Abstract
Psoriasis is a chronic skin condition that can significantly impact the quality of life of those affected. As an autoimmune disease, it can lead to itchy, painful, and scaly patches on the skin. Although various treatments, including topical creams, phototherapy, and systemic medications, are currently available, they may not always offer effective relief and can have side effects. Researchers have thus been exploring the potential benefits of non-psychoactive compounds such as CBD, found in Cannabis sativa plants, for treating psoriasis. CBD treatment may reduce inflammation, oxidative stress, itching, abnormal proliferation of keratinocytes, and may increase hydration. This review aims to provide an overview of the existing literature on the potential uses of CBD for psoriasis treatment.
Introduction
Psoriasis is a chronic skin condition that can significantly impact the quality of life of those affected. It is a multifactorial disease involving genetic predisposition, immune dysregulation, and environmental triggers. As an autoimmune disease, it can lead to itchy, painful, and scaly patches on the skin. Although various treatments, including topical creams, phototherapy, and systemic medications, are currently available, they may not always offer effective relief and can have side effects.Citation1,Citation2
Research has shown that psoriasis is associated with specific genetic markers, including variations in the HLA-Cw6 allele and immune response genes that affect skin barrier function.Citation3 Additionally, environmental factors such as stress, infection, and certain medications can exacerbate symptoms.Citation4 When immune cells activate abnormally, this can lead to rapid skin cell production, resulting in the formation of thickened patches on the skin that eventually become scaly and inflamed.Citation5
Individuals with psoriasis are at an increased risk of comorbidities, including psoriatic arthritis, cardiovascular diseases, and metabolic syndrome.Citation6,Citation7 Managing psoriasis necessitates a personalized approach that takes into account the severity of the disease, patient preferences, and responses to previous treatments. For mild to moderate cases, topical therapies such as corticosteroids, vitamin D analogs, and retinoids may be prescribed.Citation8
Phototherapy, including ultraviolet B (UVB) and psoralen plus ultraviolet A (PUVA), is an effective therapy for more extensive disease involvement.Citation9 Meanwhile, systemic therapies, including biologic agents that target specific immune pathways, have transformed psoriasis treatment. Tumor necrosis factor alpha (TNF-α) inhibitors such as adalimumab and etanercept, and interleukin (IL)-17A inhibitors like secukinumab, as well as ustekinumab, have demonstrated remarkable efficacy in improving skin symptoms and quality of life.Citation10–13
However, conventional treatments often fail to yield satisfactory results and may cause adverse side effects. Recently, cannabidiol (CBD), a non-psychoactive compound derived from Cannabis sativa plants, has gained attention as an innovative therapeutic option for psoriasis treatment.
Several species of Cannabis are recognized, including Cannabis sativa, Cannabis indica, Cannabis ruderalis, and the monospecific species Cannabis sativa L., along with various subspecies. The classification of cannabis species and subspecies remains a topic of debate.Citation14,Citation15
The term “cannabis” encompasses both hemp and marijuana, which are types of plants derived from different cultivars or chemotypes of the Cannabis sativa L. species, having low or high Δ9-THC content, respectively.Citation16 Within Cannabis sativa, tetrahydrocannabinol (THC) is known for its muscle-relaxing, appetite-stimulating, and analgesic effects, while cannabidiol (CBD) is recognized for amplifying antispastic activity properties.Citation17–19
Recent research highlights the potential benefits of cannabidiol (CBD) in managing psoriasis through its immunomodulatory and anti-inflammatory effects. Studies indicate that CBD might play a role in lowering cytokine production and inflammatory responses within psoriatic cells, which could help alleviate the symptoms associated with the disease. Specifically, it has been observed that CBD reduces the secretion of inflammatory cytokines such as IFNγ and TNFα in peripheral blood mononuclear cells (PBMCs) from individuals with psoriasis, which are key factors in the disease’s pathophysiology.Citation20
Additionally, CBD has demonstrated potential in minimizing oxidative stress in keratinocytes from psoriatic skin, which could help avert the metabolic effects related to peroxidation. This beneficial action may stem from CBD’s capability to influence the activity of enzymes that promote pro-oxidative conditions and to bolster the antioxidant defenses in these cells.Citation21
Clinical research suggests that using CBD in the form of transdermal ointments may significantly lessen the severity of symptoms in psoriasis, as measured by indices like the Psoriasis Area Severity Index (PASI). These findings support the potential of topical CBD treatments to provide relief for individuals with this condition.Citation22
The scope of this article is to review the current scientific evidence regarding the potential of CBD in psoriasis therapy, highlighting key studies and providing an overview of the underlying mechanisms.
Materials and Methods
We have conducted this review based on an electronic search of all references in English languages using the keywords “psoriasis” and “cannabidiol”. Considering that it is a niche subject, which has been studied very little, we did not apply any filters regarding the years of publication. The search was conducted in PubMed, Web of Science, and Scopus databases to identify articles that reported a relationship between psoriasis and cannabidiol, with a focus on therapeutic options. The search strategy was as follows: (psoriasis [Title/Abstract]) AND (cannabidiol [Title/Abstract]). The study was considered exempt from institutional board approval because it was review of literature with nonhuman subject research. All references were explored by hand.
We have included all studies in English describing the mechanism of action and the potential use of cannabidiol as therapy in psoriasis. Case-control, cohort, and cross-sectional studies were included. Excluded were conference proceedings, abstracts and book chapters.
The following information was extracted: author, year of study publication, country of the studied population, study design, objective of this study, number of patients with psoriasis and number of cases control for case-control studies, type and severity of psoriasis, reported treatment with CBD, the beneficial effects of the treatment, the adverse effects of the treatment.
