Abstract
Psoriasis management may be challenging, particularly for moderate-to-severe forms of the disease. Indeed, conventional systemic treatments are often avoided for contraindications or the risk of adverse events as well as phototherapy is often limited by logistic concerns. Despite the development of biological drugs and small molecules revolutionized the treatment options showing promising results in terms of safety and effectiveness, some limitations remain. Thus, there is still a need for new therapies that are always welcome in order to tailor the treatment to the patient and to have a higher level of performance, especially in order to maintain long-term effectiveness. In this scenario, deucravacitinib, an oral small molecule which selectively inhibits Tyrosine Kinase 2, may represent a promising weapon in psoriasis management. The aim of our manuscript is to review the current knowledge on the efficacy and safety of deucravacitinib for the management of psoriasis.
Introduction
Psoriasis is a chronic inflammatory skin condition, with a worldwide prevalence of about 3%.Citation1 Plaque psoriasis is the most frequent disease form, accounting for up to 90% of cases and being clinically characterized by sharply demarcated erythematous plaques covered by silvery-white lamellar scales.Citation2 Several comorbidities may be associated with psoriasis such as metabolic syndrome (diabetes, obesity, hypertension, dyslipidemia), psoriatic arthritis, cardiovascular disease, nonalcoholic fatty liver disease, inflammatory bowel disease, and depression.Citation1,Citation3,Citation4 Moreover, psoriasis strongly affects patients’ quality of life.Citation5 Thus, an effective and personalized treatment is needed.Citation6 In this scenario, even if mild psoriasis is often controlled with topical therapies, systemic treatments are required for moderate-to-severe forms.Citation7 However, traditional systemic treatments (cyclosporine, acitretin, methotrexate, fumarates) are often avoided for contraindications (eg cardiovascular disease, renal or hepatic failure, etc.) or for the risk of adverse events (AEs).Citation8,Citation9 Phototherapy may be a therapeutic opportunity, but it is limited by logistic concerns.Citation8,Citation9 Recently, the introduction of small molecules (apremilast) and biologic drugs targeting different cytokines [tumor necrosis factor (TNF) α, interleukin (IL)-12/23,-17,-23] revolutionized the management of psoriasis showing promising results in terms of efficacy and safety.Citation10–16 However, unmet needs or some limitations may still remain. On one hand, even if apremilast does not require parental administration, it shows lower and slower effectiveness compared to biologics with possible gastrointestinal AEs.Citation17,Citation18 On the other hand, biologic drugs require parental administration, which may be linked to increased risk of infection (upper respiratory tract infection) with some biologics being contraindicated in certain condition (eg multiple sclerosis for anti-TNF and inflammatory bowel diseases for anti-IL17). Finally, both apremilast and biologics have high costs and may present variable degree of risk of loss of efficacy over time.Citation17,Citation18 Thus, there is still a need for accessible, effective, and safe oral drugs for patients with moderate-to-severe forms of psoriasis. In this context, deucravacitinib, a recently developed oral small molecule (OSM) that selectively inhibits Tyrosine Kinase 2 (TYK2), may represent a valuable weapon in psoriasis management.Citation19,Citation20 The aim of our manuscript is to review the current knowledge on the efficacy and safety of deucravacitinib for the management of psoriasis.
Materials and Methods
Literature research on the PubMed, clinicaltrials.gov, Cochrane Skin and Embase databases (until February 16, 2023) was carried out. Manuscripts were found, screened and analyzed for relevant data following the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) guidelines ().Citation21 The following keywords were used for the research: “psoriasis”, “oral small molecules”, “deucravacitinib”, “efficacy”, “safety”. Investigated manuscripts included reviews, metanalyses, clinical trials, real-life experiences, case reports and series. The most relevant articles were selected. Manuscripts regarding the use of deucravacitinib for other dermatological disease were excluded. Then, the abstracts and the texts of selected articles were reviewed to refine the research. References were also analyzed to include manuscripts that could have been missed. Finally, only English language articles were investigated. This manuscript is based on previously performed studies and does not contain any studies with human or animals participants carried out by any of the authors.
Figure 1 PRISMA flowchart of study analysis.
