Abstract
Background
The quality of life in psoriatic patients is significantly impaired. Since this century, there have been biologics as a treatment for psoriasis. These biologics reduce symptoms, but more knowledge is needed about potential improvements in quality of life. As a result, biological therapy may be more valuable for patients who experience a lot of burden from their chronic skin condition in daily life. The aim of this systematic review was to investigate the possible improvement of the Dermatology Life Quality Index (DLQI) in psoriatic patients using biologics.
Materials and Methods
An online search was performed in the PubMed database to identify relevant articles. Inclusion criteria for studies were psoriatic patients, a measurement of DLQI with biologics and without biologics. Exclusion criteria for studies were abstracts not written in English, publications before 2012, full text unavailable, quality of life measurements other than DLQI. Results from the studies with different biologics were combined into the outcome measure: ≥5 points of improvement in the DLQI score. Results of the studies in which biologics were compared with (conventional) systemic therapy were combined in the outcome measure: improvement of the DLQI score is better with biologics than with systemic therapy.
Results
There were nine included articles with a total of 19.926 patients. Adalimumab, alefacept, etanercept, infliximab, ustekinumab and secukinumab were included biologics. Six studies measured the change in DLQI of different biologics in number of points. Of these six studies, 22 sub-analyses were performed and 20 of them showed a DLQI improvement of ≥5 points. The improvement in DLQI was better with biologics than with systemic therapy in two of the three measured studies.
Conclusion
Quality of life of psoriatic patients will be improved by the studied biologics. In the future, more research is needed into biologics on patient and quality of life characteristics.
Keywords:
Introduction
About 2% of the world population has the chronic skin disorder psoriasis.Citation1 This immune-mediated skin disorder causes symptoms and signs such as redness, plaques, white-silver flakes, and a lot of itching.Citation2,Citation3 There are negative consequences for the quality of life of psoriatic patients due to stigma and the observable marked skin.Citation4 For example, less self-consciousness and self-esteem can be caused by misconceptions in society, because it is still thought to be contagious.Citation4 Moreover, the severity of the skin disorder is underestimated, as it is not a fatal disease in itself.Citation4 Psoriatic patients also have an increased risk of developing depression, obesity and metabolic syndrome.Citation1,Citation4 If this kind of experiences causes stress in patients, the skin will also exacerbate.Citation4 As a result, the patient can end up in a vicious circle.Citation4
The pathogenesis is complicated, as epigenetic factors, immune cells and cytokine activation are involved in the development of psoriasis.Citation5 Topical therapy, traditional systemic therapy and biologics have been proven to be effective in reducing the symptoms of the disease,Citation6,Citation7 however little is known about the quality of life improvement in patients who are taking biologics. Research about this is important, because psoriasis can also cause social and psychological complaints that are not visible from the outside of people and biologics may be able to reduce complaints in this regard.
Biologic therapy exists almost two decades, as since 2003 the first biologic treatment alefacept was approved by the United States Food and Drug Administration.Citation6 After this, more and more biologics have been developed. Biologics can interfere with the action of cytokines, reducing the inflammatory response.Citation5 For instance, biologics are used when systemic therapy has too many side effects.Citation6 Nevertheless, the costs of biologic therapy are much higher than the traditional systemic therapy.Citation6 These costs vary between $13,000 and $30,000 USD for one patientCitation6 and therefore it is also necessary to research whether the quality of life improves significantly with the use of biologics. There are different types of questionnaires to assess quality of life, a questionnaire focused on dermatology can best be used for psoriasis. This outcome can then be used by physicians and patients.Citation6 If physicians know more about possible improvements in quality of life, besides improving of the skin, this therapy may be more valuable than is thought. Certainly, for patients who experience a lot of impact of their chronic skin condition in daily life. Moreover, these results of therapy may be of interest to society so that there can be greater understanding of psoriasis.
The aim of this systematic review is to investigate the possible improvement of the Dermatology Life Quality Index (DLQI) in psoriatic patients using biologics.
Materials and Methods
Literature Search
An online search was performed in the PubMed database on September 10th, 2021, to identify all articles that measured quality of life in psoriatic patients using biologics.
