non-biologics and may be affected by previous treatment with psoralen and ultraviolet A (PUVA) therapy and photo-therapy.Citation169
Methotrexate is among the first-line systemic treatment options for psoriasis in many countriesMethotrexate treatment may be associated with a number of side-effects including an increased risk of liver damage.Citation151,Citation170
Biologics are associated with slightly increased risk of infections, and anti-IL17 agents are associated with an increased risk of mucocutaneous candidiasis.Citation171 Risks of serious infections are generally slightly higher for patients on anti-TNF than on anti-IL17 and anti-IL23 agents.Citation158 Increased awareness of infections and candidiasis and prescription of relevant treatment are warranted in patients on biologics and other immunomodulators.Citation158,Citation171
Conclusion
Psoriasis is associated with an increased risk of several comorbidities including cardiovascular disease, psoriatic arthritis, mental disorders and other immune-mediated diseases, and these associations require the treating physician’s attention.
A holistic approach to patients with psoriatic disease is recommended; a focus not only on skin symptoms but on all aspects of the disease including comorbidities may improve disease management and prevent long-term mental and physical impairment. Arguably, attention to comorbidities may improve the quality of life of some patients with psoriasis. Managing all comorbidities associated with psoriasis may constitute a considerable task for the clinician. The use of screening tools may possibly help discover patients in need of referral to other specialties or for further investigation.
Throughout this article, we have attempted to provide relevant considerations and guidance for physicians providing care for patients with psoriasis.
Acknowledgments
Louise Faurskov Møller was co-developer of .
Disclosure
Lars Iversen has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by: AbbVie, Almirall, Amgen, Astra Zeneca, BMS, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, Janssen Cilag, Kyowa, Leo Pharma, MSD, Novartis, Pfizer, Regranion, Samsung, Union Therapeutics, UCB. Kasper Hjuler has been a consultant and advisor for the following companies: AbbVie, Bristol Myers Squibb (BMS), Janssen, LEO Pharma, UCB, Novartis and has received speaking fees or grants from: AbbVie, Eli Lilly, LEO Pharma, Novartis, Janssen. The authors report no other conflicts of interest in this work.
References
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273(2):197–204. doi:10.1111/j.1365-2796.2012.02593.x
- Ahlehoff O, Gislason GH, Lindhardsen J, et al. Prognosis following first-time myocardial infarction in patients with psoriasis: a Danish nationwide cohort study: psoriasis and prognosis following myocardial infarction. J Intern Med. 2011;270(3):237–244. doi:10.1111/j.1365-2796.2011.02368.x
- Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735. doi:10.1001/jama.296.14.1735
- Khalid U, Egeberg A, Ahlehoff O, Smedegaard L, Gislason GH, Hansen PR. Nationwide study on the risk of abdominal aortic aneurysms in patients with psoriasis. Arterioscler Thromb Vasc Biol. 2016;36(5):1043–1048. doi:10.1161/ATVBAHA.116.307449
- Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study: psoriasis and cardiovascular risk. J Intern Med. 2011;270(2):147–157. doi:10.1111/j.1365-2796.2010.02310.x
- Ahlehoff O, Gislason GH, Jørgensen CH, et al. Psoriasis and risk of atrial fibrillation and ischaemic stroke: a Danish Nationwide Cohort Study. Eur Heart J. 2012;33(16):2054–2064. doi:10.1093/eurheartj/ehr285
- Ahlehoff O, Gislason G, Lamberts M, et al. Risk of thromboembolism and fatal stroke in patients with psoriasis and nonvalvular atrial fibrillation: a Danish nationwide cohort study. J Intern Med. 2015;277(4):447–455. doi:10.1111/joim.12272
- Khalid U, Ahlehoff O, Gislason GH, Skov L, Torp-Pedersen C, Hansen PR. Increased risk of aortic valve stenosis in patients with psoriasis: a nationwide cohort study. Eur Heart J. 2015;36(32):2177–2183. doi:10.1093/eurheartj/ehv185
- Tinggaard AB, Hjuler KF, Andersen IT, Winther S, Iversen L, Bøttcher M. Prevalence and severity of coronary artery disease linked to prognosis in psoriasis and psoriatic arthritis patients: a multi‐centre cohort study. J Intern Med. 2021;290:693–703. doi:10.1111/joim.13311
- Miller IM et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013Dec;69(6):1014-24. doi:10.1016/j.jaad.2013.06.053. PMID: 24238156.
- Kragballe K, Skov L, Kofoed K, et al. Anbefalinger for vurdering af risiko for kardiovaskulær sygdom hos patienter med psoriasis og psoriasis artrit Rapport udvalg nedsat af Dansk Dermatologisk Selskab, Dansk Cardiologisk Selskab og Dansk Reumatologisk Selskab. [Recommendations for assessing the risk of cardiovascular disease in patients with psoriasis and psoriatic arthritis Report committee set up by the Danish Dermatological Society, the Danish Cardiological Society and the Danish Rheumatological Society]. Available from: http://dds.nu/wp-content/uploads/2012/08/Anbefalinger-for-vurdering-af-risikoen-for-kardiovaskulær-sygdom.pdf. Accessed March 25, 2022.