Endocannabinoid System (ESC) - Significance and Therapeutic Potential
The endocannabinoid system (ECS) was first discovered in the 1990s and has since emerged as a crucial modulatory system in the human body.Citation23 This system comprises endogenous cannabinoids (endocannabinoids), their receptors, and the enzymes responsible for their synthesis and degradation. Understanding the ECS has illuminated various physiological processes, including pain modulation, appetite regulation, immune function, and neuronal signaling.Citation24
The ECS consists of three main components: endocannabinoids, cannabinoid receptors, and enzymes. Endocannabinoids, such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG), are lipid-based molecules synthesized on demand in response to physiological stimuli.Citation25 Cannabinoid receptors, primarily CB1 and CB2 receptors, are found throughout the body and are responsible for mediating the effects of endocannabinoids.Citation23 Enzymes such as fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) regulate the production and breakdown of endocannabinoids.Citation26
The ECS plays a vital role in maintaining homeostasis within the body. It is involved in the regulation of pain sensation, inflammation, mood, and appetite.Citation24 Activation of cannabinoid receptors can modulate neuronal excitability and neurotransmitter release, contributing to the regulation of synaptic plasticity and neuroprotection.Citation27 Furthermore, the ECS has been implicated in immune function, with cannabinoids exhibiting immunomodulatory effects.Citation26
Given its involvement in various physiological processes, the ECS has garnered significant attention as a potential therapeutic target. Modulating the ECS through exogenous cannabinoids or by targeting the enzymes involved in endocannabinoid metabolism holds promise for the treatment of conditions such as chronic pain, neurodegenerative disorders, and psychiatric conditions.Citation28 Furthermore, the ECS has been implicated in the regulation of appetite, making it a potential target for managing eating disorders and obesity.Citation25
Receptors from the ECS have been identified in skin tissue, and the systemic misuse of synthetic cannabinoids or their analogs has been associated with dermatological disorders, indicating their influence on cutaneous biology.Citation29
Recent studies have indicated the presence of a functional ECS in the skin, implicating it in various biological processes such as proliferation, differentiation, growth, apoptosis, and cytokine production. The primary physiological role of the cutaneous ECS appears to be the constant regulation of the proper and balanced proliferation, differentiation, and survival, as well as the immune competence/tolerance, of skin cells. Disruption of this equilibrium may lead to the development of multiple pathological conditions and diseases of the skin, including psoriasis, acne, allergic dermatitis, seborrhea, itching and pain, systemic sclerosis, hair growth disorders, and cancer.Citation30,Citation31
In recent years, the ECS has garnered attention as a potential therapeutic avenue in psoriasis treatment due to its regulatory effects on immune function and inflammation. The ECS consists of endogenous cannabinoids (endocannabinoids), cannabinoid receptors, and enzymes involved in their synthesis and degradation. Several studies have reported dysregulation of the ECS in psoriasis, with alterations observed in the levels of endocannabinoids such as anandamide and 2-arachidonoylglycerol.Citation32 Dysfunctional cannabinoid receptors, particularly CB1 and CB2 receptors, have also been implicated in the pathophysiology of psoriasis.Citation33,Citation34
Furthermore, enzymes involved in endocannabinoid metabolism, including fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), may play a role in psoriatic inflammation.Citation35
The ECS exerts immunomodulatory and anti-inflammatory effects, which have significant implications for psoriasis. Activation of cannabinoid receptors on immune cells can modulate cytokine production, inhibit immune cell migration, and regulate the balance between pro-inflammatory and anti-inflammatory responses.Citation36 Endocannabinoids have been shown to suppress the release of pro-inflammatory mediators and attenuate immune cell activation, suggesting their potential as therapeutic agents in psoriasis.Citation32
Preclinical and clinical studies have explored the use of cannabinoids and ECS modulators in the management of psoriasis. Cannabinoids, including tetrahydrocannabinol (THC) and cannabidiol (CBD), have demonstrated anti-inflammatory and anti-proliferative effects on keratinocytes and immune cells.Citation32 Topical formulations containing cannabinoids have shown promise in reducing psoriatic plaques and improving symptoms.Citation37 Furthermore, targeting ECS-related enzymes, such as FAAH and MAGL, has emerged as a potential therapeutic strategy to enhance endocannabinoid levels and alleviate psoriatic inflammation.Citation38
Mechanisms of Action of Cannabidiol Treatment in Psoriasis
The mechanisms of action underlying the therapeutic effects of CBD in psoriasis are multifaceted, involving its anti-inflammatory, immunomodulatory, anti-proliferative, and neuroimmunomodulatory properties (). CBD interacts with the ECS and various signaling pathways, leading to the regulation of immune responses, keratinocyte proliferation and differentiation, and neurogenic inflammation.
Figure 1 The figure highlights the possible mechanisms of action of cannabidiol in the treatment of psoriasis. CBD action may reduce inflammation, oxidative stress, itching, abnormal proliferation of keratinocytes, and may increase hydration. Adapted from from smart SERVIER MEDICAL. INTEGUMENTARY SYSTEM ART. 2024. Available from https://smart.servier.com/smart_image/integumentary-system/, licensed under Creative Commons Attribution 4.0 https://creativecommons.org/licenses/by/4.0/.Citation39
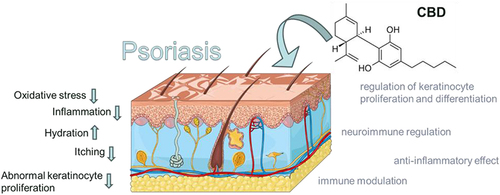
Possible Mechanisms of Action of Cannabidiol Treatment in Psoriasis.
