Abstract
Psoriasis is a prevalent, chronic inflammatory disease of the skin, mediated by crosstalk between epidermal keratinocytes, dermal vascular cells, and immunocytes such as antigen presenting cells (APCs) and T cells. Exclusive cellular “responsibility” for the induction and maintenance of psoriatic plaques has not been clearly defined. Increased proliferation of keratinocytes and endothelial cells in conjunction with APC/T cell/monocyte/macrophage inflammation leads to the distinct epidermal and vascular hyperplasia that is characteristic of lesional psoriatic skin. Despite the identification of numerous susceptibility loci, no single genetic determinant has been identified as responsible for the induction of psoriasis. Thus, numerous other triggers of disease, such as environmental, microbial and complex cellular interactions must also be considered as participants in the development of this multifactorial disease. Recent advances in therapeutics, especially systemic so-called “biologics” have provided new hope for identifying the critical cellular targets that drive psoriasis pathogenesis. Recent recognition of the numerous co-morbidities and other autoimmune disorders associated with psoriasis, including inflammatory bowel disease, multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus suggest common signaling elements and cellular mediators may direct disease pathogenesis. In this review, we discuss common cellular pathways and participants that mediate psoriasis and other autoimmune disorders that share these cellular signaling pathways.
Keywords:
Introduction
Psoriasis is a prevalent, chronic inflammatory skin disease that affects approximately 0.5%–1% of children and 2%–3% of the world’s population.Citation1 Psoriasis is a bi-modally distributed disease with one major age of onset at 20–30 years of age as well as a later smaller peak of onset at 50–60 years.Citation2,Citation3 Even though psoriasis etiology remains unknown, it is believed to be multifactorial with numerous key components including genetic susceptibility, environmental triggers in combination with skin barrier disruption and immune dysfunction.Citation4 There are five subtypes of psoriasis: vulgaris (plaque), guttate, pustular, inverse, and erythrodermic. The most common variation of psoriasis is plaque psoriasis, which affects approximately 85%–90% of psoriatic patients.Citation5
Psoriasis confers significant physical and psychological distress and impairment usually resulting in a detrimental impact on patient quality of life.Citation6,Citation7 Psoriasis patients often express feeling shame and guilt and are stigmatized by the disease.Citation8 Psoriasis has been reported to affect the interpersonal relationships of patients as well as impacting sexual well-being and capacity for intimacy.Citation9,Citation10 In the work place, psoriasis has a negative social impact which may manifest as discrimination and difficulty finding employment. Nearly 60% of employed psoriasis patients reported lost time from work in the previous year due to psoriasis.Citation11
Complications arising from psoriasis lead to numerous deaths in the United States annually.Citation12 Perhaps the most detrimental aspect of psoriasis is the concurrent physical comorbidities such as cardiovascular disease (CVD), obesity, diabetes, metabolic syndrome, inflammatory bowel disease (IBD), and psoriatic arthritis that contribute to decreased longevity.Citation13–Citation16
Skin phenotype and histology of psoriasis
Phenotype
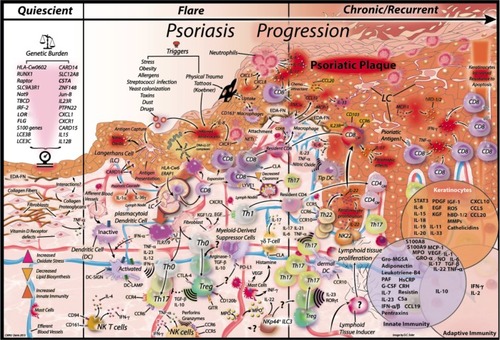
Plaque psoriasis is the most common presentation of the disease. It is phenotypically characterized by red, scaly, well-defined, silvery-white, dry plaques that preferentially appear on elbows, knees, scalp, and the lumbar area. The often symmetrical plaques can be erythematous and are often categorized by patients as itching intensely. Severity of the plaques is quantified by two major scoring systems; the psoriasis area and severity index (PASI) and the physician’s global assessment (PGA). The PASI scale allows quantification of the extent of disease as a measure of area and intensity (erythema, induration, desquamation, and body surface area). Given the complicated nature of the PASI calculation, the PGA was introduced as an alternative assessment measure. The PGA calculates either a static or dynamic global assessment of lesion severity.Citation17 Interestingly, and a key feature of chronic plaque psoriasis, is the normal appearing “uninvolved” skin immediately adjacent to psoriatic plaques of affected individuals. Several recent studies have demonstrated that uninvolved tissue may contain a unique signature of genes, that when activated may account for the conversion of uninvolved skin to involved plaque. However, given the myriad cell types known to participate in this conversion, isolation of a single triggering factor is unlikely ().
Figure 1 Key signals in psoriasis development.
Abbreviations: CD8 CM, CD8 central memory; Treg, regulatory T cell; NK, natural killer; Th, T helper; TipDC, TNF-iNOS-producing DC; NETs, neutrophil extracellular traps.
Histological manifestation
Plaque psoriasis is characterized by pronounced keratinocyte and dermal vascular endothelial cell proliferation followed by an inflammatory response. The classical histological manifestation includes marked epidermal thickening (acanthosis) due to keratinocytes’ accelerated movement through the epidermis, thickening of stratum corneum (hyperkeratosis), retention of nuclei in upper layers of the skin which causes squamous cell layer thickening (parakeratosis), elongation of epidermal rete ridges, increase in the number and size of dermal blood vessels and an increased inflammatory cell infiltrate consisting mostly of neutrophils in the stratum corneum and epidermis (Munro’s microabscesses and Kogoj pustules), significant mononuclear infiltrates in the epidermis as well as leukocyte infiltration (mostly T cells and dendritic cells [DCs]) into the dermis.
Potential causes/triggers of psoriasis
Genes
Genetics studies have shown that psoriasis patients have diverse gene polymorphisms related to immune and skin barrier function.Citation18 Analyses of psoriasis incidence demonstrated 70% probability of monozygotic twins to be affected by psoriasis and 20% probability in dizygotic twins.Citation19 Pedigree studies have shown that children have a 20% chance of developing psoriasis if one parent is affected and 65% if both parents are affected. More than 30 single nucleotide polymorphisms (SNPs) have been associated to contribute to psoriasis risk but only two gene mutations have been found to independently induce psoriasis (IL36RN and CARD14) by affecting both the skin and immune system.Citation20
PSORS1
The first associated psoriasis susceptibility (PSORS) locus was PSORS1 on chromosome 6p21. This region has been shown to have the greatest impact on psoriasis heritability but the identity of its gene is controversial. PSORS1 is located within the major histocompatibility complex class 1 region with a consensus of HLA-C being the most likely PSORS1 gene.Citation21 HLA-haplotypes that have been described as over-represented in psoriasis patients are encoded within this susceptible locus, including HLACw6, the most popular and strongest disease loci associated haplotype.Citation22 More than 60% of psoriatic individuals carry the HLA-Cw0602 allele, which confers a 20-fold increased risk of developing psoriasis.Citation23 However, even when there is a strong genetic association, the exact role of HLA-C and HLA-Cw6 in psoriasis development remains uncertain. Furthermore, HLA-C accounts for only 50% of the familial clustering observed in psoriasis.