Terms included in the search were psoriasis, quality of life, DLQI (Dermatology Life Quality Index) and biological therapy. The complete literature search was: (“psoriasis”[MeSH Terms] AND (“quality of life”[MeSH Terms] OR “DLQI”[Title/Abstract]) AND “biological therapy”[MeSH Terms]).
Inclusion and Exclusion Criteria
The abstracts that were written other than English and published before 2012 were excluded. The remaining publications were screened on title and abstract by one author.
Inclusion criteria were studies with psoriatic patients, a measurement of DLQI with biologics and without biologics. Exclusion criteria were studies where full text was unavailable, with quality of life measurements other than DLQI. The reference lists of included studies were searched for other possibly relevant studies.
Endnote X9 was used for the selection of the studies.
The primary outcome was the DLQI in psoriatic patients using biologics. Randomized controlled trials (RCT) would best suit the research question, but none were available, so this did not count towards the inclusion criteria.
Critical Appraisal
All included studies were critically appraised by one author (Chanel Claudine de Ruiter). The Joanna Briggs checklist for cohort studies, cross-sectional studies and case series were used to set the risk of bias.Citation8 Questions of the checklist were answered with yes, no, and unclear. Sometimes a question in the cohort studies has been modified with a word or two to make the checklist appropriate to the research design.
Data Extraction
Per study, study design, number of patients (and % males), mean age, follow-up period, mean duration of psoriasis, therapy intervention and mean baseline DLQI score were extracted if available. The outcomes per study were collected in a separate table. This process has been done by the same author of the critical appraisal (Chanel Claudine de Ruiter).
Data Analysis
A narrative analysis of the results was performed. The results of the studies with different biologics were combined into one outcome measure: ≥5 points of improvement in the DLQI score. This outcome measure (an improvement ≥5 points in the DLQI score) was chosen because the results are then clinically meaningful according to the study of Frieder et al.Citation9
The results of the studies in which biologics are compared with (conventional) systemic therapy are combined in the outcome measure: the improvement of the DLQI score is better with biologics than with systemic therapy. These two outcome results are sometimes obtained by manually converting the results. The outcomes of the DLQI score improvement of the studies were considered statistically significant when p < 0.05.
Results
Selection of the Studies
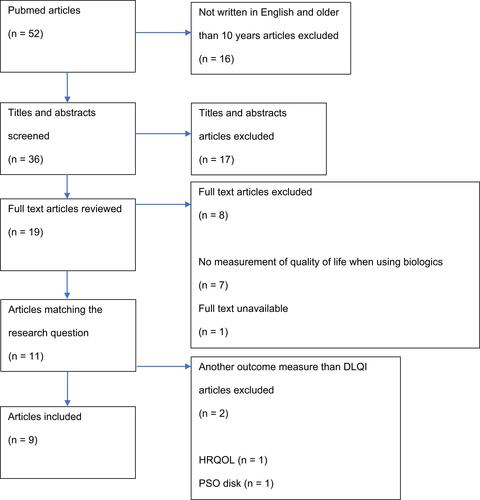
Search and selection process are given in . The search started with 52 articles, of which 16 were excluded because they were not written in English and older than 10 years (n = 16). Therefore, 36 titles and abstracts are screened for eligibility, and then 19 articles were read in full text. Ten articles were excluded for the reason: no measurement of quality of life when using biologics (n = 7), full text unavailable (n = 1) and another outcome measure than DLQI (n = 2). Finally, 9 articles were included.Citation6,Citation9–Citation16 The DLQI score is taken as quality of life outcome, because this score was the most used in the studies found. No other potentially relevant studies were found in the reference lists of included studies. The list of excluded studies after reviewing full text can be found in Appendix 1.
Critical Appraisal and Study Characteristics
Results of the critical appraisal are shown in –. The overall quality of the studies was considered good. Included studies are published between 2013 and 2018, and these are five cohort studies, three cross-sectional studies and one case series. Unfortunately, almost no confounding factors have been identified and strategies for dealing with confounding factors have never been mentioned (see and ).