Cannabidiol (CBD) engages with the cellular pathways of psoriasis mainly through indirect mechanisms, avoiding direct interaction with a specific receptor unique to psoriasis. Demonstrating its potential in immune modulation, CBD plays a critical role since psoriasis is fundamentally an autoimmune disorder. It regulates cytokine production—proteins from the immune system that can trigger inflammation. Through its modulation of cytokines, CBD may effectively reduce the inflammatory activities that are a hallmark of psoriasis.Citation40
CBD might serve as an antagonist to the GPR55 receptor, a G protein-coupled receptor implicated in inflammation. Blocking GPR55 may diminish inflammation in psoriatic lesions because this receptor’s overactivity is linked to increased inflammatory reactions. Additionally, CBD is known to activate transient receptor potential vanilloid (TRPV) receptors, such as TRPV1. These receptors play roles in conveying sensations of pain, heat, and itch, which are prevalent in psoriasis. By activating these receptors, CBD could potentially ease these bothersome symptoms.Citation41,Citation42
The stimulation of the peroxisome proliferator-activated receptor-gamma (PPAR-gamma) by CBD highlights its potential impact on critical processes of psoriasis such as skin cell proliferation and differentiation. PPAR-gamma, which is involved in lipid metabolism and glucose regulation, also plays a crucial role in the development and maturation of skin cells. By activating PPAR-gamma, CBD might aid in stabilizing the growth cycle of skin cells. Furthermore, the antioxidative and neuroprotective characteristics of CBD could help in mitigating oxidative stress and nerve-related irritation in psoriasis, both of which are known to worsen the condition.Citation40,Citation43
The multifaceted actions of CBD in managing psoriasis indicate that it targets multiple cellular pathways, thereby modulating various symptoms and potentially influencing the progression of the disease. CBD does not interact with a unique receptor specifically for psoriasis, but its wide-ranging pharmacological effects allow it to impact several relevant targets within the disease’s framework.
Mechanisms of CBD’s Anti-Inflammatory Effects
Cannabinoids and their derivatives are known for their anti-inflammatory properties and are reported to inhibit rapidly proliferating tumorigenic cell lines. CBD exerts its anti-inflammatory effects through various mechanisms.Citation21 It modulates immune responses by suppressing the activation and migration of immune cells, including T cells and dendritic cells.Citation44 Importantly, cannabinoids have been shown to shift the predominantly pro-inflammatory Th1 type expression to a more anti-inflammatory Th2 type profile.Citation45
CBD also affects the production of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6), thereby attenuating inflammatory responses.Citation46 Furthermore, CBD has been shown to inhibit the activation of nuclear factor-kappa B (NF-κB) and other signaling pathways involved in inflammation.Citation47
The proposed mechanism of palmitoylethanolamide (PEA) and CB2 receptor agonists is to inhibit a functional response to mast cell activation in vivo, demonstrating anti-inflammatory effects with the potential to prevent stress-induced exacerbation of skin disorders such as psoriasis.Citation48–50
Cannabis and cannabinoids are known for their anti-inflammatory activity in autoimmune diseases with characteristics similar to psoriasis, such as rheumatoid arthritis and Crohn’s disease, and have been shown to alter immune function by influencing cytokine expression.Citation51 Cannabidiol (CBD), the main non-psychotropic component of Cannabis sativa, was recently reported to exhibit high anti-inflammatory activity, particularly against acute models of inflammation and diabetic retinopathy.Citation52
CBD has demonstrated its capability to antagonize the GPR55 receptor and may also interact with GPR18, although its effects on the latter are not as well-defined. GPR55 is known to play a role in various inflammatory processes, and blocking this receptor might help reduce inflammation, which is vital in treating conditions like psoriasis. Additionally, GPR55 is involved in modulating other signaling pathways, such as NF-kB, which is crucial for regulating inflammation and immune responses.Citation53
CBD interacts with the transient receptor potential vanilloid (TRPV) channels, notably TRPV1, which are key in detecting temperature, pain, and itch-symptoms frequently experienced in psoriasis. By activating TRPV1, CBD could not only help soothe these discomforts but may also indirectly affect other signaling pathways such as NF-kB through its anti-inflammatory and stress response effects.Citation54
CBD boosts adenosine levels by blocking its absorption, thereby indirectly stimulating adenosine receptors. This stimulation has anti-inflammatory effects since adenosine receptors significantly contribute to the regulation of inflammation, which could influence signaling pathways like NF-kB.Citation55
Other anti-inflammatory and immunological effects of cannabinoids have been observed in a variety of in vivo and in vitro models, providing evidence that supports their potential application for psoriasis. Recent studies have demonstrated the presence of CB receptors in human skin and that anandamide, an endogenous CB receptor ligand, inhibits epidermal keratinocyte differentiation.Citation56,Citation57
Mechanisms of CBD’s Immune Modulation Effects
Although the etiology and pathogenesis of psoriasis are not fully understood, psoriasis can be characterized by epidermal keratinocyte hyperproliferation accompanied by the infiltration and increased expression of proinflammatory mediators in the skin, including a dominant Th1 cytokine profile.Citation58