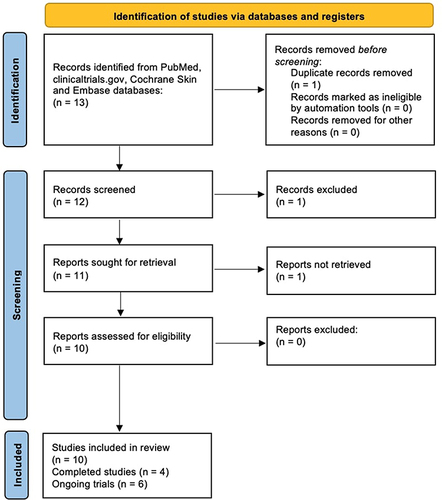
Results
A total of 4 completed trials and 6 ongoing studies were considered in the current review.Citation22–31 Among the completed studies with available results, two Phase II studies and two Phase III trials were identified.Citation22–25 Main results in terms of effectiveness and safety are summarized in and , respectively.
Table 1 Effectiveness of Deucravacitinib for Psoriasis Management in Clinical Trials
Table 2 Safety of Deucravacitinib for Psoriasis Management in Clinical Trials
Papp et al showed the results of a Phase II, double-blind trial enrolling 267 subjects randomly assigned to receive deucravacitinib at a dose of 3mg every other day (QAD) (n = 44), 3mg daily (QD) (n = 44), 3mg BID (BID) (n = 45), 6mg BID (n = 45), 12mg QD (n = 44), or placebo (n = 45) with the aim of assessing the reduction of Psoriasis Area Severity Index (PASI) of at least 75% (PASI75) after 12 weeks of treatment.Citation22 Globally, PASI75 was reached by 3 (6.7%), 4 (9.1%), 17 (38.6%), 31 (68.9%), 30 (66.7%) and 33 (75.0%) patients receiving placebo or deucravacitinib at the dose of 3mg QAD (not statistically significant compared with placebo), 3mg QD (p < 0.001 vs placebo), 3mg BID (p < 0.001 vs placebo), 6mg BID (p < 0.001 vs placebo), and 12mg QD (p < 0.001 vs placebo), respectively.Citation22 Secondary outcomes showed promising results in terms of PASI90 and PASI100 achievement (). As regards the safety, AEs were collected in 51% of the subjects in the placebo group and 55% to 80% of the subjects receiving deucravacitinib, with the highest percentage in the 6mg BID cohort.Citation22 The commonest AEs were nasopharyngitis, nausea, diarrhea, headache, and upper respiratory tract infection, leading to study discontinuation in 4% of subjects in the placebo cohort and from 2% to 7% of patients receiving deucravacitinib at different dosage.Citation22 Finally, 3 (1.4%) serious AEs were reported in patients receiving deucravacitinib, in addition to a case of malignant melanoma 96 days after the beginning of the treatment.Citation22 Moreover, a post-hoc analysis of this study including the 3 most efficacious dosage groups (3mg BID, 6mg BID, 12mg QD) and placebo was performed to assess the impact of deucravacitinib on quality-of-life (QoL).Citation23 The authors reported that the improvement in QoL, evaluated by the achievement of a Dermatology Life Quality Index (DLQI) overall score of 0/1 (no effect at all on patient’s life), had a similar trend to deucravacitinib related clinical results over 12 weeks.Citation23 Globally, patients with greater psoriasis improvements also reported greater QoL improvement.Citation23 Of note, the authors highlighted that PASI100 was not mandatory for reaching DLQI 0/1, as patients may reach DLQI0/1, neverthless the absence of complete skin clearance or BSA involvement >3%.Citation23
The safety and efficacy of deucravacitinib was also studied in a 52-week, randomized, double-blinded, placebo-controlled phase III trial (POETYK PSO-1) enrolling 666 patients randomized to receive deucravacitinib 6mg every day (n = 332), placebo BID (n = 166), or apremilast 30mg BID (n = 168).Citation24 The two main aims of the study were the achievement of PASI75 response and static Physician’s Global Assessment (sPGA) 0/1 (clear/almost clear) at week 16.Citation24 After 16 weeks of treatment, subjects randomized to placebo switched to deucravacitinib whereas at week 24, patients receiving apremilast who did not reach PASI50 crossed over to deucravacitinib up to week 52.Citation24 Globally, 194 (58.4%) patients reached PASI75 at week 16 in the deucravacitinib cohort compared with 21 (12.7%) in placebo cohort (p < 0.0001) and 59 (35.1%) in the apremilast group (p < 0.0001).Citation24 Similarly, sPGA 0/1 was reached in 178 (53.6%) patients receiving deucravacitinib compared with 12 (7.2%; p < 0.0001) and 54 (32.1%; p < 0.0001) patients receiving placebo and apremilast at the same time point, respectively.Citation24 