PSORS2/CARD14 mutations
Genome-wide linkage scans and family association mapping identified PSORS2 on chromosome 17q25.Citation24,Citation25 Recently, next generation sequencing of patients with familial psoriasis found a gain-of-function mutation in CARD14 on this locus.Citation26,Citation27 Furthermore, several missense CARD14 mutations were found among psoriatic individualsCitation26 as well as 2.7-fold increase of CARD14 mRNA in psoriasis transcriptomeCitation27 and CARD14 SNP.Citation28 CARD14 mutations may cause psoriasis through increased induction of NF-kβ, which leads to enhanced expression of key psoriatic chemokines including CCL20, CXCL8/IL-8, and IL-36γ/IL-1F9.Citation20 However, an additional environmental trigger is also suspected to be necessary to initiate the psoriatic cascadeCitation27 ().
IL36RN mutations
IL36RN, also known as IL-1F5, encodes for the anti-inflammatory protein IL-36Ra (IL-1F9), a natural IL36R antagonist. IL-36 family members have been demonstrated to be highly upregulated in psoriasis.Citation29,Citation30 The three IL-36 stimulating cytokines (IL-36α, IL-36β, and IL-36γ) belong to the IL-1 family, and they bind to IL-36R and activating NF-kβ. Mutations in IL-36Ra lead to loss of active protein which results in unrestrained proinflammatory effects of the IL-36 stimulating cytokines. Absence of IL-36Ra then leads to excessive neutrophil accumulation as observed in pustular psoriasis.Citation31 In the case of psoriasis vulgaris, IL-36Ra has been shown to be abundant, which may possibly attenuate agonist activity.Citation29,Citation31
SNPs
SNPs are substitutions of one base pair for another in more than 1% of the population.Citation32 SNPs are usually found in non-coding regions of the genome.Citation32 Using high-throughput technologies and statistical methods, large genome-wide association studies (GWAS) have compared the frequencies of hundreds of thousands different SNPs in psoriasis patients and healthy control individuals.Citation33 GWAS had identified significant SNPs in psoriasis, several of which are associated with the IL-23/IL-17 axis.Citation33 A recent publication identified an SNP in the IL23 locus that may have functional importance because of its IL-17 response gradient to T cell stimulation by IL-23 in control and psoriasis patients.Citation34 Furthermore, a recent meta-GWAS analysis confirmed 21 SNPs and identified 15 new SNPs in psoriasis patients and controls.Citation28 These SNPs were associated with numerous immunological processes implicated in psoriasis pathogenesis including keratinocyte differentiation, T cell and natural killer (NK) cell proliferation, cytokine responses, JAK-STAT cascade, T helper (Th)1 and Th17 cell regulation and leukocyte adhesion.Citation28
Environmental triggers of psoriasis
Several environmental factors including physical trauma, drug reactivity, infection as well as modifiable variables such as psychological stress, obesity, smoking, and alcohol have been associated with a predisposition toward psoriasis development and exacerbation of the disease.
Physical trauma
Heinrich Koebner first described physical trauma as a trigger and exacerbating factor for psoriasis in 1872.Citation35 He observed the development of psoriatic lesions after a direct cutaneous injury, such as excoriation, tattoos, burns, and animal or insect bites, in previously normal-appearing skin (, “Flare”). The new psoriatic lesion was characterized morphologically as identical to the injury site, known as an isomorphic response. The Koebner response has been observed with other dermal diseases such as vitiligo and lichen planus, but the frequency for its manifestation is higher among psoriasis patients. The prevalence of Koebner response in psoriasis patients ranges from 24%–51%.Citation36
Psoriasis onset following an injury may take anywhere from 3 days to 2 years to develop, and may be dependent on seasonal variation (more frequently in winter) as well as disease severity (pre-existing and stability of psoriasis).Citation37,Citation38
Drug-induced psoriasis
Several medications have been associated with psoriasis onset as well as exacerbation of disease. The most commonly reported drugs to trigger psoriasis are lithium, beta-blockers, anti-malarials, tetracyclines, and non-steroidal anti-inflammatory medications.Citation39–Citation42 In recent years, TNFα blockers, IL-6R blockers, and medications against IFNs (alpha, beta, gamma) as well as the TLR7 agonist imiquimod, have all been reported to induce or exacerbate psoriasis.Citation43–Citation49
Other reported medications that exacerbate psoriasis include ACE inhibitors, calcium channel blockers, and IL-2 among others.Citation50–Citation52
Infections
Considerable data suggest that infections are an important trigger for psoriasis, especially among children. Guttate psoriasis has been associated with Streptococcus pyogenes infection through both pharyngeal and skin routes.Citation53–Citation55 A recent publication reports that streptococcal throat infections can trigger psoriasis onset and exacerbate chronic psoriasis. Additionally, patients with psoriasis are more prone to develop sore throats than non-psoriatic patients. Furthermore, Staphylococcus aureus, Malassezia and Candida albicans colonization in the gut and/or skin have also been linked to psoriasis exacerbation.Citation56–Citation58 In addition, several researchers have observed an association between disease severity as measured by PASI with status regarding infection with Helicobacter pylori.Citation59–Citation61 Given the relationship of these infections and psoriasis, the exotoxins of these microorganisms as well as peptidoglycans derived from bacterial sources have been reported as possible candidates for activating T cells and leading to psoriasis development through an abnormal immunological response.Citation62–Citation65
Stress
Psychological stress is known to aggravate psoriasis by altering the immune system. Numerous authors have suggested that increases in stress hormone levels due to activation of the hypothalamus–pituitary–adrenal axis may cause psoriasis exacerbation.Citation66–Citation69 Corticotrophin-release hormone (CRH) is a central component of the hypothalamus–pituitary–adrenal axis that is important in the coordination of systemic stress responses as well as modulation of inflammatory response. Cutaneous CRH and CRH-receptor 1 have been shown to regulate local homeostasis in the skin and in psoriasis, expression of CRH is significantly increased.Citation70 Currently, the proinflammatory effects of CRH on skin are not clear, CRH may stimulate production of IL-6 or IL-11 in keratinocytes during cutaneous stress, therefore, it is possible that CRH is acting on keratinocytes to further exacerbate psoriasis.Citation71
Alcohol and smoking
The association between alcohol consumption and psoriasis is complex and controversial. Studies indicate that the prevalence of psoriasis among patients who abuse alcohol is increased. Furthermore, a recent meta-analysis of case control studies showed that alcohol consumption is associated with increased risk of psoriasis. Epidemiological studies suggest that patients with moderate to severe psoriasis have an increased incidence of alcohol-related diseases and mortality.Citation72–Citation74 The mechanism(s) by which alcohol consumption might trigger psoriasis remain unknown. However, in vitro studies have shown that 0.05% ethanol can activate T cells and induce keratinocyte hyperproliferation by directly stimulating TGFα, IL-6, and IFNα production. Furthermore, ethanol and acetone have been demonstrated to upregulate mRNA expression of ITGA5, cyclinD1, and keratinocyte growth factor receptor leading to non-tumorigenic human keratinocytes to proliferate in vitro.Citation75
Previous reports have shown a strong correlation between smoking and risk of psoriasis. One study indicates that this risk is higher among women than men, while another study showed that the risk for developing psoriasis is higher in former and current smokers than in those individuals who have never smoked.Citation76,Citation77
Genetic susceptibility to psoriasis has been linked to smoking. Based on a recent study, smokers with HLA-Cw6 haplotype have an 11-fold increased risk of developing psoriasis compared to non-smokers without the HLA-Cw6 haplotype.Citation78 A separate study demonstrated that SNP genetic variants in the genome-wide psoriasis-associated loci CSMD1 and TNIP/ANXA6 increase the risk for psoriasis when combined with smoking and alcohol use.Citation79
Obesity
Obesity has been shown to be a risk factor for psoriasis. Correlation between obesity and psoriasis severity has been observed although the mechanism by which obesity promotes psoriasis is not well understood.Citation80,Citation81 It is possible that the mechanism involves the adipocyte-derived cytokines, leptin and resistin. Previous publications have reported that leptin and resistin are found in high concentrations in psoriasis patients.Citation82,Citation83 Furthermore, these adipokines can induce monocytes to produce proinflammatory cytokines including IL-8, TNFα, and IL-1β. Using an ex vivo organotypic culture system these investigators reported that exogenous addition of leptin induced psoriatic skin to produce AREG, an EGF family member that has been shown to cause keratinocyte proliferation in vitro and to promote inflammatory hyperplasia in transgenic mice that overexpress dermal leptin. Interestingly, psoriasis improvement after bypass surgery has been observed, however, worsening of psoriasis after weight loss as well as weight-loss surgery has also been observed.Citation84–Citation86 Therefore, more studies are needed to improve our understanding of the effect of obesity and weight loss on psoriasis.