Table 1 Critical Appraisal of Included Cohort Studies
Table 2 Critical Appraisal of Included Cross Sectional Studies
Table 3 Critical Appraisal of Included Case Series
The details of the study characteristics are demonstrated in and the results of included studies are demonstrated in .
Table 4 Characteristics of Included Studies
Table 5 Results of Included Studies
There were different therapy interventions including for biologics: adalimumab,Citation6,Citation9,Citation13–Citation15 alefacept,Citation6 etanercept,Citation6,Citation9,Citation14–Citation16 infliximab,Citation6,Citation9,Citation15,Citation16 ustekinumabCitation6,Citation9,Citation14–Citation16 and secukinumab.Citation9,Citation15,Citation16 The studies comparing biologics to (conventional) systemic therapy did not specify which biologics and systemic therapy were intended.Citation10–Citation12
A total of 19.926 patients participated in the nine studies of which 17.836 patients received biologics and 2.090 patients received systemic therapy. The number of patients varied widely between studies (see ). If the percentage of males was available, it was always a majority (>50%).Citation6,Citation12,Citation13,Citation16
The duration of having psoriasis was not available in all studies, only in three studies. These studiesCitation6,Citation12,Citation14 indicate that patients have the skin disease on average 16.9 years in a study with systemic therapyCitation12 and on average at least 17.5 years for adalimumab in biologic therapy studies.Citation6 22.9 (SD: ± 12.1) years taking etanercept is the mean maximum disease duration of included studies using biologics.Citation6
The mean age was identified in five studies,Citation6,Citation12–Citation14,Citation16 the youngest mean age was 38.20 years using ustekinumab,Citation16 and the oldest mean age was 51.9 years using adalimumab.Citation13 It was remarkable that these therapies almost do not involve young people, given these mean ages.
In four studies, the baseline DLQI is given,Citation6,Citation13,Citation14,Citation16 and in six studies, there is a follow-up.Citation6,Citation9,Citation13–Citation16 The mean DLQI score for biological therapy ranged from 10.7 for alefaceptCitation6 to 17.70 for etanercept.Citation16 If known, the follow-up period varied from 12 weeks using adalimumab,Citation6 alefacept,Citation6 etanercept,Citation6,Citation9 infliximab,Citation6,Citation9 ustekinumabCitation6,Citation9 and secukinumabCitation9 to 12 months using adalimumab,Citation14 etanerceptCitation14 and ustekinumab.Citation14
Data Analysis
There were six studies that measured the change in DLQI of different biologics in number of pointsCitation6,Citation9,Citation13–Citation16 and there were three studies comparing DLQI score change between biologics and systemic therapy.Citation10–Citation12 The results per outcome are demonstrated in . The p values were available from these studies, except from Ahn et al,Citation6 Radtke et alCitation10 and Reich et alCitation12 of these studies only the 95% CI or standard deviation were available.
Table 6 Results per Outcome
In the studies comparing different biologics, the biologics that were included are adalimumab, alefacept, etanercept, infliximab, ustekinumab, secukinumab. Of alefacept, there were only results from one study,Citation6 so no further research of DLQI change for this biologic could be done.
An improvement of ≥5 points in DLQI score was reported in four adalimumab treatment studies.Citation6,Citation9,Citation13,Citation14 The greatest improvement in DLQI score was seen in the study of Iskandar et alCitation14 with a median improvement of 14 (p < 0001). The smallest improvement was seen in the study by Frieder et alCitation9 with a mean improvement of 8.4 (p < 0.001) at a dose of 40 mg every other week. Of the 5 adalimumab studies, there was not ≥5 points of improvement in the DLQI score in Norris et al,Citation15 which was 3.68 (p 0.040).
For etanercept, there was ≥5 points DLQI improvement in four studies.Citation6,Citation9,Citation14,Citation16 Solberg et alCitation16 showed the biggest improvement of these studies, namely 12.50 points (p ≤ 0.01) on average. Ahn et alCitation6 showed the least improvement, averaging 5.80 points at a dose of 25 mg subcutaneously once weekly. The DLQI improvement of ≥5 points was observed in 77% (p < 0.0001) of patients in the study by Frieder et alCitation9, they received 50 mg twice weekly. This is the highest percentage of patients with a DLQI score improvement of ≥5 points. This same study indicated that 50% of patients (p < 0.0001) on a dose of 25 mg weekly improved DLQI by ≥5 points, which was the lowest reported percentage of patients with a DLQI improvement ≥5 points.Citation9 In the study of Norris et al,Citation15 the mean DLQI improvement was 2.99 (p 0.245), accordingly less than 5 and not statistically significant.