Globally, PASI75 response and sPGA 0/1 continued to improve up to week 24 and these responses were maintained to week 52 in patients receiving deucravacitinib.Citation24 Moreover, at week 16 PASI90 was reached by 118 (35.5%) patients receiving deucravacitinib compared with 7 (4.2%) subjects receiving placebo (p < 0.0001) and 32 (19.0%) treated with apremilast (p = 0.0002), respectively.Citation24 Finally, 47 (14.2%) patients undergoing treatment with deucravacitinib reached PASI100 at week 16 compared with 1 (0.6%) and 5 (3.0%) subjects in the placebo and apremilast cohort, respectively (p < 0.0001 for both).Citation24 As regards the safety, AEs rates were similar among the three cohorts. In particular, the commonest AEs in deucravacitinib group were upper respiratory tract infection and nasopharyngitis.Citation24 Discontinuations due to AEs over weeks 0‒16 were lower with deucravacitinib (14, 1.8%) versus placebo (7, 4.2%) and apremilast (10, 6.0%).Citation24 No AEs leading to interruption occurred in >1 patient receiving deucravacitinib.Citation24
These results were confirmed by another 52-week, double-blinded, Phase 3 trial (POETYK PSO-2) investigating the achievement of PASI75 response and sPGA 0/1 at week 16 on 1020 patients randomized to receive deucravacitinib 6mg every day (n = 511), placebo (n = 255), or apremilast 30mg BID (n = 254).Citation25 Placebo cohort was switched to deucravacitinib at week 16 whereas apremilast group was rerandomized 1:1 to continue deucravacitinib or placebo at week 24.Citation25 Globally, 271 (53.0%) patients reached PASI75 at week 16 in the deucravacitinib group compared with 24 (9.4%) in placebo cohort (p < 0.0001) and 101 (39.8%) in the apremilast group (p = 0.0004).Citation25 Similarly, sPGA 0/1 was reached in 253 (49.5%) patients receiving deucravacitinib compared with 22 (8.6%, p < 0.0001) and 86 (33.9%, p < 0.0001) patients receiving placebo and apremilast, respectively, at the same time point.Citation25 Of note, PASI75 response and sPGA 0/1 continued to improve up to week 24 and these responses were maintained to week 52 with continuous treatment.Citation25 Moreover, at week 16 PASI90 was reached by 138 (27.0%) patients receiving deucravacitinib vs 7 (2.7%) and 46 (18.1%) treated with placebo (p < 0.0001) and apremilast (p = 0.0046), respectively.Citation25 Finally, 52 (10.2%) patients undergoing treatment with deucravacitinib reached PASI100 at week 16 compared with 3 (1.2%) and 11 (4.3%) subjects in the placebo and apremilast cohort, respectively (p < 0.0001 for placebo and p = 0.0051 for apremilast).Citation25 AEs [placebo: 138 (54.3%); deucravacitinib: 293 (57.5%); apremilast: 150 (59.1%)] and serious AEs [placebo: 3 (1.2%); deucravacitinib: 8 (1.6%); apremilast: 1 (0.4%)] rate was similar across the treatment groups.
Similarly, AEs-related treatment interruptions were lower in the deucravacitinib (n = 14, 2.7%) versus placebo (n = 9, 3.5%) or apremilast (n = 12, 4.7%) groups.Citation25 Nasopharyngitis (n = 55, 10.8%) and upper respiratory tract infection (n = 25, 4.9%) were the most common AEs in patients receiving deucravacitinib up to week 16.Citation25
To date, preliminary results from a Phase III, open-label, long-term extension (LTE) trial (POETYK PSO-LTE) with the purpose of assessing the long-term efficacy and safety of deucravacitinib in patients with psoriasis showed that the benefits of deucravacitinib treatment seem to maintain over the longer term (up to 2 years).Citation26 In particular, in 262 deucravacitinib patients from POETYK PSO-1 who transitioned into POETYK PSO-LTE, PASI75, PASI90 and sPGA 0/1 were reached by 82.4%, 55.2% and 66.5% subjects, respectively, following 112 weeks of treatment.Citation27
Finally, several clinical trials investigating the safety and efficacy of deucravacitinib for psoriasis management in scalp psoriasis,Citation28 palmoplantar pustulosis,Citation29 nail psoriasisCitation30 and in pediatric populationCitation31 are ongoing.