Microbiota in psoriasis
Given that the skin acts as a barrier that is also in contact with the outside environment, it is colonized by different microorganisms including bacteria, fungi, viruses, and mites.Citation87–Citation89 An estimated 1 million different bacterial species inhabit one square centimeter of skin.Citation90 Several factors including age, genetics, immune reactivity, climate, and hygiene influence the composition of the microbiota communities of the skin. Also, differences in skin thickness, density of hair follicles and skin invaginations cause different habitats with differential microbiota composition. Under healthy conditions, symbiotic relationships develop that permit skin protection against invasion by more pathogenic and harmful microorganisms to the host as well as by educating and priming resident T cells.Citation91 Therefore, alterations in microbial communities of the skin have been associated with disease.
Using 16S-based genomic sequencing, differences in the composition of the bacterialCitation92,Citation93 and fungalCitation94,Citation95 communities in lesional and non-lesional psoriatic skin have been observed.
Psoriatic lesional skin has been shown to have increased bacterial diversity including increased S. pyogenesCitation93 and S. aureusCitation96 when compared to healthy and non-lesional skin. Three studies on skin microbiome in psoriasis, one using biopsiesCitation92 and the other two using swabsCitation93,Citation97 have been performed. Streptococcus was the most common species found in biopsies,Citation92 while the most common bacterial species detected using swabs were Corynebacteria.Citation93,Citation97 As previously stated, tonsil infections have been shown to be a possible trigger for psoriasis development. A study performed by Diluvio et al comparing T cell receptor (TCR) β-chain rearrangements in psoriatic skin lesions, blood, tonsils, and tonsillar T cells fractions showed that psoriatic lesions were dominated by clonal T cell expansions, which might be the link between Streptococcus tonsillitis and inflammation in psoriasis.Citation98 The authors suggest that S. pyogenes infections prime and select tonsillar T cells to migrate into the skin, where they are reactivated and expand promoting psoriatic skin lesion formations.Citation98 This mechanism was supported by long-term remission of psoriatic skin inflammation for more than 3 years after tonsillectomy.Citation98 However, this study was performed using a small number of patients (n=3), therefore the significance of the findings needs further evaluation.
Fungal composition differences have also been shown in psoriasis. Several studies suggest the Malassezia yeasts as possible triggers for elicitation,Citation99–Citation102 as well as for exacerbation of psoriatic plaques.Citation103–Citation106 Furthermore, several studies have shown improvement of scalp psoriasis after antifungal agent ketoconazole treatment.Citation101,Citation107,Citation108 Given that Malassezia yeasts are part of the healthy human cutaneous flora, it is important to determine what causes them to become aggravators of psoriasis.
Another commensal yeast species that has been associated with psoriasis is Candida spp. Significantly higher prevalence of Candida spp. colonization in both the oral cavity,Citation109,Citation110 as well as in lesional skinCitation109 on psoriasis patients have been described. Furthermore, super-antigens and toxins released from Candida spp. have been associated with exacerbation of psoriasis by activating T cells and keratinocytes cytokines secretion.Citation111 The most common Candida spp. found in oral cavity and psoriatic lesional skin is C. albicans.Citation109 C. albicans is one of the most common commensal yeasts and it is the most prevalent to cause different diseases under predisposing conditions.Citation112
Even when microbiota has been shown to be differential between psoriasis and homeostasis, an association between microbiome and psoriasis has yet to be established. Furthermore, recent studies have shown that skin microbiota varies in dry, moist and sebaceous sites but how this variation might affect psoriasis development remains unknown.Citation91
Cellular participants in psoriasis
The skin is a dynamic organ that serves as a front line defense against insults, injuries, and microbial pathogens. Given its constant exposure to the environment, immune surveillance and immune tolerance are key roles of the skin. The skin consists of the epidermis, dermis, and adipose tissue or subcutis layers. The epidermis is mainly composed of keratinocytes and Langerhans cells (LCs).Citation20
Keratinocytes
Keratinocytes are the main components of the epidermis, where they maintain a mechanical barrier and participate in the initiation and maintenance of the skin’s immune response. Keratinocytes interact with immune cells during the development of psoriasis. Psoriatic skin is characterized by increased proliferation and abnormal differentiation of keratinocytes.Citation3 In psoriasis, keratinocyte differentiation is incomplete and keratinocyte stem cell proliferation pathways are dysregulated, causing preferential activation and proliferation of rapidly matured cells with reduced lipids and keratohyalin granules.Citation3
Neutrophils
Neutrophils or polymorphonuclear leukocytes are the most abundant circulating leukocytes in humans.Citation113 Cellular infiltration in psoriasis includes neutrophils from dermal papillae towards the epidermis and accumulation of neutrophils in the stratum corneum (Munro microabscesses).Citation114 Neutrophils are attracted to the skin by an array of chemotactic factors including IL-8, gro-MGSA, complement product C5a, leukotriene B4 and platelet activating factor.Citation115–Citation117 Stimulation of neutrophils by GM-CSFCitation118,Citation119 results in rapid upregulation of surface integrin CD11b/CD18 which causes extravasation and localization via ICAM1 expressing activated epidermal keratinocytes.Citation120
A previous publication described rapid improvement of long-standing psoriasis during agranulocytosis wherein, after blood neutrophil recovery, psoriatic plaques reappear.Citation114 Therefore, blood neutrophil counts and agranulocytosis may be correlated with psoriasis activity, which might indicate a requirement for neutrophils in psoriatic plaques development.