- Elnabawi YA, Dey AK, Goyal A, et al. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res. 2019;115(4):721–728. doi:10.1093/cvr/cvz009
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 Pt 1):556–562. doi:10.1038/jid.2011.365
- Oliveira M, Rocha BO, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90(1):9–20. doi:10.1590/abd1806-4841.20153038
- Gelfand JM, Yeung H. Metabolic syndrome in patients with psoriatic disease. J Rheumatol Suppl. 2012;89:24–28. doi:10.3899/jrheum.120237
- Alberti KGM, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi:10.1016/S0140-6736(05)67402-8
- Gisondi P, Bellinato F, Girolomoni G, Albanesi C. Pathogenesis of chronic plaque psoriasis and its intersection with cardio-metabolic comorbidities. Front Pharmacol. 2020;11. doi:10.3389/fphar.2020.00117
- Dowlatshahi EA, van der Voort E, Arends LR, Nijsten T. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169(2):266–282. doi:10.1111/bjd.12355
- Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55(5):829–835. doi:10.1016/j.jaad.2006.08.040
- Ahlehoff O, Lindhardsen J, Gislason GH, et al. Prognosis after percutaneous coronary intervention in patients with psoriasis: a cohort study using Danish nationwide registries. BMC Cardiovasc Disord. 2012;12(1). doi:10.1186/1471-2261-12-79
- Hjuler K, Gormsen L, Vendelbo M, Egeberg A, Nielsen J, Iversen L. Systemic inflammation and evidence of a cardio-splenic axis in patients with psoriasis. Acta Dermato Venereologica. 2018;98(4):390–395. doi:10.2340/00015555-2873
- Stern RS, Nijsten T. Going beyond associative studies of psoriasis and cardiovascular disease. J Invest Dermatol. 2012;132(3 Pt 1):499–501. doi:10.1038/jid.2011.452
- Ahlehoff O, Skov L, Gislason G, et al. Pharmacological undertreatment of coronary risk factors in patients with psoriasis: observational study of the Danish nationwide registries. PLoS One. 2012;7(4):e36342. doi:10.1371/journal.pone.0036342
- National klinisk retningslinje for psoriasis - Sundhedsstyrelsen. [National Recommendations for Psoriasis. Danish Health Authority]. Available from: https://www.sst.dk/-/media/Udgivelser/2016/NKR-psoriasis/National-klinisk-retningslinje-psoriasis.ashx?la=da&hash=09EF41C7A2EDD0BE7DB1FCD1644718E75C7B6E3A. Accessed March 25, 2022.
- Daudén E, Castañeda S, Suárez C, et al. Clinical practice guideline for an integrated approach to comorbidity in patients with psoriasis: approach to comorbidity in psoriasis. J Eur Acad Dermatol Venereol. 2013;27(11):1387–1404. doi:10.1111/jdv.12024
- Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur J Prev Cardiol. 2021;zwab154. doi:10.1093/eurjpc/zwab154
- Psoriasis - assessment and management of psoriasis. Clinical Guideline. Methods, evidence and recommendations; 2012. Available from: https://www.nice.org.uk/guidance/cg153/evidence/full-guideline-pdf-188351533. Accessed March 25, 2022.
- Wu JJ, Joshi AA, Reddy SP, et al. Anti-inflammatory therapy with tumour necrosis factor inhibitors is associated with reduced risk of major adverse cardiovascular events in psoriasis. J Eur Acad Dermatol Venereol. 2018;32(8):1320–1326. doi:10.1111/jdv.14951
- Ahlehoff O, Hansen PR, Gislason GH, et al. Myocardial function and effects of biologic therapy in patients with severe psoriasis: a prospective echocardiographic study. J Eur Acad Dermatol Venereol. 2016;30(5):819–823. doi:10.1111/jdv.13152
- Hjuler KF, Bøttcher M, Vestergaard C, Bøtker HE, Iversen L, Kragballe K. Association between changes in coronary artery disease progression and treatment with biologic agents for severe psoriasis. JAMA Dermatol. 2016;152(10):1114. doi:10.1001/jamadermatol.2016.1984
- Gelfand JM, Shin DB, Alavi A, et al. A phase IV, randomized, double-blind, placebo-controlled crossover study of the effects of ustekinumab on vascular inflammation in psoriasis (the VIP-U trial). J Invest Dermatol. 2020;140(1):85–93.e2. doi:10.1016/j.jid.2019.07.679
- Mehta NN, Shin DB, Joshi AA, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circulation. 2018;11(6). doi:10.1161/CIRCIMAGING.117.007394
- Bissonnette R, Harel F, Krueger JG, et al. TNF-α antagonist and vascular inflammation in patients with psoriasis vulgaris: a randomized placebo-controlled study. J Invest Dermatol. 2017;137(8):1638–1645. doi:10.1016/j.jid.2017.02.977
- Lebwohl M. Does treatment of psoriasis reduce cardiovascular comorbidities? J Investig Dermatol. 2017;137(8):1612–1613. doi:10.1016/j.jid.2017.06.001
- Gisondi P. Does systemic treatment of psoriasis reduce the risk of comorbidities? Br J Dermatol. 2020;182(4):823–824. doi:10.1111/bjd.18456
- Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(3):480–489. doi:10.1136/annrheumdis-2014-206624
- von Stebut E, Reich K, Thaçi D, et al. Impact of secukinumab on endothelial dysfunction and other cardiovascular disease parameters in psoriasis patients over 52 weeks. J Invest Dermatol. 2019;139(5):1054–1062. doi:10.1016/j.jid.2018.10.042
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes. 2015;39(8):1197–1202. doi:10.1038/ijo.2015.64
- Klingberg E, Björkman S, Eliasson B, Larsson I, Bilberg A. Weight loss is associated with sustained improvement of disease activity and cardiovascular risk factors in patients with psoriatic arthritis and obesity: a prospective intervention study with two years of follow-up. Arthritis Res Ther. 2020;22(1):254. doi:10.1186/s13075-020-02350-5
- Ritchlin CT, Colbert RA, Gladman DD. Psoriatic ArthritisN Engl J Med. 2017 Mar 9;376(10):957-970. doi: 10.1056/NEJMra1505557. Erratum in: N Engl J Med. 2017 May 25;376(21):2097. PMID: 28273019.
- Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum. 2009;61(2):233–239. doi:10.1002/art.24172
- Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80(1):251–265.e19. doi:10.1016/j.jaad.2018.06.027
- Ocampo DV, Gladman D. Psoriatic arthritis. F1000Res. 2019;8:F1000Faculty Rev–1665. doi:10.12688/f1000research.19144.1
- Menter A. Psoriasis and psoriatic arthritis overview. Am J Manag Care. 2016;22(8 Suppl):s216–224.
- Gladman DD. Recent advances in understanding and managing psoriatic arthritis. F1000Res. 2016;5:2670. doi:10.12688/f1000research.9592.1
- Khraishi M, Chouela E, Bejar M, et al. High prevalence of psoriatic arthritis in a cohort of patients with psoriasis seen in a dermatology practice. J Cutan Med Surg. 2012;16(2):122–127. doi:10.2310/7750.2011.10101
- Mahajan VK, Sharma AL, Chauhan PS, Mehta KS, Sharma NL. Early treatment with addition of low dose prednisolone to methotrexate improves therapeutic outcome in severe psoriatic arthritis. Indian J Dermatol. 2013;58(3):240. doi:10.4103/0019-5154.110847
- Kleinert S, Feuchtenberger M, Kneitz C, Tony HP. Psoriatic arthritis: clinical spectrum and diagnostic procedures. Clin Dermatol. 2007;25(6):519–523. doi:10.1016/j.clindermatol.2007.08.004
- Gottlieb A, Korman NJ, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol. 2008;58(5):851–864. doi:10.1016/j.jaad.2008.02.040
- Scarpa R, Oriente P, Pucino A, et al. Psoriatic arthritis in psoriatic patients. Br J Rheumatol1984;23:246–50) + (Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA) – an analysis of 220 patients. Q J Med. 1987;62:127–41.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74(6):1045–1050. doi:10.1136/annrheumdis-2013-204858
- Theander E, Husmark T, Alenius GM, et al. Early psoriatic arthritis: short symptom duration, male gender and preserved physical functioning at presentation predict favourable outcome at 5-year follow-up. Results from the Swedish Early Psoriatic Arthritis Register (SwePsA). Ann Rheum Dis. 2014;73(2):407–413. doi:10.1136/annrheumdis-2012-201972
- van der Heijde D, Gladman DD, Kavanaugh A, Mease PJ. Assessing structural damage progression in psoriatic arthritis and its role as an outcome in research. Arthritis Res Ther. 2020;22(1):18. doi:10.1186/s13075-020-2103-8
- Urruticoechea-Arana A, Benavent D, León F, et al. Psoriatic arthritis screening: a systematic literature review and experts’ recommendations. PLoS One. 2021;16(3):e0248571. doi:10.1371/journal.pone.0248571
- Ruderman EM, Tambar S. Psoriatic arthritis: prevalence, diagnosis, and review of therapy for the dermatologist. Dermatol Clin. 2004;22(4):477–486, x. doi:10.1016/S0733-8635(03)00127-X
- Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3(1):55–78. doi:10.1016/0049-0172(73)90035-8
- Hukuda S, Minami M, Saito T, et al. Spondyloarthropathies in Japan: nationwide questionnaire survey performed by the Japan Ankylosing Spondylitis Society. J Rheumatol. 2001;28(3):554–559.
- Fournié B, Crognier L, Arnaud C, et al. Proposed classification criteria of psoriatic arthritis. A preliminary study in 260 patients. Rev Rhum Engl Ed. 1999;66(10):446–456.