Discussion
Psoriasis management may be challenging, particularly for moderate-to-severe forms of the disease.Citation32,Citation33 Despite the use of biological drugs and OSM revolutionized the treatment options showing promising results in terms of effectiveness and safety,Citation34–36 also during the COVID-19 pandemic period which brought further challenges to the daily clinical practice,Citation37–41 there is still a need for new therapies that are always welcome in order to tailor the treatment to the patient and to have a higher level of performance, especially in order to maintain long-term effectiveness. Indeed, conventional systemic treatments are often avoided for contraindications or the risk of AEs,Citation8,Citation9 phototherapy is often limited by logistic concerns.Citation8,Citation9 As regards OSM, apremilast is the only innovative drug with oral administration, which specifically inhibits the phosphodiesterase-4 enzyme, modulating the immune system through the raise of the intracellular cyclic adenosine monophosphate levels and inhibiting the production of TNF, IL-2 and 8.Citation42 Despite the high profile of safety of apremilast (it could be used in patients with hepatitis, tuberculosis or cancer), it has lower and slower effectiveness compared to biologics, with significant lower results in terms of PASI 90 and 100 response.Citation17,Citation18 Similarly, even if biologics have widely showed their effectiveness and safety, few limitations (eg parental administration which may represent an obstacle in the treatment of patients who are needle-phobic, costs, etc.) may reduce their use.Citation17,Citation18
In this context, new data on psoriasis pathogenesis allowed the development of new selective therapies.Citation7 Among these, new scientific findings on TYK2, an intracellular kinase that mediates signaling of IL-23 and other cytokines implicated in psoriasis pathogenesis, particularly IL-12 and type I interferonsCitation43,Citation44 made TYK2 an interesting target for novel psoriasis therapeutic options.
Recently, based on a review of a large, randomized safety clinical trial comparing tofacitinib with anti-TNF for the management of arthritis, US Food and Drug Administration alerted that there seems to be an increased risk of serious heart-related events such as heart attack or stroke, blood clots, cancer, and death. Subsequently, a warning has been made for two other Janus Kinase (JAK) inhibitors: baricitinib and upadacitinib.Citation45 Of interest, deucravacitinib was not involved in these warnings.Citation45 Indeed, it selectively inhibits TYK2 by uniquely binding to the TYK2 regulatory pseudokinase domain (allosteric inhibitions), which has a specific configuration in each JAK (JAK1, JAK2, JAK3 and TYK2), without binding to the conserved active domain (competitive inhibitors), which is similar in all JAK inhibitors. Thus, the use of deucravacitinib does not affect JAK 1, 2 and 3 biomarkers.Citation46 Therefore, differently from JAK inhibitors which may be sometimes linked to an increased risk of changes in laboratory parameters (neutrophils, hemoglobin, lipid panel, platelets, creatine phosphokinase), thromboembolic events, malignancy, and infections, the high selectivity for TYK2 is expected to avoid the AEs deriving from direct JAK inhibition.Citation46
Deucravacitinib, a new OSM that selectively inhibits TYK2, seems to be a valuable option among the armamentarium of drugs for psoriasis management. Currently, it has been approved by the Food and Drug Administration for the management of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy at the dosage of 6mg orally once daily, with or without food.Citation46 Despite real-life data are still absent, clinical trials showed promising data in terms of effectiveness and safety, showing also it superiority against apremilast.Citation22–25 Moreover, several studies are ongoing.Citation26–31
Globally, in the Phase 2 trial, deucravacitinib at the dosages of 3 mg daily and higher resulted in greater clearance of psoriasis compared to placebo.Citation22 Analogous results were observed in QoL improvement.Citation23 Furthermore, both completed phase 3 trials showed that the effectiveness of deucravacitinib 6mg once daily was superior to that of apremilast 30mg twice daily or placebo, at week 16 and week 24.Citation24,Citation25 Of note, clinical response was maintained in patients who continuously received deucravacitinib up to 52 weeks while patients who switched from placebo to deucravacitinib at week 16 had similar responses to those who received continuous deucravacitinib treatment from day 1.Citation24,Citation25
The drug package insert recommends considering risks and benefits of treatment prior to initiating deucravacitinib in patients with chronic or recurrent infection or are predisposed to infections as this drug may increase the risk of infection, avoiding the drug in subjects with an active or serious infection.Citation47 The benefits and risks should be considered also in patients with malignancies before starting treatment.Citation47 Moreover, creatine phosphokinase, triglyceride and liver enzymes should be monitored during treatments since their values may be increased by the drug use. Finally, no dose adjustment is required in patients with renal impairment, but treatment should be avoided in patients with severe hepatic failure.Citation47 Of note, unlike apremilast, screening for tuberculosis and viral hepatitis is necessary to start deucravacitinib therapy, as the case of biologics.