CBD interacts with various immune cell types implicated in psoriasis, including T cells, dendritic cells, and macrophages. CBD has been shown to modulate T cell activation and differentiation, leading to a shift from a pro-inflammatory Th1/Th17 phenotype towards an anti-inflammatory Th2/Treg phenotype.Citation45 Additionally, CBD can inhibit dendritic cell activation and maturation, reducing their ability to prime and activate T cells.Citation59 CBD’s effects on macrophages involve the suppression of pro-inflammatory M1 polarization and the promotion of anti-inflammatory M2 polarization.Citation60
Cytokines play a crucial role in the pathogenesis of psoriasis, and CBD has been shown to modulate their production and signaling pathways. CBD can suppress the production of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6).Citation46 Moreover, CBD can inhibit signaling pathways such as the nuclear factor-kappa B (NF-κB) pathway, which is involved in the production of pro-inflammatory cytokines.Citation47
CBD targets PPARs, particularly PPAR-gamma, which when activated by CBD, exhibits anti-proliferative properties on keratinocytes—these are the main cells in the epidermis and are often affected in psoriasis. The activation of PPAR-gamma results in the suppression of cell growth and adjustments to immune responses, influencing crucial pathways such as Wnt and MAPK that are essential for cell differentiation and proliferation.Citation43
CBD’s immunomodulatory effects in psoriasis include regulating T cell function. CBD has been shown to suppress T cell proliferation and cytokine production, thereby dampening the inflammatory response. Additionally, CBD can modulate the balance between pro-inflammatory Th17 cells and anti-inflammatory regulatory T cells, contributing to the attenuation of inflammation in psoriasis.Citation61,Citation62
Mechanisms of CBD’s Regulation of Keratinocyte Proliferation and Differentiation Effects
CBD interacts with key signaling pathways involved in keratinocyte homeostasis. It has been shown to modulate the Wnt/β-catenin pathway, which is crucial for regulating keratinocyte proliferation and differentiation.Citation63 CBD can also affect mitogen-activated protein kinase (MAPK) signaling pathways, including the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK pathways, which are involved in keratinocyte proliferation and inflammation.Citation57 Furthermore, CBD’s impact on epigenetic regulation, such as histone modifications and DNA methylation, may contribute to the modulation of keratinocyte functions.Citation64
CBD has been shown to regulate the expression of keratinocyte differentiation markers and growth factors implicated in psoriasis. It can promote the expression of keratinocyte differentiation markers, such as involucrin and filaggrin, leading to enhanced keratinocyte differentiation. Wilkinson and Williamson tested isolated phytocannabinoids, including Δ-9 THC, CBN, CBD, and CBG, all of which inhibited keratinocyte proliferation in a concentration-dependent manner. These compounds showed anti-proliferative potencies of the same order in this cell line, with CBG and CBD eliciting the greatest overall activity. CBD had the lowest maximum inhibitory concentration, and also the lowest IC50 value.Citation32
Additionally, CBD can modulate the expression of growth factors, including transforming growth factor-beta (TGF-β) and epidermal growth factor (EGF), which are involved in keratinocyte proliferation and differentiation.Citation65 CBD’s anti-inflammatory properties may contribute to its effects on keratinocyte functions in psoriasis. By reducing inflammatory mediators, CBD may help restore keratinocyte homeostasis and attenuate psoriatic symptoms.Citation66
Mechanisms of CBD’s Neuroimmunomodulation Effects
CBD interacts with the endocannabinoid system (ECS), which plays a crucial role in regulating immune and neural functions. CBD has been shown to modulate ECS signaling by inhibiting endocannabinoid degradation enzymes, such as fatty acid amide hydrolase, leading to increased endocannabinoid levels.Citation53 The modulation of ECS activity by CBD may contribute to its neuroimmunomodulatory effects in psoriasis.
Neuroinflammation plays a key role in the pathogenesis of psoriasis. CBD has been shown to modulate neuroinflammatory pathways, including the nuclear factor-kappa B (NF-κB) pathway, the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway, and the mitogen-activated protein kinase (MAPK) pathway.Citation67 By attenuating neuroinflammation, CBD may help alleviate neuroimmune dysregulation in psoriasis.
CBD can modulate the expression and release of neurotrophic factors implicated in neuroimmune interactions. It has been shown to upregulate the expression of brain-derived neurotrophic factor (BDNF), which plays a crucial role in neuronal survival, plasticity, and immune regulation.Citation68 Additionally, CBD can regulate the release of pro-inflammatory cytokines and chemokines from neuronal cells, thereby modulating the neuroimmune response in psoriasis.
Neurogenic inflammation, itch, and pain are common features of psoriasis. CBD has been reported to attenuate neurogenic inflammation by inhibiting the release of neuropeptides, such as substance P and calcitonin gene-related peptide (CGRP), from sensory nerves.Citation69 Furthermore, CBD’s analgesic and anti-pruritic properties may contribute to its therapeutic potential in alleviating itch and pain in psoriatic lesions.
Evidence of the Use of Cannabidiol in the Treatment of Psoriasis
Building on the hypothesis of Norooznezhad and Norooznezhad, besides inhibiting keratinocyte proliferation, cannabinoids target two main pathways in psoriasis pathogenesis: angiogenesis and inflammation. It has been theoretically suggested that JWH-133 could represent a potential oral or topical treatment for psoriasis.Citation70
Topical formulations containing cannabidiol show promise as an effective and safe therapeutic option for treating psoriasis (). The anti-inflammatory, anti-proliferative, and immunomodulatory properties of CBD make it a potential approach to address the multifaceted aspects of psoriasis pathogenesis.