- Helliwell PS, Ruderman EM. Natural history, prognosis, and socioeconomic aspects of psoriatic arthritis. Rheum Dis Clin North Am. 2015;41(4):581–591. doi:10.1016/j.rdc.2015.07.004
- Villani AP, Rouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73(2):242–248. doi:10.1016/j.jaad.2015.05.001
- Girolomoni G, Gisondi P. Psoriasis and systemic inflammation: underdiagnosed enthesopathy. J Eur Acad Dermatol Venereol. 2009;23(Suppl 1):3–8. doi:10.1111/j.1468-3083.2009.03361.x
- Gladman DD, Thavaneswaran A, Chandran V, Cook RJ. Do patients with psoriatic arthritis who present early fare better than those presenting later in the disease? Ann Rheum Dis. 2011;70(12):2152–2154. doi:10.1136/ard.2011.150938
- Alten R, Conaghan PG, Strand V, et al. Unmet needs in psoriatic arthritis patients receiving immunomodulatory therapy: results from a large multinational real-world study. Clin Rheumatol. 2019;38(6):1615–1626. doi:10.1007/s10067-019-04446-z
- Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–2673. doi:10.1002/art.21972
- Tillett W, Costa L, Jadon D, et al. The ClASsification for Psoriatic ARthritis (CASPAR) criteria – a retrospective feasibility, sensitivity, and specificity study: table 1. J Rheumatol. 2012;39(1):154–156. doi:10.3899/jrheum.110845
- Dennis McGonagle, Kay-Geert A. Hermann, Ai Lyn Tan, Differentiation between osteoarthritis and psoriatic arthritis: implications for pathogenesis and treatment in the biologic therapy era, Rheumatology, Volume 54, Issue 1, January 2015, Pages 29–38.
- Chandran V, Cook RJ, Edwin J, et al. Soluble biomarkers differentiate patients with psoriatic arthritis from those with psoriasis without arthritis. Rheumatology. 2010;49(7):1399–1405. doi:10.1093/rheumatology/keq105
- Dominguez P, Gladman DD, Helliwell P, Mease PJ, Husni ME, Qureshi AA. Development of screening tools to identify psoriatic arthritis. Curr Rheumatol Rep. 2010;12(4):295–299. doi:10.1007/s11926-010-0113-2
- Tinazzi I, Adami S, Zanolin EM, et al. The early psoriatic arthritis screening questionnaire: a simple and fast method for the identification of arthritis in patients with psoriasis. Rheumatology. 2012;51(11):2058–2063. doi:10.1093/rheumatology/kes187
- Eder L, Jayakar J, Thavaneswaran A, et al. Is the Madrid Sonographic Enthesitis Index useful for differentiating psoriatic arthritis from psoriasis alone and healthy controls? J Rheumatol. 2014;41(3):466–472. doi:10.3899/jrheum.130949
- Ibrahim, G.H., et al., Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol, 2009. 27(3): p. 469-74.
- Audureau, E., et al., Psoriatic arthritis screening by the dermatologist: development and first validation of the 'PURE-4 scale'. J Eur Acad Dermatol Venereol, 2018. 32(11): p. 1950-1953.
- Gladman DD, Schentag CT, Tom BD, Chandran V, Brockbank J, Rosen C, Farewell VT. Development and initial validation of a screening questionnaire for psoriatic arthritis: the Toronto Psoriatic Arthritis Screen (ToPAS). Ann Rheum Dis. 2009 Apr;68(4):497-501. doi:10.1136/ard.2008.089441. Epub 2008 Apr 29. PMID: 18445625.
- Gisondi P, Tinazzi I, El-Dalati G, et al. Lower limb enthesopathy in patients with psoriasis without clinical signs of arthropathy: a Hospital-based case-control study. Ann Rheum Dis. 2008;67(1):26–30. doi:10.1136/ard.2007.075101
- Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):499–510.