All four infliximab studies showed ≥5 points of DLQI improvement.Citation6,Citation9,Citation15,Citation16 Solberg et alCitation16 showed the greatest improvement of these studies with a mean improvement of 14.00 (p ≤ 0.01) points. The least improvement was seen in the study by Ahn et alCitation6 with a mean improvement of 9.3 (SD 7.0) at a dose of 3 mg/kg intravenously for 3 infusions.
An improvement of ≥5 points in DLQI score was reported in all five ustekinumab treatment studies.Citation6,Citation9,Citation14–Citation16 The biggest improvement in DLQI score was seen in the study of Iskandar et alCitation14 with a median improvement of 14 (p < 0001). Norris et alCitation15 showed the smallest improvement, averaging 7.51 points (p < 0.001).
Secukinumab therapy indicated a ≥5-point improvement of DLQI in all three studies reviewed.Citation9,Citation15,Citation16 The DLQI improvement was largest in a study by Frieder et al.Citation9 The ERASURE study by Frieder et alCitation9 showed that patients receiving 300 mg of secukinumab had an average DLQI improvement of 11.4. The DLQI improvement was smallest in a study by Solberg et alCitation16 this was 8.60 points (p ≤ 0.01).
The improvement in DLQI is better with biologics than with systemic therapy in two studies.Citation10,Citation11 Fernández-Torres et alCitation11 showed that patients receiving biologics had 0.809 (p 0.053) fewer impairments in DLQI than patients receiving systemic therapy. Radtke et alCitation10 showed that in patients receiving biologics their DLQI is 0.7 points better than in patients receiving systemic therapy. However, the study of Reich et alCitation12 showed that DLQI in patients receiving biological therapy is 0.7 worse than in patients receiving systemic therapy.
Accordingly, of the six studies with different biologics, 22 sub-analyses were performed and 20 of them showed a DLQI improvement of ≥5 points. Therefore, biologics strongly appear to improve the DLQI score by more than 5 points, sometimes up to 14-point median for adalimumabCitation14 or 14-point mean for infliximab.Citation16
It was also noted that when the dose was indicated, a higher or more frequent dose often gave a better DLQI score and sometimes not (for details see ). The study of Frieder et alCitation9 () showed that patients who received placebos improved on average up to 1.9 points in DLQI for adalimumab and secukinumab (FIXTURE study). The DLQI improved with placebos at least 0.4 points on average for infliximab.Citation9 This also shows that biologics improves the quality of life in psoriatic patients.
Discussion
In this literature research, biologics have been shown to improve the DLQI score, often by much more than 5 points. Adalimumab, etanercept, infliximab, ustekinumab and secukinumab were measured for this outcome measure. In addition, in two out of three studies more improvement was seen in DLQI score with biologics than with systemic therapy. These results are clinically meaningful when there is an improvement of ≥5 points in the DLQI score according to the study of Frieder et al.Citation9 The purpose of the DLQI questionnaire is to estimate how much psoriasis has affected patients’ life in the past week before the questionnaire was taken.Citation17 This questionnaire consists of ten questions, where a minimum score is 0 and a maximum score is 30. By 0 points, there is no effect of psoriasis on patients’ life and by 30 points there is an extremely large effect of psoriasis on patients’ life.Citation17 Therefore, if the score is lower, there is more improvement in quality of life. For example, with a five-point improvement, the effect on life due to psoriasis can go from very large to moderate.Citation17 Moreover, with a 10-point improvement, the effect on life due to psoriasis can go from very large to small.Citation17
However, biologics are only given after the failure of conventional therapies according to the guidelines.Citation18 So a DLQI score at baseline (before starting a biologic therapy) also means a DLQI score in ineffective conventional systemic therapy. The included studies also include more “difficult to treat” psoriatic patients, because these patients have already used other biologics or/ and conventional therapies.