Currently, there are many therapeutic options for the management of moderate-to-severe forms of psoriasis when the use of conventional systemic treatment is contraindicated or ineffective. However, medicine is moving more and more to a personalized approach making the goal of treatment the use of the right drug for the right patient at the right moment. In this scenario, the introduction of apremilast and biologics changed daily clinical practice. However, even if apremilast may be use in patients with multiple comorbidities including cancer, latent tuberculosis and hepatitis, its use may be limited by gastrointestinal AEs as well as the use of biologics may have some limitations.Citation17,Citation18 Thus, the introduction of new innovative safe and effective option is always desirable to extend psoriasis treatment armamentarium and let the dermatologist able to choose the best therapy for the patient following his peculiarities and preferences.Citation48
In this context, deucravacitinib may be a valuable option for psoriasis management. Indeed, promising results have been reported in clinical trials, with a statistically significant higher effectiveness than placebo and apremilast with PASI 75 response in 58.4% of subjects at week 16 and 69.3% of patients at week 24 in POETYK PSO-1 and 53.0% and 58.7% of subjects at the same timepoints in POETYK PSO-2, respectively, with comparable safety to apremilast in both studies.Citation24,Citation25 However, real-life data are still absent.
To sum up, deucravacitinib seems to be an innovative and valuable weapon for the management of psoriasis due to the promising results in terms of effectiveness and safety reported by clinical trials, offering an oral alternative to existing systemic therapies. Certainly, additional studies are required to confirm these results and to compare deucravacitinib versus biologic drugs to establish the exact role of this treatment in the management of psoriasis.
Disclosure
The authors report no conflicts of interest in this work.
References
- Raharja A, Mahil SK, Barker JN. Psoriasis: a brief overview. Clin Med. 2021;21(3):170–173. doi:10.7861/clinmed.2021-0257
- Kamiya K, Kishimoto M, Sugai J, Komine M, Ohtsuki M. Risk factors for the development of psoriasis. Int J Mol Sci. 2019;20(18):18. doi:10.3390/ijms20184347
- Amin M, Lee EB, Tsai TF, Wu JJ. Psoriasis and co-morbidity. Acta Derm Venereol. 2020;100(3):adv00033. doi:10.2340/00015555-3387
- Korman NJ. Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol. 2020;182(4):840–848. doi:10.1111/bjd.18245
- Kouris A, Platsidaki E, Kouskoukis C, Christodoulou C. Psychological parameters of psoriasis. Psychiatrike. 2017;28(1):54–59. doi:10.22365/jpsych.2017.281.54
- Camela E, Potestio L, Ruggiero A, Ocampo-Garza SS, Fabbrocini G, Megna M. Towards personalized medicine in psoriasis: current progress. Psoriasis. 2022;12:231–250. doi:10.2147/PTT.S328460
- Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6):1475. doi:10.3390/ijms20061475
- Nast A, Smith C, Spuls PI, et al. EuroGuiDerm guideline on the systemic treatment of psoriasis vulgaris – part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereol. 2020;34(11):2461–2498. doi:10.1111/jdv.16915
- Nast A, Smith C, Spuls PI, et al. EuroGuiDerm guideline on the systemic treatment of psoriasis vulgaris – part 2: specific clinical and comorbid situations. J Eur Acad Dermatol Venereol. 2021;35(2):281–317. doi:10.1111/jdv.16926