Table 1 Topical Formulations with Cannabidiol
In 2003, Iliev and Broshtilova described a formula known as “Overcome Psoriasis Formula”, which includes cannabis, a component long used in traditional Chinese medicine (TCM) to treat psoriasis induced by Blood Dryness. This formula, named “Ke yin fang”, comprises the following ingredients: Radix Rehmanniae Glutinosae 30g, Radix Scrophulariae Ningpoensis 30g, Semen Cannabis Sativae 10g, Rhizoma Menispermi Daurici 10g, and Radix Sophorae Flavescentis 10g.71
Hadjiminaglou and Bolcato attempted to use a formula based on essential oils as an alternative to topical steroids. The selection of essential oils was based on factors closely related to the inflammatory reaction. Essential oil of Cannabis sativa, obtained from the whole fresh plant, contains Myrcene (33.0%), trans-β-Ocimene (15.0%), Terpinolene (13.0%), β-Caryophyllene (11.3%), and Caryophyllene oxide (1.4%) as its main chemical components. It has been demonstrated that Cannabis sativa essential oil is an in vitro inhibitor of 5-lipoxygenase.Citation72,Citation75
Another study utilized a shampoo containing Broad-Spectrum Cannabidiol for patients with mild to moderate scalp psoriasis or Seborrheic Dermatitis (SD). The shampoo’s ingredients included 0.075% broad-spectrum cannabidiol, known for its anti-inflammatory effects on the pilosebaceous unit and potential to reduce oxidative stress, ketoconazole (to reduce Malassezia spp.), and ingredients that promote hair growth.Citation76–78 The study demonstrated the shampoo’s high efficacy in reducing signs of scalp inflammation, as well as symptoms of erythema, scaling, itching, and burning, after 2 weeks of use. Baseline severity was similar in males and females, with no side effects reported.Citation73
Changoer and Anastassov tested a method that comprises the topical application of a composition containing cannabinoids, specifically cannabidiol (CBD) and cannabigerol (CBG) at concentrations of 3% and 15% by weight of the composition. The topical application was applied at least twice daily for six weeks. The treatment with 3% CBG/CBD oil showed no improvement in the lesions. However, the 15% CBG/CBD oil treatment resulted in a 16% improvement in Subject 1 and a 33% improvement in Subject 2, both with psoriasis vulgaris.Citation74
In 2020, a case of vulgar psoriasis was treated with a regimen including a tetrahydrocannabinol (THC) distillate cream with medium chain triglyceride (MCT) oil, beeswax, and sunflower lecithin; THC soap infused with scent-free hemp soap, 5 mg/mL; and a hair oil with THC distillate dissolved into jojoba oil, 5 mg/mL, applied daily. Visible results were observed two days after starting the treatment. After seven months, the patient continued using the soap as a preventive measure to inhibit activity in areas with a high density of hair follicles and applied the creams every few days to manage skin dryness.Citation75
In vitro research investigated the plant cannabinoids Δ-9 tetrahydrocannabinol (THC), cannabidiol (CBD), cannabinol (CBN), and cannabigerol (CBG) for their ability to inhibit the proliferation of a hyper-proliferating human keratinocyte cell line and for any involvement of cannabinoid receptors. All cannabinoids tested inhibited keratinocyte proliferation in a concentration-dependent manner, demonstrating therapeutic potential for the treatment of psoriasis.Citation32
Another in vitro and in situ study suggested that cannabinoids and their receptors constitute a novel, clinically relevant control element of human K6 and K16 expression. Therefore, cannabimimetic agents might be relevant for the treatment of several skin conditions related to aberrant K6/K16 expression, such as psoriasis and wound healing.Citation38
One study confirmed that when entering keratinocytes, CBD—especially after exposure to UVA radiation—accumulates mainly in the membranes of keratinocytes, particularly in those of patients with psoriasis. CBD treatment leads to the accumulation of CBD primarily in the membranes of keratinocytes, especially in UV-irradiated psoriasis cells, and reduces oxidative phospholipid modifications and their consequences. By increasing the level of PEA, CBD can contribute to reducing inflammation.Citation79
Sangiovanni et al demonstrated in vitro that Cannabis sativa L. ethanolic extract (CSE), standardized in CBD, is able to inhibit the release of mediators of inflammation involved in wound healing and inflammatory processes occurring in the skin. The mode of action appears to involve impairment of the NF-κB pathway, as the extract was able to inhibit TNFα-induced NF-κB-driven transcription in both HDF and HaCaT cells.Citation80
Casares et al indicated that psoriasis is characterized by chronic inflammation and keratinocyte hyperproliferation, with high levels of keratin 16 and 17. Therefore, despite CBD’s anti-inflammatory effects, the use of CBD in psoriasis should be approached with caution due to its potential pro-proliferative effects in vivo.Citation81
The Perception of the Use of Cannabidiol in the Treatment of Psoriasis
Although studies validating the use of medical cannabis in chronic inflammatory skin conditions are limited, in Connecticut (USA), medical cannabis is approved for conditions such as psoriasis and psoriatic arthritis.Citation22
A survey in the USA among dermatologists revealed that 83.3% were uncertain if psoriasis was an indication for medical cannabis, and 79.2% reported they did not have sufficient knowledge to explain the risks and benefits of medical cannabis. Only 16.7% of respondents had prior education on medical cannabis, yet 100% expressed interest in learning about its potential uses. Additionally, 55.5% of Medical Advisory Board dermatologists believe medical cannabis is safe for their patients, with no respondents considering it unsafe.Citation82
It’s crucial to acknowledge that research into CBD’s impact on psoriasis is still in its early stages. The medical community has yet to establish definitive guidelines regarding the most effective usage and potential long-term consequences of CBD for treating psoriasis. Additionally, the effectiveness of CBD may differ based on how it is administered, its concentration, and individual differences among patients.