Furthermore, the “flaky skin” psoriasis mouse model (fsn/fsn) exhibits prominent neutrophil infiltration and microabscesses within a hyperproliferative epidermis. In this model, neutrophil depletion caused dramatic reduction of epidermal thickening, neutrophil infiltration into the skin, down regulation of TNFα and IL-1β as well as elimination of microabscesses.Citation121
Moreover, neutrophils can produce extracellular traps, which are composed of DNA and antimicrobial peptides.Citation122 Their physiological function is to kill invading microorganisms while preventing tissue damage.Citation122 These neutrophil extracellular traps are induced by IL-23 and IL-1β and during their formation neutrophils release IL-17, a major cytokine associated with psoriasis development.Citation123 This is particularly important because although IL-17 production by T cells is widely studied, appreciation of innate immune cells producing IL-17 is a fairly new area of research, which requires further studies.
Mast cells
Early psoriatic skin lesion has been reported to typically include degranulated mast cells.Citation124,Citation125 The number of mast cellsCitation126–Citation129 as well as histamine concentrationCitation130,Citation131 are increased in psoriatic skin. A particular subset of mast cells, tryptase and chymase producers (MCTCs), have been shown to be enriched in the papillary dermis of psoriatic skin.Citation127,Citation132 Mast cells have been termed “ghost cells” in early psoriasis lesions because they are frequently activated and degranulated. Furthermore, approximately 70% of mast cells in psoriatic skin are IFNγ positive, which suggests an important role for triggering psoriasis.Citation132
In an early study, initial phases of psoriasis triggered by Koebner phenomenon showed that mast cells were significantly increased at day 4 when compared to control skin. Mast cells’ increased peak was at day 14 which was simultaneous with the manifestation of psoriasis.Citation133 Also, mast cells’ numbers decrease in psoriasis lesions after successful therapy with anthralin, psoralen plus UVA light therapy and cyclosporine.Citation129,Citation134,Citation135
A recent article demonstrated that mast cells and neutrophils increased number in psoriasis contribute to the release of IL-17 through the formation of extracellular traps, which may be triggered by IL-23 and IL-1β.Citation123 More recently, mast cells have been shown to be major producers of IL-22 in psoriasis and atomic dermatitis.Citation136 Therefore, innate immune cells’ release of IL-17 is a new and exciting topic that needs further evaluation for determining how it may be triggering psoriasis.
DCs
DCs are antigen presenting cells (APCs) crucial for efficient T and B cell activation. In the skin, there are three main DC populations: epidermal LCs, resident dermal myeloid DCs (mDCs), and plasmacytoid DCs (pDCs). During inflammation, a fourth population, inflammatory DCs, can be observed.Citation137
In psoriasis, an overall increase in DCs has been reported in the epidermis and dermis.Citation138,Citation139 High numbers of pDCs, immature as well as mature DCs, iNOS- and TNF-producing DCs (TipDCs), and inflammatory epidermal DCs have been observed in psoriatic lesional skin.Citation140
LCs
LCs are resident conventional DCs present in the suprabasal layers of the epidermis, in close contact with keratinocytes.Citation137 Activation of LCs (eg, antigen acquisition) causes their migration out of the epidermis toward draining lymph nodes where T cell activation can be initiatedCitation20 (). LCs represent approximately 3% of the epidermal cellsCitation141 and are characterized by expression of CD207, CD1a, e-cadherin, and EpCAM.Citation137 It was recently shown that LCs have two subtypes, short-term and long-term LCs, which are present in the steady state and during inflammation, respectively.Citation142
The functional role of LCs in psoriasis is not fully understood. Previous studies have shown LC reduction in psoriatic skin, which can be restored to normal levels after therapy.Citation143
A recent publication demonstrated that LCs are reduced in lesional psoriatic skin of patients as well as in the skin of the KRT5 specific deletion of Jun and JunB (DKO) psoriatic mouse model.Citation144 LC depletion can aggravate psoriatic-like inflammation in DKO mice in an IL-10 and PD-L1 dependent manner.Citation144
pDCs
pDCs account for less than 0.1% of PBMCs; however, they are the primary source of IFNα.Citation145 In contrast to other DC subsets, pDCs do not express TLRs 2–5 on their surface; instead they uniquely express endosomal TLR7 and TLR9, which respond to their respective ligands single stranded RNA and unmethylated CpG.Citation146 Hence, IFNα secretion in response to in vivo CpG changes has been shown to be exclusively mediated by pDCs.Citation145 This is particularly important because CpG methylation has been demonstrated to change in psoriatic involved skin.Citation147
pDCs have been shown to be highly expressed within lesional psoriatic tissueCitation148 and their presence in non-lesional skin has also been reported by some investigators,Citation148 but not observed by others.Citation144 Blockade of IFN production by pDCs has been shown to inhibit psoriatic lesional development in a xenograft skin mouse model.Citation148 Furthermore, it was found that pDCs were necessary for the initiation of psoriatic disease in the DKO psoriatic-like inflammation mouse model but not for the sustainability of their chronic inflammation.Citation144
In psoriasis, keratinocytes produce elevated levels of the antimicrobial peptide LL-37, which forms complexes with self-DNA/RNA released by stressed/damaged cells. This causes activation of pDCs in a TLR7 and 9 dependent manner.Citation149 Also, pDC activation causes release of IFNα which in combination with IL-1β, IL-6, and TNFα are thought to activate conventional DCs (cDCs) causing their migration to cutaneous lymph nodes where they can prime Th17 and Th22 differentiationCitation149 ().
mDCs
Although pDCs are believed to be initiators of psoriatic skin development, myeloid DCs (mDC) are thought to have an important role in the maintenance and amplification of psoriasis.Citation150
The first report of mDCs in psoriasis demonstrated that dermal DCs derived from lesional skin could stimulate T cell responses by producing IL-2 and IFNγ, thereby contributing to Th1-type responses.Citation151
Dermal mDCs are identified by CD11c expression.Citation152 Skin inflammation in psoriasis causes a 30-fold increase in CD11c+ DCs in the dermis, which is nearly equivalent to the number of lesional T cells.Citation137
Two populations of mDCs have been identified: classical resident mDCs (CD11c+ BDCA-1+) and inflammatory mDCs (CD11c+ BDCA-1neg). BDCA or CD-1c is part of the major histocompatibility complex that participates in lipid antigen presentation to T cells;Citation153 the absence of BDCA expression is the current phenotype for classifying inflammatory mDCs.