- Ruggiero A, Camela E, Potestio L, Fabbrocini G, Megna M. Drug safety evaluation of tildrakizumab for psoriasis: a review of the current knowledge. Expert Opin Drug Saf. 2022;21(12):1445–1451. doi:10.1080/14740338.2022.2160447
- Megna M, Potestio L, Fabbrocini G, Camela E. Treating psoriasis in the elderly: biologics and small molecules. Expert Opin Biol Ther. 2022;1–18. doi:10.1080/14712598.2022.2089020
- Ruggiero A, Potestio L, Camela E, Fabbrocini G, Megna M. Bimekizumab for the treatment of psoriasis: a review of the current knowledge. Psoriasis. 2022;12:127–137. doi:10.2147/PTT.S367744
- Ruggiero A, Picone V, Martora F, Fabbrocini G, Megna M. Guselkumab, risankizumab, and tildrakizumab in the management of psoriasis: a review of the real-world evidence. Clin Cosmet Investig Dermatol. 2022;15:1649–1658. doi:10.2147/CCID.S364640
- Megna M, Potestio L, Fabbrocini G, Cinelli E. Tildrakizumab: a new therapeutic option for erythrodermic psoriasis? Dermatol Ther. 2021;34(5):e15030. doi:10.1111/dth.15030
- Megna M, Potestio L, Fabbrocini G, Ruggiero A. Long-term efficacy and safety of guselkumab for moderate to severe psoriasis: a 3-year real-life retrospective study. Psoriasis. 2022;12:205–212. doi:10.2147/PTT.S372262
- Marasca C, Fornaro L, Martora F, Picone V, Fabbrocini G, Megna M. Onset of vitiligo in a psoriasis patient on ixekizumab. Dermatol Ther. 2021;34(5):e15102. doi:10.1111/dth.15102
- Balogh EA, Bashyam AM, Ghamrawi RI, Feldman SR. Emerging systemic drugs in the treatment of plaque psoriasis. Expert Opin Emerg Drugs. 2020;25(2):89–100. doi:10.1080/14728214.2020.1745773
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2015;151(9):961–969. doi:10.1001/jamadermatol.2015.0718
- Catlett IM, Hu Y, Gao L, Banerjee S, Gordon K, Krueger JG. Molecular and clinical effects of selective tyrosine kinase 2 inhibition with deucravacitinib in psoriasis. J Allergy Clin Immunol. 2022;149(6):2010–2020.e8. doi:10.1016/j.jaci.2021.11.001
- Estevinho T, Lé AM, Torres T. Deucravacitinib in the treatment of psoriasis. J Dermatolog Treat. 2023;34(1):2154122. doi:10.1080/09546634.2022.2154122
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379(14):1313–1321. doi:10.1056/NEJMoa1806382
- Thaçi D, Strober B, Gordon KB, et al. Deucravacitinib in moderate to severe psoriasis: clinical and quality-of-life outcomes in a phase 2 trial. Dermatol Ther. 2022;12(2):495–510. doi:10.1007/s13555-021-00649-y
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88(1):29–39. doi:10.1016/j.jaad.2022.07.002
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88(1):40–51. doi:10.1016/j.jaad.2022.08.061
- Bristol Myers Squibb. New two-year deucravacitinib data rein- force durable efficacy and consistent safety profile in treatment of moderate to severe plaque psoriasis [media release]; 2022. Available from: http://www.bms.com. Accessed May 2, 2023.
- Bristol Myers Squibb. Bristol Myers Squibb announces new SotyktuTM (deucravacitinib) long-term data showing clinical efficacy maintained for up to two years with continuous treat- ment in moderate-to-severe plaque psoriasis [media release]; 2022. h.
- Efficacy and safety of deucravacitinib versus placebo in participants with moderate-to-severe scalp psoriasis. Available from: https://clinicaltrials.gov/ct2/show/NCT05478499?cond=deucravacitinib+psoriasis&draw=2&rank=2. Accessed February 19, 2023.
- Deucravacitinib for the treatment of palmoplantar pustulosis. Available from: https://clinicaltrials.gov/ct2/show/NCT05710185?cond=deucravacitinib+psoriasis&draw=2&rank=8. Accessed February 19, 2023.