Conclusion
In conclusion, the current literature on the use of cannabidiol (CBD) in psoriasis therapy suggests its potential as a promising treatment option. The mechanisms of action underlying CBD’s therapeutic effects in psoriasis are not yet fully understood. Despite the promising findings, further research is required to establish the optimal dosage, formulation, and long-term safety profile of CBD-based treatments for psoriasis. Large-scale clinical trials are necessary to validate the efficacy of CBD and determine its potential as a standalone therapy or as an adjunct to existing treatment modalities.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
“Publication of this paper was supported by the University of Medicine and Pharmacy Carol Davila, through the institutional program Publish not Perish”.
References
- Stanescu AMA, Simionescu AA, Florea M, Diaconu CC. Is metformin a possible beneficial treatment for psoriasis? A scoping review. J Pers Med. 2021;11(4):251. doi:10.3390/jpm11040251
- Stanescu AMA, Simionescu AA, Diaconu CC. Oral vitamin D therapy in patients with psoriasis. Nutrients. 2021;13(1):163. doi:10.3390/nu13010163
- Tsoi LC, Spain SL. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet. 2012;44:(12):1341–1348. doi:10.1038/ng.2467
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512–516. doi:10.1016/j.jaad.2013.11.013
- Costache RS, Georgescu M, Ghilencea A, Feroiu O, Costache DO. The role of inflammation in the pathogenesis of psoriasis. Rom J Milit Med. 2023;3:245–253. doi:10.55453/rjmm.2023.126.3.2
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–385. doi:10.1038/jid.2012.339
- Bucur Ș, Savu AP, Stănescu AMA, et al. Oversight and management of women with psoriasis in childbearing age. Medicina. 2022;58:780. doi:10.3390/medicina58060780
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643–659. doi:10.1016/j.jaad.2008.12.032
- Pathirana D, Ormerod AD, Saiag P, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23(Suppl 2):1–70. doi:10.1111/j.1468-3083.2009.03389.x
- Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled Phase II dose-ranging study. Br J Dermatol. 2013;168:412–421. doi:10.1111/bjd.12110
- Reich K, Langley RG, Papp KA, et al. A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. N Engl J Med. 2011;365:1586–1596. doi:10.1056/NEJMoa1010858
- Peneş NO, Weber B, Păun SD. Role of genetic polymorphism in nutritional supplementation therapy in personalized medicine. Rom J Morphol Embryol. 2017;58:53–58.
- Bucur S, Mutu CC, Costache RS, et al. Correlations between etiopathogenic factors and persistence on anti-IL-17 therapies in patients with psoriasis vulgaris. Rom J Milit Med. 2024;4:255–262. doi:10.55453/rjmm.2024.127.4.1
- Pollio A. The name of cannabis: a short guide for nonbotanists. Cannabis Cannabinoid Res. 2016;1:234–238. doi:10.1089/can.2016.0027
- Small E, Cronquist A. A practical and natural taxonomy for cannabis. Taxon. 1976;25(4):405–435. doi:10.2307/1220524
- Johnson R. Defining Hemp: a Fact Sheet. Washington, D.C: Congressional Research Service, 44742. Available from: https://crsreports.congress.gov/product/pdf/R/R44742. Accessed January 11, 2023.
- Baker D, Pryce G, Croxford JL, et al. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature. 2000;404:84–87. doi:10.1038/35003583
- Williamson EM, Evans FJ. Cannabinoids in clinical practice. Drugs. 2000;60:1303–1314. doi:10.2165/00003495-200060060-00005
- Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology. 1982;76:245–250. doi:10.1007/BF00432554
- Pagano C, Ciaglia E, Coppola L, et al. Cannabidiol exerts multitarget immunomodulatory effects on PBMCs from individuals with psoriasis vulgaris. Front Immunol. 2024;15:1373435. doi:10.3389/fimmu.2024.1373435
- Wroński A, Jarocka-Karpowicz I, Stasiewicz A, Skrzydlewska E. Phytocannabinoids in the Pharmacotherapy of Psoriasis. Molecules. 2023;28:1192. doi:10.3390/molecules28031192
- Nussbaum D, Desai S, Gonzalez-Lopez A, Hawkes JE, Gondo G, Friedman A. Practices and perspectives on medical cannabis and cannabinoids: a survey of the national psoriasis foundation medical advisory board. JAAD Int. 2022;9:23–25. doi:10.1016/j.jdin.2022.07.001
- Mackie K. Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol. 2006;46:101–122. doi:10.1146/annurev.pharmtox.46.120604.141254y
- Pertwee R. Endocannabinoids and Their Pharmacological Actions. Handb Exp Pharmacol. 2015;231:1–37. doi:10.1007/978-3-319-20825-1_1
- Di Marzo V, Piscitelli F, Mechoulam R. Cannabinoids and endocannabinoids in metabolic disorders with focus on diabetes. Handb Exp Pharmacol. 2011;75–104. doi:10.1007/978-3-642-17214-4_4
- Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi:10.1124/pr.58.3.2
- Katona I, Freund TF. Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci. 2012;35:529–558. doi:10.1146/annurev-neuro-062111-150420
- Fine PG, Rosenfeld MJ. The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Med J. 2013;4:e0022. doi:10.5041/RMMJ.10129
- Baswan SM, Klosner AE, Glynn K, et al. Therapeutic Potential of Cannabidiol (CBD) for skin health and disorders. Clin Cosmet Invest Dermatol. 2020;13:927–942. doi:10.2147/CCID.S286411
- Bíró T, Tóth BI, Haskó G, Paus R, Pacher P. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30:411–420. doi:10.1016/j.tips.2009.05.004