CD11c+ BDCA-DCs are present in high numbers in psoriatic lesional skinCitation154 and include TipDCsCitation138 as well as IL-20- and IL-23-producing DCs.Citation150 TipDCs appear to be important in the pathogenesis of psoriasis (); rapid down-modulation of the TipDC products TNF, iNOS, IL-20, and IL-23 are observed after effective TNF-blocking therapy.Citation155 Furthermore, TipDCs are immunostimulatory and capable of Th17 polarizationCitation154 as well as IL-12p40, IL-23p19, and IL-20 production in psoriatic skin.Citation156 A recent publication proposed that mDCs expressing 6-sulfo-LacNac (Slan DC) are the inflammatory DCs’ precursors in psoriasis capable of driving Th1 and Th17 responses.Citation157
T cells
Teff and memory cells
One of the known characteristics of psoriasis is the recurrence of lesions at the site of initial onset after therapy has been discontinued. Although the ultimate cause for this phenomenon is not clearly understood, the involvement of different types of cells, including sessile effector memory T cells is suspectedCitation158–Citation160 (). More specifically, there have been reports that the effector memory T cells responsible for this phenomenon could be IL-17-expressing skin-resident CD8 T cells.Citation161 However, the presence of these IL-17-expressing CD8 T cell clones would suggest that an auto-antigen must exist, a contentious point in the field of psoriasis.Citation162–Citation166 The presence of tissue-resident memory T cell clones that live permanently in the skinCitation160 could explain the recurrence phenomenon observed in psoriasis patients. Since psoriasis patients can go into remission following biologic therapy, new treatments focusing on eliminating these skin-resident memory T cells could foreseeably accomplish permanent skin clearance in psoriatic patients even after treatment discontinuation.
Suarez-Farinas et alCitation167 recently reported numerous genes that did not return to normal levels following psoriatic skin improvement with anti-TNFα therapy. This list of genes suggests that there may be factors beyond skin-resident auto-reactive T cells. For example, the LYVE1 gene participates in lymph vessel drainage. In normal skin, the presence of this gene was reported in wide-open lumens of lymphatic channels located in the upper reticular dermis. This could be an important factor contributing to the failure of healed psoriatic skin to clear infiltrating cells, since down regulation of LYVE1 may contribute to the collapsed morphology of draining lymphoid vessels present in psoriatic healed skin.Citation167,Citation168 This decreased draining capacity could contribute to leaving pathogenic T cells that would be de-mobilized otherwise.
Th17 cells: protective and pathogenic
Recent exciting advances in cellular immunology have provided new targets for therapy in immunological diseases, including pathogenic Th17 cells,Citation169 which are associated with initiation of autoimmune and inflammatory conditionsCitation170 including psoriasis.Citation154,Citation171–Citation174 The complex interplay among regulatory T cell (Treg), effector memory T cells (Tmem)/Teff, DC/APC, Th1 and Th17 cells ultimately defines the skin’s immune response at rest, during challenge, and in diseases such as psoriasis.
The Th1/Th2 paradigm proposed by Mosmann et al established the two canonical subsets of helper T cells.Citation175 Th1 cells are classically defined by activation of the transcription factors STAT4 and T-bet, secretion of IFNγ, and participation in directed immune responses to pathogens as well as coordination of cell mediated immune response. Th2 cells, conversely, are controlled by the transcription factor Gata-3, produce IL-4, IL-5, and IL-13, and mediate humoral immunity. Recently however, the discovery of a new, distinct Th-subset has been revealed;Citation176 termed Th17 cells, these cells were demonstrated to occur in the absence of Th1- or Th2-specific transcription factors and cytokines.Citation177,Citation178 Several groups have implicated cytokines in the differentiation of Th17 cells, including TGFβ, IL-6, IL-1, and IL-21, and have shown the necessity of IL-23 for maintenance and proliferation of established Th17 cells (). IL-23R expression on Th17 cells is directed by TGFβ and, in combination with IL-6 and IL-21, signals through STAT3 to direct Th17 differentiation. TGFβ and IL-1 plus IL-6 or IL-21 act on Th17 cells as differentiation factors and IL-23 promotes growth and stabilization. STAT3, ROR-γt, and ROR-α have been identified as transcription factors active in Th17 development, and these are activated by the cytokines discussed (). The relationship between TGFβ and Th17 cells likely indicates a further connection to CD4+CD25+Foxp3+ Tregs since TGFβ also induces differentiation of naïve T cells into Foxp3+ Tregs in the absence of IL-6 or IL-23.
Cytokines secreted from Th17 auto-reactive cells include IL-17A, IL-17F, IL-22, TNFα, and IL-6, which in combination with IL-19, IL-20 and IL-24 from mononuclear cells, likely participate in the pathogenesis of psoriasis.Citation179 The complex interplay of proinflammatory cytokines, chemokines, growth factors, and chemical mediators initiated by Th17 cells may well be critical for inducing the keratinocyte hyperplasia, angiogenesis and influx of neutrophils that ultimately culminates in excess keratinocyte proliferation and characteristic features of psoriatic plaques.Citation162,Citation180
Tregs
We previously examined Tregs’ function in psoriasis patients using cells isolated from both PBMC and skin biopsy tissue.Citation181 Selection of Treg cells by negative CD4 bead selection, followed by positive CD25 bead selection after resting the cells to allow non-constitutive CD25 to become negative, allowed us to utilize a population of Treg cells that was identical between normal and psoriatic samples in terms of numbers and Foxp3 expression. Treg cells isolated from both blood and lesional skin of psoriasis patients exhibited significantly less effective suppression of allogeneic Teff cells than Tregs isolated from normal (healthy) individuals. Our observation of psoriatic Treg dysfunction included “criss-cross” experiments demonstrating that psoriatic Tregs were deficient in suppressing both psoriatic and normal Teff cells. In addition, as shown in our previous paper, Teff cells obtained from psoriasis patients are hyperproliferative in response to alloantigen. This likely contributes to the escape of Tmem/eff psoriatic T cells and to the decreased restraint observed in psoriatic T cell co-culture experiments. Our recent work indicates that there is a defect localized in the psoriatic Treg subset that expresses CCR5, the high potency subset of Treg cells that chemo-attracts to MIP1, 2.
NK T cells
The exact role for NK T cells in psoriasis pathogenesis remains controversial, however at least one study has demonstrated CD56+ cells infiltrating psoriatic tissue.Citation182 In addition to immature CD56hi CD16neg/lo cells mature CD56lo CD16+ cells are also present, and each population can produce IFNγ contributing to the proinflammatory loop.Citation183 Keratinocytes secrete chemokines known to attract NK T cells including CXCL10, CCL5, and CCL20. Secretion of these chemokines results in the recruitment of NK T cells to the skin where upon activation the NK T cells may contribute to the inflammatory skin milieu.