- Brüner M, Dige A, Loft AG, et al. Spondylitis-psoriasis-enthesitis-enterocolitis-dactylitis-uveitis-peripheral synovitis (SPEED-UP) treatment. Autoimmun Rev. 2021;20(2):102731. doi:10.1016/j.autrev.2020.102731
- Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79(6):700.1–712. doi:10.1136/annrheumdis-2020-217159
- Kavanaugh A, Coates LC, van der Windt DA, Corp N, Soriano ER. GRAPPA treatment recommendations: updates and methods. J Rheumatol. 2020;96:41–45. doi:10.3899/jrheum.200126
- Vena GA, Vestita M, Cassano N. Can early treatment with biologicals modify the natural history of comorbidities? Dermatol Ther. 2010;23(2):181–193. doi:10.1111/j.1529-8019.2010.01313.x
- Makredes M, Robinson D, Bala M, Kimball AB. The burden of autoimmune disease: a comparison of prevalence ratios in patients with psoriatic arthritis and psoriasis. J Am Acad Dermatol. 2009;61(3):405–410. doi:10.1016/j.jaad.2009.02.015
- Wu JJ, Nguyen TU, Poon KYT, Herrinton LJ. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67(5):924–930. doi:10.1016/j.jaad.2012.04.039
- Ellinghaus D, Ellinghaus E, Nair RP, et al. Combined analysis of genome-wide association studies for Crohn disease and psoriasis identifies seven shared susceptibility loci. Am J Hum Genet. 2012;90(4):636–647. doi:10.1016/j.ajhg.2012.02.020
- Najarian DJ, Gottlieb AB. Connections between psoriasis and Crohn’s disease. J Am Acad Dermatol. 2003;48(6):805–821;quiz 822–824. doi:10.1067/mjd.2003.540
- Yen H, Chi CC. Association between psoriasis and vitiligo: a systematic review and meta-analysis. Am J Clin Dermatol. 2019;20(1):31–40. doi:10.1007/s40257-018-0394-1
- Blegvad C, Egeberg A, Nielsen T, et al. Autoimmune disease in children and adolescents with psoriasis: a cross-sectional study in Denmark. Acta Derm Venerol. 2017;97(10):1225–1229. doi:10.2340/00015555-2743
- Ayala-Fontánez N, Soler DC, McCormick TS. Current knowledge on psoriasis and autoimmune diseases. Psoriasis. 2016;6:7–32. doi:10.2147/PTT.S64950
- Christophers E. Psoriasis–epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26(4):314–320. doi:10.1046/j.1365-2230.2001.00832.x
- Sacks D, Baxter B, Campbell BC, et al.; From the American Association of Neurological Surgeons (AANS), American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE), Canadian Interventional Radiology Association (CIRA), Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR), European Stroke Organization (ESO), Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS), and World Stroke Organization (WSO). Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612–632. doi:10.1177/1747493018778713
- Cottone M, Sapienza C, Macaluso FS, Cannizzaro M. Psoriasis and inflammatory bowel disease. Dig Dis. 2019;37(6):451–457. doi:10.1159/000500116
- Egeberg A, Mallbris L, Warren RB, et al. Association between psoriasis and inflammatory bowel disease: a Danish nationwide cohort study. Br J Dermatol. 2016;175(3):487–492. doi:10.1111/bjd.14528
- Persson PG, Leijonmarck CE, Bernell O, Hellers G, Ahlbom A. Risk indicators for inflammatory bowel disease. Int J Epidemiol. 1993;22(2):268–272. doi:10.1093/ije/22.2.268
- Egeberg A, Thyssen JP, Burisch J, Colombel JF. Incidence and risk of inflammatory bowel disease in patients with psoriasis-a nationwide 20-year cohort study. J Invest Dermatol. 2019;139(2):316–323. doi:10.1016/j.jid.2018.07.029
- Whitlock SM, Enos CW, Armstrong AW, et al. Management of psoriasis in patients with inflammatory bowel disease: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2018;78(2):383–394. doi:10.1016/j.jaad.2017.06.043
- Andersen YMF, Wu JJ, Thyssen JP, Egeberg A. Chronologic order of appearance of immune-mediated inflammatory diseases relative to diagnosis of psoriasis. J Am Acad Dermatol. 2019;81(6):1283–1291. doi:10.1016/j.jaad.2019.04.033
- Bucalo A, Rega F, Zangrilli A, et al. Paradoxical psoriasis induced by anti-TNFα treatment: evaluation of disease-specific clinical and genetic markers. IJMS. 2020;21(21):7873. doi:10.3390/ijms21217873
- Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psorias Psoriat Arthritis. 2019;4(2):70–80. doi:10.1177/2475530318810851
- Mylonas A, Conrad C. Psoriasis: classical vs. paradoxical. The Yin-Yang of TNF and type I interferon. Front Immunol. 2018;9:2746. doi:10.3389/fimmu.2018.02746
- Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61(12):1693–1700. doi:10.1136/gutjnl-2011-301668
- Nast A, Smith C, Spuls PI, et al. EuroGuiDerm guideline on the systemic treatment of Psoriasis vulgaris - part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereol. 2020;34(11):2461–2498. doi:10.1111/jdv.16915
- Targan SR, Feagan B, Vermeire S, et al. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2016;111(11):1599–1607. doi:10.1038/ajg.2016.298
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154(12):1417–1423. doi:10.1001/jamadermatol.2018.3631
- Puig L, Kirby B, Mallbris L, Strohal R. Psoriasis beyond the skin: a review of the literature on cardiometabolic and psychological co-morbidities of psoriasis. Eur J Dermatol. 2014;24(3):305–311. doi:10.1684/ejd.2014.2299
- González-Parra S, Daudén E. Psoriasis and depression: the role of inflammation. Actas Dermosifiliogr. 2019;110(1):12–19. doi:10.1016/j.ad.2018.05.009
- Nicholas MN, Gooderham M. Psoriasis, depression, and suicidality. Skin Therapy Lett. 2017;22(3):1–4.