- Nickles MA, Lio PA. Cannabinoids in dermatology: hope or hype. Cannabis Cannabinoid Res. 2020;5:279–282. doi:10.1089/can.2019.0097
- Wilkinson JD, Williamson EM. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J Dermatol Sci. 2007;45:87–92. doi:10.1016/j.jdermsci.2006.10.009
- Ständer S, Schmelz M, Metze D, Luger T, Rukwied R. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177–188. doi:10.1016/j.jdermsci.2005.01.007
- Mnekin L, Ripoll L. Topical use of cannabis sativa L. Biochem Cosme. 2021;8:85. doi:10.3390/cosmetics8030085
- Schlosburg JE, Blankman JL, Long JZ, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–1119. doi:10.1038/nn.2616
- Fowler CJ, Rojo ML, Rodriguez-Gaztelumendi A. Modulation of the endocannabinoid system: neuroprotection or neurotoxicity. Exp Neurol. 2010;224:37–47. doi:10.1016/j.expneurol.2010.03.021
- Palmieri B, Laurino C, Vadalà M. A therapeutic effect of cbd-enriched ointment in inflammatory skin diseases and cutaneous scars. Clin Ter. 2019;170:e93–e99. doi:10.7417/CT.2019.2116
- Ramot Y, Sugawara K, Zákány N, Tóth BI, Bíró T, Paus R. A novel control of human keratin expression: cannabinoid receptor 1-mediated signaling down-regulates the expression of keratins K6 and K16 in human keratinocytes in vitro and in situ. PeerJ. 2013;1:e40. doi:10.7717/peerj.40
- SERVIER MEDICAL. INTEGUMENTARY SYSTEM ART. 2024. Available from https://smart.servier.com/smart_image/integumentary-system/. Accessed June 18.
- Atalay S, Jarocka-Karpowicz I, Skrzydlewska E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants. 2020;9(1):21.
- Ryberg E, Larsson N, Sjögren S, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152(7):1092–1101.
- Tóth KF, Ádám D, Bíró T, Oláh A. Cannabinoid signaling in the skin: therapeutic potential of the ”C(ut)annabinoid. System. Molecules. 2019;24(5):918.
- O’Sullivan SE. An update on PPAR activation by cannabinoids. Br J Pharmacol. 2016;173(12):1899–1910.
- Adhikary S, Li H, Heller J, et al. Modulation of inflammatory responses by a cannabinoid-2-selective agonist after spinal cord injury. J Neurotrauma. 2011;28:2417–2427. doi:10.1089/neu.2011.1853
- Klein TW, Newton C, Larsen K, et al. Cannabinoid receptors and T helper cells. J Neuroimmunol. 2004;147:91–94. doi:10.1016/j.jneuroim.2003.10.019
- Lowe H, Toyang N, Steele B, Bryant J, Ngwa W. The endocannabinoid system: a potential target for the treatment of various diseases. Int J Mol Sci. 2021;22(9472). doi:10.3390/ijms22179472
- Rajan TS, Giacoppo S, Scionti D, et al. Cannabidiol activates neuronal precursor genes in human gingival mesenchymal stromal cells. J Cell Biochem. 2017;118(6):1531–1546. doi:10.1002/jcb.25815
- Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78. doi:10.1111/j.1600-065X.2007.00519.x
- Facci L, Dal Toso R, Romanello S, Buriani A, Skaper SD, Leon A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc Natl Acad Sci U S A. 1995;92:3376–3380. doi:10.1073/pnas.92.8.3376
- Scarampella F, Abramo F, Noli C. Clinical and histological evaluation of an analogue of palmitoylethanolamide, PLR 120 (comicronized Palmidrol INN) in cats with eosinophilic granuloma and eosinophilic plaque: a pilot study. Vet Dermatol. 2001;12:29–39. doi:10.1046/j.1365-3164.2001.00214.x
- Klein TW, Newton C, Larsen K, et al. The cannabinoid system and immune modulation. J Leukoc Biol. 2003;74:486–496. doi:10.1189/jlb.0303101
- Ibsen MS, Connor M, Glass M. Cannabinoid CB1 and CB2 receptor signaling and bias. Cannabis Cannabinoid Res. 2017;2(1):48–60. doi:10.1089/can.2016.00
- De Petrocellis L, Ligresti A, Moriello AS, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–1494. doi:10.1111/j.1476-5381.2010.01166.x
- Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci. 2006;103(20):7895–7900. doi:10.1073/pnas.0511232103
- El-Remessy AB, Al-Shabrawey M, Khalifa Y, Tsai NT, Caldwell RB, Liou GI. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am J Pathol. 2006;168:235–244. doi:10.2353/ajpath.2006.050500
- Maccarrone M, Di Rienzo M, Battista N, et al. The endocannabinoid system in human keratinocytes. Evidence that anandamide inhibits epidermal differentiation through CB1 receptor-dependent inhibition of protein kinase C, activation protein-1, and transglutaminase. J Biol Chem. 2003;278:33896–33903. doi:10.1074/jbc.M303994200
- Casanova ML, Blázquez C, Martínez-Palacio J, et al. Inhibition of skin tumor growth and angiogenesis in vivo by activation of cannabinoid receptors. J Clin Invest. 2003;111:43–50. doi:10.1172/JCI16116
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64(Suppl 2):ii30–ii36. doi:10.1136/ard.2004.031120
- Yang EJ, Hendricks AJ, Beck KM, Shi VY. Bioactive: a new era of bioactive ingredients in topical formulations for inflammatory dermatoses. Dermatol Ther. 2019;32:e13101. doi:10.1111/dth.13101
- Vomund S, Schäfer A, Parnham MJ, Brüne B, von Knethen A. Nrf2, the master regulator of anti-oxidative responses. Int J Mol Sci. 2017;18:2772. doi:10.3390/ijms18122772
- Maida V, Shi RB, Fazzari FGT, Zomparelli L. Promoting wound healing of uremic calciphylaxis leg ulcers using topical cannabis-based medicines. Dermatol Ther. 2020;33:e14419. doi:10.1111/dth.14419
- Kozela E, Juknat A, Vogel Z. Modulation of inflammatory responses by cannabinoids. In: Handbook of Experimental Pharmacology, Martin CM. Vol. 231. Springer; 2017:1–25.