γδ T cells
T cells express 1 of 2 major TCR subtypes composed of heterodimeric alpha/beta (αβ) or gamma/delta (γδ) proteins. The majority of T cells in the blood and draining lymph nodes are of the αβ type while γδ T cells are increased in epithelial tissues and exhibit less TCR variability, however, this characterization has been largely surmised from murine studies. In humans, the isolation and characterization of γδ T cells is vastly less. Nevertheless, there are reports of γδ T cells found in human psoriatic tissue and a concomitant reduction in circulating cells.Citation184
Monocytes, macrophages, and myeloid-derived suppressor cells
Monocytes
Monocytes are known precursors for DCs and macrophages and as such, they have been described to be important cellular contributors to psoriatic pathology. It has been suggested that psoriatic monocytes engulf low density lipoprotein leading to overproduction of inflammatory cytokines.Citation185 Additionally, psoriatic monocytes have also been described to possess increased phagocytic capabilities due to an imbalance in the ratio of cAMP/cGMP found in lesional skin.Citation186 More recently an increase in CD14+ CD16+ intermediate monocytes – termed Mon2 – has been described in a cohort of human psoriatic patients.Citation187,Citation188 Interestingly, Mon2 monocytes also have been shown to be linked to an increased risk of CVD and to be predictive of myocardial infarction and death.Citation189–Citation191
Macrophages
Infiltration of macrophages at the dermal epidermal junction is a well-known characteristic of psoriasis ().Citation192,Citation193 Although there is general agreement classifying psoriasis as a T cell mediated pathology, the role for macrophages in disease pathology has been described recently in psoriasiform mice models.Citation180,Citation194,Citation195 Research has shown that downregulation of CD18 in mice spontaneously creates chronically inflamed skin resembling human psoriasis that contains large numbers of TNFα−releasing macrophages. Interestingly, when macrophages were depleted using clodronate liposomes, the skin showed a significant improvement in inflammation.Citation195 Similarly, a deletion of IKK2 in mice leads to chronic skin inflammation with psoriasis-like characteristics. The inflamed skin contained large amounts of macrophages and the inflammation was reported to be T cell independent.Citation194 Macrophages have also been identified as a major source of the proinflammatory cytokine MCP-1 and mature CD163+ macrophages have been shown to react to tattoo chemicals in psoriasis-prone patients potentially contributing to the initiation of a psoriatic flare ().
Myeloid-derived suppressor cells (MDSCs)
Under healthy physiologic circumstances myeloid progenitor cells are created in the bone marrow and egress to the peripheral blood supply. In particular, neutrophils and monocytes are in turn recruited into local tissues where they either undergo activation or in the case of monocytes, differentiate into macrophages, DCs, and other myeloid effector subtypes. However, under pathological conditions, this differentiation step is halted and either neutrophils or monocytes stay undifferentiated for longer periods of time, becoming the so-called MDSCs. These cells are currently considered a heterogeneous cell population divided into CD33+ CD11b+ CD15+ CD14neg HLA-DRneg/low granulocytic MDSC and CD14+ CD15neg HLA-DRneg/low monocytic MDSC (Mo-MDSC), such as the ones described in . While both granulocytic MDSCs and Mo-MDSCs have the ability to display suppressive mechanisms such as inhibiting the proliferation of CD8 T cells,Citation196–Citation198 only Mo-MDSCs have been shown to possess the ability to induce Tregs.Citation199 Mo-MDSCs express several regulatory molecules such as ARG1, IL-10, CTLA4, and nitrous oxide. Although MDSCs were first described in cancer patients and the “MDSC” designation was coined in 2007,Citation200 their role in autoimmune diseases has begun to be described recently.Citation201,Citation202
Topical and systemic therapeutics in psoriasis
Topical treatments
Mild psoriasis is typically treated with topical therapy alone or in combination with other agents.Citation203 Moderate to severe psoriasis patients are usually treated with phototherapy and systemic biologics but the use of topical in combination with these therapies may be helpful in reducing the amount of other therapies required to achieve disease control. The six principal classes of topical therapies in psoriasis are coal tar, dithranol (anthralin), vitamin D analogs, corticosteroids, keratolytics, calcineurin inhibitors, and retinoids.
Coal tar has been used as a psoriasis treatment for more than a hundred years. However, its mechanism of action remains unknown, although anti-proliferative actions have been shown.Citation204 Adverse effects of coal tar include poor tolerance in patients due to its odor and staining, skin irritation, contact dermatitis, folliculitis and photosensitivityCitation203 and potential carcinogenicity in humans as well as in several animal models.Citation203,Citation205,Citation206
Dithranol or anthralin is an anthracycline that has been used as psoriasis treatment for a long time. However, its use has declined due to more cosmetically acceptable therapies.Citation203,Citation206 The response rate for dithranol has been shown to vary from 30%–70% but it is not recommended as long-term therapy.Citation207 Some of its side effects include irritation and discoloration of skin, as well as blistering and necrosis when used in excess.Citation206
Vitamin D analogs are used for the treatment of mild to moderate psoriasis.Citation203 The global estimate of efficacy showed that between 30%–50% of patients treated significantly improved or had complete clearance after 4–6 weeks of use.Citation203,Citation206 Vitamin D analogs bind to intracellular vitamin D receptor; upon binding, downstream effects include direct regulation of genes involved in epidermal proliferation, inflammation, and keratinization.Citation204 Currently, the vitamin D analogs available for treating psoriasis are calcitriol, tacalcitol, and calcipotriol.Citation204 Skin irritation and photosensitivity are the most common side effects of vitamin D analogs.Citation203,Citation206
Corticosteroids include the most important and frequently used topical medications for psoriasis. They are used for all grades of plaque psoriasis as monotherapy or complementary to systemic therapies. Corticosteroids are classified into four groups depending on their efficacy: super-potent, potent, moderately potent, and mild.Citation204 Given the wide range of corticosteroids used, their effect on cellular metabolism varies. However, their therapeutic response is mediated by vasoconstriction, anti-inflammatory, and immunosuppressive effects.Citation204 Their efficacy and side effects depend on the potency, vehicle, occlusion, and patient compliance.Citation203 The higher the potency, the more efficacy is observed, but to minimize side effects, typically the strength of the corticosteroid is decreased after improvement of psoriasis begins.Citation206 Some systemic side effects observed after corticosteroid therapies include hypertension, osteoporosis, cataracts, glaucoma, and diabetes, among others.
Keratolytics agents such as salicylic acid, urea, propylene glycol, and glycolic acids are another important group of topical treatment in psoriasis. When used in conjunction with other topical treatments they show increased efficacy but also increased toxicity.Citation203,Citation207,Citation208
Calcineurin inhibitors such as tacrolimus and pimecrolimus have been used off-label to treat facial and intertriginous lesions.Citation203,Citation208 Calcineurin is a protein phosphatase essential for lymphocyte proliferation through upregulation of IL-2.Citation209 Therefore, inhibition of calcineurin causes immunosuppression. Their most common side effects include self-limited pruritus, burning sensation at site of application, and breakdown products found in breast milk.Citation203,Citation208
Topical retinoids such as tazarotene were developed after association of oral retinoids with several adverse effects such as teratogenicity, serum lipid and transaminase elevations, mucocutaneous toxicity, hair loss, and skeletal changes.Citation210 Approximately half the patients have 50% improvement after 0.1% tazarotene gel application for 12 weeks.Citation203,Citation206
Also, these medications work best when combined with topical vitamin D analogs or topical steroids.Citation207,Citation208 The mechanism of action of retinoids is through binding of the retinoic acid receptor (RAR) preferentially binding RAR-α, RAR-β and RAR-γCitation211 but not to retinoid X receptor.Citation212 The engagement of these receptors causes reduction of epidermal hyperproliferation, decrease of inflammation, and decrease of keratinocytes’ differentiation.Citation213 The most common adverse effects include local inflammation and photosensitization.Citation207
Phototherapy
Phototherapy is commonly used as treatment for moderate to severe psoriasis. The use of UV light developed from the observation that natural and artificial light has beneficial effects over psoriasis severity. Phototherapy consists of UVB and psoralen plus UVA (PUVA).