- Bouguéon K, Misery L. [Depression and psoriasis]. Ann Dermatol Venereol. 2008;135(Suppl 4):S254–S258. French. doi:10.1016/S0151-9638(08)70544-1
- Tohid H, Aleem D, Jackson C. Major depression and psoriasis: a psychodermatological phenomenon. Skin Pharmacol Physiol. 2016;29(4):220–230. doi:10.1159/000448122
- Matiushenko VP, Kutasevych YF, Havryliuk OA, Jafferany M. Effectiveness of psychopharmacotherapy in psoriasis patients with associated anxiety and depression. Dermatol Ther. 2020;33(6):e14292. doi:10.1111/dth.14292
- Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29(7):805–813. doi:10.1515/revneuro-2017-0108
- Gupta MA, Gupta AK, Kirkby S, et al. A psychocutaneous profile of psoriasis patients who are stress reactors. A study of 127 patients. Gen Hosp Psychiatry. 1989;11(3):166–173. doi:10.1016/0163-8343(89)90036-4
- Dowlatshahi EA, Wakkee M, Arends LR, Nijsten T. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134(6):1542–1551. doi:10.1038/jid.2013.508
- Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146(8):891–895. doi:10.1001/archdermatol.2010.186
- Kouris A, Platsidaki E, Kouskoukis C, Christodoulou C. Psychological parameters of psoriasis. Psychiatriki. 2017;28(1):54–59. doi:10.22365/jpsych.2017.281.54
- Luca M, Musumeci ML, D’Agata E, Micali G. Depression and sleep quality in psoriatic patients: impact of psoriasis severity. Int J Psychiatry Clin Pract. 2020;24(1):102–104. doi:10.1080/13651501.2019.1659372
- Rapp SR, Feldman SR, Exum ML, Fleischer AB, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3 Pt 1):401–407. doi:10.1016/s0190-9622(99)70112-x
- Patel N, Nadkarni A, Cardwell LA, et al. Psoriasis, depression, and inflammatory overlap: a review. Am J Clin Dermatol. 2017;18(5):613–620. doi:10.1007/s40257-017-0279-8
- Soliman MM. Depressive, anxiety, stress, and insomnia symptoms in patients with psoriasis: a cross-sectional study. Postepy Dermatol Alergol. 2021;38(3):510–519. doi:10.5114/ada.2020.98726
- Lamb RC, Matcham F, Turner MA, et al. Screening for anxiety and depression in people with psoriasis: a cross-sectional study in a tertiary referral setting. Br J Dermatol. 2017;176(4):1028–1034. doi:10.1111/bjd.14833
- Singhal A, Ross J, Seminog O, Hawton K, Goldacre MJ. Risk of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med. 2014;107(5):194–204. doi:10.1177/0141076814522033
- Egeberg A, Hansen PR, Gislason GH, Skov L, Mallbris L. Risk of self-harm and nonfatal suicide attempts, and completed suicide in patients with psoriasis: a population-based cohort study. Br J Dermatol. 2016;175(3):493–500. doi:10.1111/bjd.14633
- Papp KA, Reich K, Paul C, et al. A prospective Phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–286. doi:10.1111/bjd.14493
- Singh S, Taylor C, Kornmehl H, Armstrong AW. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77(3):425–440.e2. doi:10.1016/j.jaad.2017.05.019
- Chamoun A, Goudetsidis L, Poot F, Bourdeaud’hui F, Titeca G. [Psoriasis and depression]. Rev Med Brux. 2015;36(1):23–28. French.
- Batko B. Patient-centered care in psoriatic arthritis-a perspective on inflammation, disease activity, and psychosocial factors. J Clin Med. 2020;9(10):E3103. doi:10.3390/jcm9103103
- Sahi FM, Masood A, Danawar NA, Mekaiel A, Malik BH. Association between psoriasis and depression: a traditional review. Cureus. 2020;12(8):e9708. doi:10.7759/cureus.9708
- Golpour M, Hosseini SH, Khademloo M, et al. Depression and anxiety disorders among patients with psoriasis: a hospital-based case-control study. Dermatol Res Pract. 2012;2012:1–5. doi:10.1155/2012/381905
- Pollo CF, Miot HA, de Matos TD, et al. Prevalence and factors associated with depression and anxiety in patients with psoriasis. J Clin Nurs. 2021;30(3–4):572–580. doi:10.1111/jocn.15577
- Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31(12):1999–2009. doi:10.1111/jdv.14460
- Skoie IM, Dalen I, Ternowitz T, et al. Fatigue in psoriasis: a controlled study. Br J Dermatol. 2017;177(2):505–512. doi:10.1111/bjd.15375
- Skoie IM, Ternowitz T, Jonsson G, Norheim K, Omdal R. Fatigue in psoriasis: a phenomenon to be explored. Br J Dermatol. 2015;172(5):1196–1203. doi:10.1111/bjd.13647
- Skoie IM, Dalen I, Omdal R. Effect of biological treatment on fatigue in psoriasis: a systematic review and meta-analysis. Am J Clin Dermatol. 2019;20(4):493–502. doi:10.1007/s40257-019-00434-w