As far as known, there have been no previous studies of DLQI change in psoriatic patients using biologics in the past ten years than the included studies. However, research has been done on the correlation between the Psoriasis Area and Severity Index (PASI) and DLQI score. A study by Mattei et alCitation19 showed that patients taking biologics who have a 45–55% mean improvement of PASI score have a 5-point mean improvement of DLQI score (p < 0.01). The same studyCitation19 found that a 85% mean improvement of PASI score gives a 10-point mean improvement of DLQI score. This positive correlation is also found in a study by Puig et al,Citation20 where a 75–89% PASI improvement for adalimumab and infliximab gives a mean DLQI improvement of 8.5 (p < 0.01) and 8.67 points (p not reported). In addition, Puig et alCitation20 found that a 90% PASI improvement with adalimumab and infliximab therapy gives a mean DLQI improvement of 10.7 (p < 0.01) and 8.95 (p not reported). If the PASI score improves 75%, it is described in studies as PASI 75 and if it improves 90%, it is described as PASI 90. In another study by Abrouk et al,Citation21 a mean improvement of 6.78 points DLQI was observed in patients using adalimumab who had a PASI 75, the observation time was 24 weeks. At the same time, a mean improvement of 12.94 points DLQI was found in patients who had a PASI 90 (p 0.01 between the two PASI groups).Citation21 Patients using ustekinumab with a PASI 75 showed a mean DLQI improvement of 12.57 points at week 24.Citation21 At the same time, the average improvement of the DLQI score for patients with a PASI 90 was 20.74 points.Citation21 The results of ustekinumab therapy by Abrouk et alCitation21 had a p value of 0.11 between the two PASI groups, meaning that these results are not statistically significant. And lastly, a study by Sondermann et alCitation22 demonstrated that patients with a low PASI score (<3) had a mean DLQI score of 3.7 and patients with a high PASI score (≥3) had a mean DLQI score of 13.0 (p < 0.001). This research demonstrated that a better PASI score for the patient gives a better DLQI score.Citation22
These four studiesCitation19–Citation22 demonstrated that there is a positive correlation between the severity of the psoriasis (PASI) and the quality of life in patients (DLQI). Although little is known about quality of life in psoriasis patients using biologics, studies have been done on the severity and the therapeutic function, focusing on the skin inflammation. Through this kind of studies, there is more insight into how much progress there is of the DLQI when the severity of the psoriasis decreases.
However, not only the PASI and DLQI are important for measuring quality of life. The patient’s personal experience should also be taken seriously.
Limitations of the Literature Study
A limitation of this systematic review is that no randomized controlled trials (RCTs) were available, as RCTs have a greater evidential value than observational research. In addition, almost no confounding factors have been identified and strategies for dealing with confounding factors have never been mentioned. Confounding factors that were missed are, for example, alcohol consumption, smoking, weight and the PASI score. Due to the different study designs and the different outcome measures, there was also a lot of heterogeneity between the nine included studies, this meant that no meta-analysis could be performed. A meta-analysis is preferred because results are more reliable then. Some study characteristics were also not always known. Examples of this are the duration of psoriasis before the biologic therapy started, dose and dosage form of the biologics and follow-up period. The dosage form, for instance, may affect the DLQI score different if it is intravenous compared to subcutaneous. The follow-up period also differed in the studies that indicated this, so that no statement can be made about the speed of DLQI improvement.
Strengths of the Literature Study
This literature study is of interest to physicians who want to prescribe biologics to their patients, so that they can also consider whether the quality of life is improving rather than only the severity of the skin condition. The information is especially important to psoriatic patients, because biologics can have a better effect on their lives. As far as is known, this is one of the first systematic reviews with this research question. Another strong point of this review is that the DLQI questionnaire used also contains a question about possible problems of the treatment, such as the dosage form or the time duration of the biologics to be administered.