- An investigator initiated open label study evaluating the efficacy and tolerability of oral deucravacitinib for the treatment of nail psoriasis. Available from: https://clinicaltrials.gov/ct2/show/NCT05124080?cond=deucravacitinib+psoriasis&draw=2&rank=5.Ac. Accessed May 2, 2023.
- A study to evaluate the drug levels, efficacy and safety of deucravacitinib in pediatric participants with moderate to severe plaque psoriasis. Available from: https://clinicaltrials.gov/ct2/show/NCT04772079?cond=deucravacitinib+psoriasis&draw=2&rank=4.Acc. Accessed May 2, 2023.
- Megna M, Camela E, Battista T, et al. Efficacy and safety of biologics and small molecules for psoriasis in pediatric and geriatric populations. Part I: focus on pediatric patients. Expert Opin Drug Saf. 2023:1–17. doi:10.1080/14740338.2023.2173170
- Megna M, Camela E, Battista T, et al. Efficacy and safety of biologics and small molecules for psoriasis in pediatric and geriatric populations. Part II: focus on elderly patients. Expert Opin Drug Saf. 2023:1–16. doi:10.1080/14740338.2023.2173171
- Megna M, Potestio L, Ruggiero A, Camela E, Fabbrocini G. Guselkumab is efficacious and safe in psoriasis patients who failed anti-IL17: a 52-week real-life study. J Dermatolog Treat. 2022;1–18. doi:10.1080/09546634.2022.2036674
- Megna M, Potestio L, Ruggiero A, Camela E, Fabbrocini G. Risankizumab treatment in psoriasis patients who failed anti-IL17: a 52-week real-life study. Dermatol Ther. 2022;35(7):e15524. doi:10.1111/dth.15524
- Megna M, Potestio L, Camela E, Fabbrocini G, Ruggiero A. Ixekizumab and brodalumab indirect comparison in the treatment of moderate to severe psoriasis: results from an Italian single-center retrospective study in a real-life setting. Dermatol Ther. 2022;35(9):e15667. doi:10.1111/dth.15667
- Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11(6):1511. doi:10.3390/jcm11061511
- Ruggiero A, Martora F, Fabbrocini G, et al. The role of teledermatology during the COVID-19 pandemic: a narrative review. Clin Cosmet Investig Dermatol. 2022;15:2785–2793. doi:10.2147/CCID.S377029
- De Lucia M, Potestio L, Costanzo L, Fabbrocini G, Gallo L. Scabies outbreak during COVID-19: an Italian experience. Int J Dermatol. 2021;60(10):1307–1308. doi:10.1111/ijd.15809
- Megna M, Camela E, Villani A, Tajani A, Fabbrocini G, Potestio L. Teledermatology: a useful tool also after COVID-19 era? J Cosmet Dermatol. 2022;21(6):2309–2310. doi:10.1111/jocd.14938
- Ruggiero A, Martora F, Picone V, et al. The impact of COVID-19 infection on patients with psoriasis treated with biologics: an Italian experience. Clin Exp Dermatol. 2022;47(12):2280–2282. doi:10.1111/ced.15336
- Nassim D, Alajmi A, Jfri A, Pehr K. Apremilast in dermatology: a review of literature. Dermatol Ther. 2020;33(6):e14261. doi:10.1111/dth.14261
- Krueger JG, McInnes IB, Blauvelt A. Tyrosine kinase 2 and Janus kinase‒signal transducer and activator of transcription signaling and inhibition in plaque psoriasis. J Am Acad Dermatol. 2022;86(1):148–157. doi:10.1016/j.jaad.2021.06.869
- Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci Transl Med. 2019;11(502):502. doi:10.1126/scitranslmed.aaw1736
- FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-requ. Accessed May 2, 2023.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective TYK2 inhibitors. Drugs. 2020;80(4):341–352. doi:10.1007/s40265-020-01261-8
- SOTYKTU US. Prescribing information. Available from: https://packageinserts.bms.com/pi/pi_sotyktu.pdf. Accessed February 19, 2023.
- Camela E, Potestio L, Fabbrocini G, Ruggiero A, Megna M. New frontiers in personalized medicine in psoriasis. Expert Opin Biol Ther. 2022;1–3. doi:10.1080/14712598.2022.2113872