- Neill J, Belan I, Ried K. Effectiveness of non-pharmacological interventions for fatigue in adults with multiple sclerosis, rheumatoid arthritis, or systemic lupus erythematosus: a systematic review. J Adv Nurs. 2006;56(6):617–635. doi:10.1111/j.1365-2648.2006.04054.x
- Graff LA, Walker JR, Russell AS, Bissonnette R, Bernstein CN. Fatigue and quality of sleep in patients with immune-mediated inflammatory disease. J Rheumatol Suppl. 2011;88:36–42. doi:10.3899/jrheum.110902
- Hewlett S, Ambler N, Almeida C, et al. Self-management of fatigue in rheumatoid arthritis: a randomised controlled trial of group cognitive-behavioural therapy. Ann Rheum Dis. 2011;70(6):1060. doi:10.1136/ard.2010.144691
- Ruggiero A, Fabbrocini G, Cacciapuoti S, Cinelli E, Gallo L, Megna M. Ocular Manifestations in Psoriasis Screening (OcMaPS) questionnaire: a useful tool to reveal misdiagnosed ocular involvement in psoriasis. J Clin Med. 2021;10(5):1031. doi:10.3390/jcm10051031
- Demerdjieva Z, Mazhdrakova I, Tsankov N. Ocular changes in patients with psoriasis. Clin Dermatol. 2019;37(6):663–667. doi:10.1016/j.clindermatol.2019.07.029
- Chimenti MS, Triggianese P, Salandri G, et al. A multimodal eye assessment in psoriatic arthritis patients sine-psoriasis: evidence for a potential association with systemic inflammation. J Clin Med. 2020;9(3):E719. doi:10.3390/jcm9030719
- Chi CC, Tung TH, Wang J, et al. Risk of uveitis among people with psoriasis: a nationwide cohort study. JAMA Ophthalmol. 2017;135(5):415–422. doi:10.1001/jamaophthalmol.2017.0569
- Selmi C. Diagnosis and classification of autoimmune uveitis. Autoimmun Rev. 2014;13(4–5):591–594. doi:10.1016/j.autrev.2014.01.006
- Trafford AM, Parisi R, Kontopantelis E, Griffiths CEM, Ashcroft DM. Association of psoriasis with the risk of developing or dying of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2019;155(12):1390. doi:10.1001/jamadermatol.2019.3056
- Brauchli YB, Jick SS, Miret M, Meier CR. Psoriasis and risk of incident cancer: an inception cohort study with a nested case-control analysis. J Invest Dermatol. 2009;129(11):2604–2612. doi:10.1038/jid.2009.113
- Gelfand JM, Shin DB, Neimann AL, Wang X, Margolis DJ, Troxel AB. The risk of lymphoma in patients with psoriasis. J Investig Dermatol. 2006;126(10):2194–2201. doi:10.1038/sj.jid.5700410
- Gelfand JM, Berlin J, Van Voorhees A, Margolis DJ. Lymphoma rates are low but increased in patients with psoriasis: results from a population-based cohort study in the United Kingdom. Arch Dermatol. 2003;139(11):1425–1429. doi:10.1001/archderm.139.11.1425
- Kim N, Thrash B, Menter A. Comorbidities in psoriasis patients. Semin Cutan Med Surg. 2010;29(1):10–15. doi:10.1016/j.sder.2010.01.002
- Stern RS. Lymphoma risk in psoriasis: results of the PUVA follow-up study. Arch Dermatol. 2006;142(9):1132–1135. doi:10.1001/archderm.142.9.1132
- Bellinato F, Gisondi P, Girolomoni G. Risk of lymphohematologic malignancies in patients with chronic plaque psoriasis: a systematic review with meta-analysis. J Am Acad Dermatol. 2022;86(1):86–96. doi:10.1016/j.jaad.2021.07.050
- Kamstrup MR, Skov L, Zachariae C, Thyssen JP, Egeberg A. Psoriasis and risk of malignant lymphoma: a population-based cohort study. Br J Dermatol. 2018;178(6):1435–1436. doi:10.1111/bjd.16245
- Johansen CB, Egeberg A, Jimenez Solem E, Vittrup I, Skov L, Francis Thomsen S. Comorbidities, socioeconomic status, drug use, and health care consumption in Danish women with psoriasis: a nationwide cross-sectional study. Int J Womens Dermatol. 2021;7(3):246–258. doi:10.1016/j.ijwd.2020.11.004
- Kim GE, Seidler E, Kimball AB. A measure of chronic quality of life predicts socioeconomic and medical outcomes in psoriasis patients. J Eur Acad Dermatol Venereol. 2015;29(2):249–254. doi:10.1111/jdv.12503
- Bardazzi F, Tengattini V, Rucci P, et al. Socio-economic status and severity of plaque psoriasis: a cross-sectional study in the metropolitan city of Bologna. Eur J Dermatol. 2019;29(2):197–202. doi:10.1684/ejd.2019.3524
- Wenk KS, Arrington KC, Ehrlich A. Psoriasis and non-alcoholic fatty liver disease. J Eur Acad Dermatol Venereol. 2011;25(4):383–391. doi:10.1111/j.1468-3083.2010.03841.x
- Paschos P, Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 2009;13(1):9–19.