Implications for Research
It is recommended that more research should be done on the effect of biologics on the quality of life in psoriatic patients. Because the last included study is this literature study is from 2018, it is also important that new research will be conducted in the short and long term. Therefore, more patient’s data should be measured in new studies. Likewise, one standard outcome measure of quality of life should be used in these new studies. In this review, the DLQI outcome measure was used, but the Health-Related Quality of Life (HRQOL) or the PSO disk (a quality of life tool for psoriasis) can also be used for example. It also needs to be further examined whether the DLQI questionnaire is the best questionnaire for measuring quality of life. For instance, depression or improvement of the skin is not included in the DLQI questionnaire. The quality of life in newer biologics should also be explored not only the biologics included in this study. In residual psoriasis, more research could be done on which body parts can cause more complaints in terms of quality of life, as was done in the study by Hjuler et al.Citation23 In addition to this, it is also interesting to investigate the duration of treatment, since a longer duration of treatment gives a better outcome in DLQI according to the study by Muslimani.Citation24
Finally, research should be done on the effect of biologics on the quality of life in patients with atopic eczema or other skin disease where biologics are used.
Implications for Practice
In clinical practice, more attention should be paid to the quality of life of patients who have psoriasis. The DLQI questionnaire can be used for this for example, by taking this questionnaire before the start of biologics and after a few weeks or months. For the individual patient, it is then possible to understand how the quality of life may improve. As a result, more patients will use these biologics if their physician recommends it, and this will improve the doctor–patient relationship. However, the personal experience of the patient will not always be clear with such a questionnaire. For this, it is therefore important to know the individual patient well as a doctor.
Conclusion
In conclusion, the quality of life of psoriatic patients will be improved by the studied biologics. These biologics show that the DLQI score improves by ≥5 points, on a scale of 0 to 30 points. However, two studies on adalimumabCitation15 and etanerceptCitation15 did not find ≥5 points DLQI improvement, but this result was negligible as compared to the positive results for the other four adalimumabCitation6,Citation9,Citation13,Citation14 and four etanerceptCitation6,Citation9,Citation14,Citation16 studies. In addition, twoCitation10,Citation11 out of threeCitation10–Citation12 studies found that biologics provide greater improvement as compared to systemic therapy.
Furthermore, more research is needed in biological therapies on patient and quality of life characteristics so that a meta-analysis can be performed. The patient’s personal experience should also be included more in future research, so that these studies can be used more in practice.
Abbreviations
DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area Severity Index.
Disclosure
The authors report no conflicts of interest in this work.
References
- Roszkiewicz M, Dopytalska K, Szymańska E, Jakimiuk A, Walecka I. Environmental risk factors and epigenetic alternations in psoriasis. Ann Agric Environ Med. 2020;27(3):335–342. doi:10.26444/aaem/112107
- Kumari R, Halder J, Sharma A, Rai VK. Recent advancement in topical drug delivery for psoriasis: clinical pertinence and potential market. Curr Drug Targets. 2021;22(13):1507–1523. doi:10.2174/1389450122666210412124256
- Ko SH, Chi CC, Yeh ML, Wang SH, Tsai YS, Hsu MY. Lifestyle changes for treating psoriasis. Cochrane Database Syst Rev. 2019;7(7):Cd011972. doi:10.1002/14651858.CD011972.pub2
- Kouris A, Platsidaki E, Kouskoukis C, Christodoulou C. Psychological parameters of psoriasis. Psychiatriki. 2017;28(1):54–59. doi:10.22365/jpsych.2017.281.54
- Deng Y, Chang C, Lu Q. The inflammatory response in psoriasis: a comprehensive review. Clin Rev Allergy Immunol. 2016;50(3):377–389. doi:10.1007/s12016-016-8535-x
- Ahn CS, Gustafson CJ, Sandoval LF, Davis SA, Feldman SR. Cost effectiveness of biologic therapies for plaque psoriasis. Am J Clin Dermatol. 2013;14(4):315–326. doi:10.1007/s40257-013-0030-z
- Rich SJ, Bello-Quintero CE. Advancements in the treatment of psoriasis: role of biologic agents. J Manag Care Pharm. 2004;10(4):318–325. doi:10.18553/jmcp.2004.10.4.318
- Critical appraisal tools. Available from: https://jbi.global/critical-appraisal-tools. Accessed May 12, 2022.