- Oláh A, Tóth BI, Borbíró I, et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J Clin Invest. 2014;124:3713–3724. doi:10.1172/JCI64628
- Chiurchiù V, Leuti A, Maccarrone M. Bioactive lipids and chronic inflammation: managing the fire within. Front Immunol. 2018;9(38). doi:10.3389/fimmu.2018.00038
- Cheng YC, Lin HY, Hsieh MT, Liao YW. Differential regulation of pro-inflammatory cytokines and growth factors by phytocannabinoids in human keratinocytes and fibroblasts. Cannabis Cannabinoid Res. 2019;4(3):167–181. doi:10.1089/can.2019.0016
- Kozela E, Juknat A, Gao F, Kaushansky N, Coppola G, Vogel Z. Pathways and gene networks mediating the regulatory effects of cannabidiol, a nonpsychoactive cannabinoid, in autoimmune T cells. J Neuroinflam. 2016;13(136). doi:10.1186/s12974-016-0603-x
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi:10.1002/glia.1107
- Campos AC, Fogaça MV, Scarante FF, et al. Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front Pharmacol. 2017;8(269). doi:10.3389/fphar.2017.00269
- Güngör Ş. Role of endocannabinoids on neurogenic responses of TRPV1 channels. Curr Neuropharmacol. 2019;17(1):99–111. doi:10.2174/1570159X15666170518151541
- Norooznezhad AH, Norooznezhad F. Cannabinoids: possible agents for treatment of psoriasis via suppression of angiogenesis and inflammation. Med Hypotheses. 2017;99:15–18. doi:10.1016/j.mehy.2016.12.003
- Iliev E, Broshtilova V. Traditional Chinese medicine principles in the pathogenesis and treatment of psoriasis vulgaris. Semin Integra Med. 2003;1(3):145–150. doi:10.1016/s1543-1150(03)00027-9
- Minaglou FH, Bolcato O. The potential role of specific essential oils in the replacement of dermacorticoid drugs (strong, medium and weak) in the treatment of acute dry or weeping dermatitis. Int J Aromather. 2005;15(2):66–73. doi:10.1016/j.ijat.2005.03.013
- Vincenzi C, Tosti A. Efficacy and tolerability of a shampoo containing broad-spectrum cannabidiol in the treatment of scalp inflammation in patients with mild to moderate scalp psoriasis or seborrheic dermatitis. Skin Appendage Disord. 2020;6:355–361. doi:10.1159/000510896
- Changoer L, Anastassov G Method to Treat Psoriasis. Patent 20190060250. New York, NY, USA: AXIM Biotechnologies, Inc; 2019. Available from: https://www.freepatentsonline.com/y2019/0060250.html. Accessed February 10, 2023.
- Friedman AJ, Momeni K, Kogan M. Topical cannabinoids for the management of psoriasis vulgaris: report of a case and review of the literature. J Drugs Dermatol. 2020;19(795). doi:10.36849/JDD.2020.5229
- Baylac S, Racine P. Inhibition of human elastase by natural fragrance extracts of aromatic plants. Int J Aromather. 2004;14:179–182. doi:10.1016/j.ijat.2004.09.008
- Piérard-Franchimont C, Goffin V, Henry F, Uhoda I, Braham C, Piérard GE. Nudging hair shedding by antidandruff shampoos. A comparison of 1% ketoconazole, 1% piroctone olamine and 1% zinc pyrithione formulations. Int J Cosmet Sci. 2002;24:249–256. doi:10.1046/j.1467-2494.2002.00145.x
- Choi FD, Juhasz MLW, Atanaskova Mesinkovska N. Topical ketoconazole: a systematic review of current dermatological applications and future developments. J DermatolTreat. 2019;30:760–771. doi:10.1080/09546634.2019.1573309
- Jarocka-Karpowicz I, Biernacki M, Wroński A, Gęgotek A, Skrzydlewska E. Cannabidiol effects on phospholipid metabolism in keratinocytes from patients with psoriasis vulgaris. Biomolecules. 2020;10(367). doi:10.3390/biom10030367
- Sangiovanni E, Fumagalli M, Pacchetti B, et al. Extract and cannabidiol inhibit in vitro mediators of skin inflammation and wound injury. Phytother Res. 2019;33:2083–2093. doi:10.1002/ptr.6400
- Casares L, García V, Garrido-Rodríguez M, et al. Cannabidiol induces antioxidant pathways in keratinocytes by targeting BACH1. Redox Biol. 2020;28:101321. doi:10.1016/j.redox.2019.101321
- Yeroushalmi S, Nelson K, Sparks A, Friedman A. Perceptions and recommendation behaviors of dermatologists for medical cannabis: a pilot survey. Complement Ther Med. 2020;55:102552. doi:10.1016/j.ctim.2020.102552