- Madanagobalane S, Anandan S. The increased prevalence of non-alcoholic fatty liver disease in psoriatic patients: a study from South India. Australas J Dermatol. 2012;53(3):190–197. doi:10.1111/j.1440-0960.2012.00905.x
- Miele L, Vallone S, Cefalo C, et al. Prevalence, characteristics and severity of non-alcoholic fatty liver disease in patients with chronic plaque psoriasis. J Hepatol. 2009;51(4):778–786. doi:10.1016/j.jhep.2009.06.008
- Mantovani A, Gisondi P, Lonardo A, Targher G. Relationship between non-alcoholic fatty liver disease and psoriasis: a novel hepato-dermal axis? Int J Mol Sci. 2016;17(2):217. doi:10.3390/ijms17020217
- Bellinato F, Goio I, Malara G, et al. Non-alcoholic fatty liver disease is associated with reduced glomerular filtration rate in patients with chronic plaque psoriasis. J Cutan Med Surg. 2021;12034754211066906. doi:10.1177/12034754211066906
- Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77(5):845–854.e5. doi:10.1016/j.jaad.2017.07.013
- Penso L, Dray-Spira R, Weill A, Pina Vegas L, Zureik M, Sbidian E. Association between biologics use and risk of serious infection in patients with psoriasis. JAMA Dermatol. 2021;157(9):1056. doi:10.1001/jamadermatol.2021.2599
- Hjuler KF, Böttcher M, Vestergaard C, et al. Increased prevalence of coronary artery disease in severe psoriasis and severe atopic dermatitis. Am J Med. 2015;128(12):1325–1334.e2. doi:10.1016/j.amjmed.2015.05.041
- Kaiser H, Abdulla J, Henningsen KMA, Skov L, Hansen PR. Coronary Artery Disease Assessed by Computed Tomography in Patients with Psoriasis: A Systematic Review and Meta-Analysis. Dermatology. 2019;235(6):478–487. doi:10.1016/j.amjmed.2015.05.041 Epub 2019 Sep 3. PMID: 31480039.
- Thaçi D, de la Cueva P, Pink AE, Jalili A, Segaert S, Hjuler KF, Calzavara-Pinton P. General practice recommendations for the topical treatment of psoriasis: a modified-Delphi approach. BJGP Open. 2020;4(5):bjgpopen20X101108. doi:10.3399/bjgpopen20X101108 PMID: 33144365; PMCID: PMC7880171.
- Generali E, Scirè CA, Favalli EG, Selmi C. Biomarkers in psoriatic arthritis: a systematic literature review. Expert Rev Clin Immunol. 2016 Jun;12(6):651–60. doi:10.1586/1744666X.2016.1147954 Epub 2016 Feb 24. PMID: 26821681.
- Elliott A, McGonagle D, Rooney M. Integrating imaging and biomarker assessment to better define psoriatic arthritis and predict response to biologic therapy. Rheumatology (Oxford). 2021 Dec 24;60(Suppl 6):vi38-vi52. doi:10.1093/rheumatology/keab504 PMID: 34951926; PMCID: PMC8709569.
- Rouzaud M, et al. J Eur Acad Dermatol Venereol. 2014;28(Suppl 5):17–26.
- Perez-Chada LM, Merola JF. Comorbidities associated with psoriatic arthritis: Review and update. Clin Immunol. 2020;214:108397. doi:10.1016/j.clim.2020.108397 Epub 2020 Mar 27. PMID: 32229290
- Beck, Aaron T, Robert A. Steer, and Margery G. Carbin. “Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation.” Clinical psychology review8. (1988): 77-100.
- Julian, Laura J. “Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A).” Arthritis care & research vol. 63 Suppl 11,0 11 (2011):S467-72. doi:10.1002/acr.20561
- Hjuler KF, Dige A, Agnholt J, Laurberg TB, Loft AG, Møller LF, Christensen R, Iversen L. Effectiveness of interdisciplinary combined dermatology-gastroenterology-rheumatology clinical care compared to usual care in patients with immune-mediated inflammatory diseases: a parallel group, non-blinded, pragmatic randomised trial. BMJ Open. 2021;11(4):e041871. doi:10.1136/bmjopen-2020-041871 PMID: 33910945; PMCID: PMC8094387.
- deShazo R, Soltani-Arabshahi R, Krishnasamy S, Langley RG, Kalia S, Ståhle M, Langholff W, Goyal K, Fakharzadeh S, Galindo C, Srivastava B, Krueger G. Non-Melanoma Skin Cancer Risk Among Patients in the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2019 Oct 1;18(10):1059-1060. PMID: 31603636
- European Medicines Agency. Annex1 Summary of Product Characteristics for Jylamvo 2 mg/ml oral solution. Available from: https://www.ema.europa.eu/en/documents/product-information/jylamvo-epar-product-information_en.pdf. Accessed June 01, 2022
- Davidson L, van den Reek JMPA, Bruno M, et al. Risk of candidiasis associated with interleukin-17 inhibitors: A real-world observational study of multiple independent sources. Lancet Reg Health Eur. 2021;13:100266. doi:10.1016/j.lanepe.2021.100266