- Frieder J, Kivelevitch D, Fiore CT, Saad S, Menter A. The impact of biologic agents on health-related quality of life outcomes in patients with psoriasis. Expert Rev Clin Immunol. 2018;14(1):1–19. doi:10.1080/1744666X.2018.1401468
- Radtke MA, Schäfer I, Blome C, Augustin M. Patient benefit index (PBI) in the treatment of psoriasis–results of the National Care Study “PsoHealth”. Eur J Dermatol. 2013;23(2):212–217. doi:10.1684/ejd.2013.1988
- Fernández-Torres RM, Pita-Fernández S, Fonseca E. Quality of life and related factors in a cohort of plaque-type psoriasis patients in La Coruña, Spain. Int J Dermatol. 2014;53(11):e507–511. doi:10.1111/ijd.12294
- Reich K, Mrowietz U, Radtke MA, et al. Drug safety of systemic treatments for psoriasis: results from The German Psoriasis Registry Psobest. Arch Dermatol Res. 2015;307(10):875–883. doi:10.1007/s00403-015-1593-8
- Buffiere-Morgado A, Couderc E, Delwail A, et al. Characterization of skin Th17 transcriptional profiles in psoriatic patients under adalimumab biotherapy. Eur J Dermatol. 2017;27(6):579–589. doi:10.1684/ejd.2017.3127
- Iskandar IYK, Ashcroft DM, Warren RB, et al. Comparative effectiveness of biological therapies on improvements in quality of life in patients with psoriasis. Br J Dermatol. 2017;177(5):1410–1421. doi:10.1111/bjd.15531
- Norris D, Photiou L, Tacey M, et al. Biologics and dermatology life quality index (DLQI) in the Australasian psoriasis population. J Dermatolog Treat. 2017;28(8):731–736. doi:10.1080/09546634.2017.1329501
- Solberg SM, Sandvik LF, Eidsheim M, Jonsson R, Bryceson YT, Appel S. Serum cytokine measurements and biological therapy of psoriasis - Prospects for personalized treatment?. Scand J Immunol. 2018;88(6):e12725. doi:10.1111/sji.12725
- Finlay AY, Khan GK. Dermatology life quality index (DLQI); 1992. Available from: https://www.bad.org.uk/page.aspx?sitesectionid=1353. Accessed May 12, 2022.
- Smith CH, Yiu ZZN, Bale T, et al. British association of dermatologists guidelines for biologic therapy for psoriasis 2020: a rapid update. Br J Dermatol. 2020;183(4):628–637. doi:10.1111/bjd.19039
- Mattei PL, Corey KC, Kimball AB. Psoriasis area severity index (PASI) and the dermatology life quality index (DLQI): the correlation between disease severity and psychological burden in patients treated with biological therapies. J Eur Acad Dermatol Venereol. 2014;28(3):333–337. doi:10.1111/jdv.12106
- Puig L, Thom H, Mollon P, Tian H, Ramakrishna GS. Clear or almost clear skin improves the quality of life in patients with moderate-to-severe psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2017;31(2):213–220. doi:10.1111/jdv.14007
- Abrouk M, Nakamura M, Zhu TH, Farahnik B, Koo J, Bhutani T. The impact of PASI 75 and PASI 90 on quality of life in moderate to severe psoriasis patients. J Dermatolog Treat. 2017;28(6):488–491. doi:10.1080/09546634.2016.1278198
- Sondermann W, Fiege O, Körber A, Scherbaum N. Psychological burden of psoriatic patients in a German university hospital dermatology department. J Dermatol. 2021;48(6):794–806. doi:10.1111/1346-8138.15721
- Hjuler KF, Iversen L, Rasmussen MK, Kofoed K, Skov L, Zachariae C. Localization of treatment-resistant areas in patients with psoriasis on biologics. Br J Dermatol. 2019;181(2):332–337. doi:10.1111/bjd.17689
- Muslimani MA, Bolcato V, de Silvestri A, Brazzelli V. Psoriatic patients undergoing long-term therapy with biologics: impact of residual localization of psoriasis on quality of life in an Italian clinical setting. Dermatol Ther. 2020;33(6):e14337. doi:10.1111/dth.14337