UVB light is used for mild to moderate psoriasis and can be used as a broadband or a narrowband.Citation214 The efficacy of both types of UVB therapy fluctuates from 50%–70% of patients achieving at least 75% PASI improvement after 4–6 weeks.Citation204 Often it is combined with additional topical or systemic therapeutics.Citation215 Some of the side effects seen with acute UVB treatment are itching, burning, and erythema, while chronic exposure to UVB may cause photoaging, carcinogenesis, solar lentigines, and telangiectasias.Citation204
UVA light is more effective than UVB for treating psoriasis. However, it is more carcinogenic and causes more photoaging.Citation207 UVA is also frequently used as photochemotherapy (PUVA), where it is administered in combination with photosensitizing psoralen compounds. Improvement with PUVA therapy can be seen within a month and clearance within several months.Citation214 However, increased incidences of cutaneous malignancies and increased risk for melanoma have been observed among long-term patients receiving high dose PUVA therapy.Citation216
Retinoids
Oral retinoids have also been used as a treatment for psoriasis since the early 1980s.Citation217 They can be administered alone or in combination with UV light treatment, the latter has been shown to be more effective in patients with psoriasis vulgaris.Citation218 Retinoids are natural and synthetic analogs of vitamin A that inhibit epidermal proliferation and differentiation.Citation219 These anti-psoriatic traditional compounds are non-specific, exhibit diverse side effects and relatively high toxicity, therefore more specific therapeutics have been developed.
More narrowly targeted specific biologic compounds were developed based upon the response to immunosuppressive agents observed in psoriasis patients. Anti-psoriatic biological agents include antibodies, soluble cytokine receptors, and fusion proteins that inhibit psoriasis immuno-pathogenesis by interfering with signaling of specific proinflammatory pathways and/or receptors, cytokines or antigens.
Systemic therapeutics
Traditional systemics
Early therapeutic modalities for the treatment of psoriasis included methotrexate and cyclosporine, which induce general immunosuppression by preventing T cell activation. Although these therapies were effective, given their non-specificity, long-term use is very toxic and therefore resulted in diminished enthusiasm for their continued therapeutic use. Methotrexate is a synthetic analog of folic acid which acts as an anti-inflammatory therapeutic as well as an immunosuppressant by reducing T cell activation as well as other non-specific components of the immune system.Citation220 Liver toxicity is the main side effect of long-term use of methotrexate; however a recent review concluded that the incidence of hepatic fibrosis due to methotrexate treatment according to the literature does not give precise risk quantification.Citation221
Another traditional first line systemic therapy is cyclosporine. As with methotrexate, even though effective for treating psoriasis,Citation222 several toxicities have been implicated in its long-term use.Citation219 Cyclosporine inhibits T cell activation and cytokine expression, which causes general immunosuppression. Furthermore, cyclosporine usage can cause hypertension, hyperkalemia, hypomagnesemia, hypercalcinuria, acidosis,Citation223,Citation224 as well as nephrotoxicity with reduced renal transplant function, arteriolopathy and interstitial fibrosis.Citation225
T cell transmigration and activation receptors
The first US Food and Drug Administration-approved biologic for the treatment of psoriasis was alefacept, which targeted T cell activation, followed by efalizumab, which targeted T cell transmigration.Citation214 Currently, both of these biologics have been discontinued. Alefacept was discontinued by the manufacturer in 2011, although no specific safety concerns were indicated. Efalizumab, a monoclonal antibody that blocked CD11a, the alpha subunit of LFA-1, which is selectively used by T cells for migration and activation was discontinued due to severe adverse events associated with fatal brain infections.Citation226,Citation227 However, it was during studies examining the mechanism of action of efalizumab that the inflammatory Tip-DCs were discovered in psoriasis lesions.Citation138
TNFα
The largest group of approved biologic therapeutics for psoriasis is TNFα inhibitors including etanercept, infliximab, and adalimumab. Biologics targeting TNFα revolutionized psoriasis therapy due to their greatly improved effectiveness compared to more global immunosuppressants. Different TNF therapeutics have been used for the treatment of psoriasis including neutralizing antibodies and TNF receptor fusion proteins. Treatment with anti-TNF antibodies such as adalimumab or infliximab has been shown to improve the PASI score of psoriasis patients by up to 75%.Citation228,Citation229 Furthermore, 34%–49% of patients achieved PASI-75 after treatment with the TNF receptor fusion protein, etanercept.Citation230 However, paradoxical development of psoriasis has been linked to anti-TNFα treatments in rheumatoid arthritis (RA) patientsCitation231 and to worsening of the disease in psoriasis patients.Citation232 Also, serious side effects such as lymphoma, infections, congestive heart failure, demyelinating diseases, induction of auto-antibodies, lupus-like syndrome, and systemic side effects have also been reported.Citation233
IL-12/IL-23p40
Early research showing IL-23 and Th17 related cytokines in skin lesions and serum of psoriasis patients, as well as the association of IL23R gene variants in psoriasis and functional role of Th17 cells in autoimmunity, raised interest in the IL-23/Th17 axis as a potential target for psoriasis immunotherapy.Citation234 Ustekinumab is an approved psoriasis biologic that neutralizes the p40 subunit shared by IL-23 and IL-12. Therefore this neutralization also partially inhibits Th17 and Th1 responses. More than 65% of patients treated with ustekinumab show a PASI-75 response at 12 weeks post-therapy.Citation235 Thus far, serious infections, malignancies or adverse cardiovascular events have not been observed in patients treated with ustekinumab.Citation236
IL-23/IL-17
Skin biopsies from psoriatic lesions show increased levels of IL-17A and T cells as well as higher IL-17A mRNA expression when compared with healthy control skin.Citation161,Citation237 IL-17 receptors are constitutively expressed on keratinocytes throughout the epidermis and on some dermal cells such as DCs, dermal fibroblasts, and endothelial cells.Citation174 Stimulation by IL-17A causes keratinocytes to express multiple chemokines including CCL20 which may directly recruit CCR6+ cells, such as Th17 and DCs to the skin, which elicit a positive loop for inflammatory cell maintenance in psoriatic lesional skin.Citation237 Th17 cells in peripheral circulation and lesional psoriatic skin have been shown to positively correlate with psoriasis disease severity.Citation238 Several IL-17A inhibitors are in clinical Phase III trials in the United States such as the monoclonal antibodies for IL-17A neutralization secukinumabCitation239 and ixekizumabCitation240 and the antibody for binding IL-17A receptor, brodalumab.Citation241 Recently, the European Medicines Agency (EMA) has approved secukinumab as a first line systemic treatment for moderate-to-severe plaque psoriasis in adults. However, even though IL17-A neutralizing antibodies have been successful, their side effects remain unknown. Additionally, several IL-23 inhibitors are currently in Phase III of clinical trials in the United States including antibodies directed against the p19 subunit of IL-23, such as guselkumab (CNTO1959) and MK-3222/SCH-900222.Citation242
JAK inhibitors
Several important cytokines in psoriasis such as IL-2, IL-6, IL-22, IL-23, and IFNγ use the janus kinase (JAK/STAT) signaling pathways.Citation243,Citation244 Therefore, JAK inhibition may interfere with key cytokine signaling causing suppression of immune cell activation and subsequent inflammation.Citation245,Citation246 A clinical JAK inhibitor, tofacitinib, has been approved for RA;Citation247 oral and topical formulations are being tested for psoriasis.Citation248,Citation249 Early results show significant response rates but the long-term safety of JAK inhibition remains unknown.
Biomarkers of psoriasis
The US National Institutes of Health Biomarkers and Surrogate Endpoint Working Group defines biomarkers as biological characteristics that are objectively measured and evaluated as indicators of specific processes either under homeostasis, pathogenesis or pharmacologic responses.Citation250 Biomarkers can be disease-related for diagnosis and prognosis or drug-related based on pharmacokinetics and pharmacodynamics. Therefore, biomarkers play an important role in diagnosis assessment, disease processes, and treatment response.Citation250 Evaluation of biomarkers in psoriasis may help to establish severity and therapeutic response. Biomarkers can be categorized into several distinct biologic classes including genetic biomarkers, serum (blood)/soluble biomarkers, tissue biomarkers, and transcriptional markers of activation associated with disease. Herein, we address only examples of serum (blood)/soluble biomarkers.
Inflammatory biomarkers
TNF
Psoriasis patients have increased levels of proinflammatory cytokines, such as TNF. TNFα is a 17 kD polypeptide that regulates innate immune responses. TNF can stimulate proinflammatory cytokines and enhance cell adhesion thereby increasing the phagocytic index for innate defense cells such as macrophages. TNF ligation through membrane-bound receptors (TNF-R1 [p55] and TNF-R2 [p75]) induces apoptosis machinery following phagocytic activation. TNFα can be produced by a plethora of cells including lymphocytes, monocytes, keratinocytes, mast cells, and APCs such as macrophages and DCs of the skin. In psoriasis, TNFα promotes innate immune cell activation and trafficking to the skin which results in accelerated keratinocyte proliferation. Targeted therapeutics, designed to inhibit TNFα activity are currently in use to treat several autoimmune conditions including psoriasis and psoriatic arthritis. Thus, monitoring the level of TNFα activity can be used as a “biomarker” for psoriasis activity, although it is not specific to only psoriasis and should be viewed as a non-specific marker of general inflammation, as are all of the outlined markers in this section.
Adiponectin
This adipose tissue specific cytokine is known to inhibit inflammatory response by reducing the production of TNFα, IL-6, IFNγ, adhesion molecules in monocytes, phagocytic activity by macrophages, and increases insulin sensitivity and repair of the vasculature.Citation81,Citation251–Citation253 However, the role of adiponectin in psoriasis is controversial. Some reports indicate a reduction of adiponectin in overweight/obese psoriatic patients,Citation254–Citation256 which was inversely correlated with PASI score;Citation257 while others have shown increased adiponectin concentration that positively correlates with PASI score.Citation258 Therefore, further studies are warranted for determining adiponectin’s role in psoriasis.
Resistin
Resistin is expressed by macrophages and peripheral monocytes in the stromal compartment of the adipose tissue. Resistin is involved in proinflammatory responses by causing an increase in the expression of TNFα and IL-6.Citation259 Resistin levels have been found to be elevated in psoriasis as well as being associated with disease severity.Citation83,Citation254,Citation257,Citation260 Recent studies have shown that the more severe the psoriasis the higher the levels of resistin found in the serumCitation254 and plasma.Citation261
MPO
MPO is a proinflammatory protein that can be stored in leukocytes and secreted upon activation of the cells during inflammatory processes. The conversion of chloride and hydrogen peroxide to hypochlorite – a strong reactive oxygen species is catalyzed by MPO. Although several cell types can produce MPO, including monocytes, macrophages, Kupffer cells, and microglial cells, nearly all of the circulating MPO is produced by polymorphonuclear neutrophils. MPO has emerged as a biomarker for CVD as well as generalized stress and inflammation.
High-sensitivity CRP
In patients with psoriasis, there is sufficient systemic inflammation to raise hepatic-derived CRP, a risk factor for CVDs.Citation262,Citation263 A number of systemic inflammatory diseases such as RA, systemic lupus erythematosus (SLE), and chronic gingivitis are associated with increased risk of CVDs and CRP elevation; although the mechanisms of this risk elevation are not elucidated.Citation264,Citation265
Emerging biomarkers
The new challenge in identifying biomarkers will be to increase the specificity of these markers for individual disease(s) rather than generic markers of inflammation. As such, genetic biomarkers are beginning to narrow the spectrum of which genes participate in different inflammatory diseases; however, many of these are also redundant to several disorders. The answer may lie in designing specific panels of markers, or molecular signatures that will be unique for each disease. Currently, the technology to accomplish this goal may slowly be reaching its potential. Increased use of sophisticated molecular, genetic, and cellular technologies such as multi-parameter flow cytometry analysis, mass spectrometry, “omics” data generated by next-generation technologies combined with computational algorithms mining the data sets will advance the comprehensive bioinformatics of diseases, allowing for improved profiling of different pathological subsets. Indeed, a systems biology approach examining immune cell interactions participating in skin disease is beginning to emerge. Employing these advanced methods should enhance the ability to design improved biomarkers for complex diseases.Citation266,Citation267
Psoriasis as an autoimmune disease
Autoimmune diseases are an amalgam of chronic conditions where the destruction or disruption of the body’s own tissues by the immune system occurs.Citation268 Even when the specific etiology of the majority of the autoimmune diseases remains unknown, they are associated with a combination of genetic and environmental factors.
The current consensus regarding psoriasis etiology is that it constitutes an inherited and an immune-mediated disease. However, controversy exists to whether psoriasis should be considered a bona fide autoimmune disease given that no auto-antigen has been conclusively discovered that triggers the disease and no self-reactive T cells have been identified.Citation269
Currently two theories about the nature of an antigen in psoriasis exist. The first one considers psoriasis to be an autoimmune disease caused by molecular mimicry,Citation270,Citation271 while the second one postulates that psoriasis is triggered by bacterial microbiota of the skin.Citation65
Some evidence for considering psoriasis as an autoimmune disease include genetic predisposition as well as the overlap of several biochemical pathways with those altered in other autoimmune diseases such as Crohn’s disease (CD), type I diabetes and RA, although the status of CD as “autoimmune” has also been recently questioned.Citation272 Also, molecular mimicry between streptococcal and keratin proteins, the existence of homologous peptides between these proteins and CD8+ T cells’ response to these homologous peptides have been shown in psoriasis.Citation271 Furthermore, several molecules in T cells that are associated with autoimmunity have been demonstrated in psoriasis. Some negative regulators of T cell activation such as CTLA4, PD1, SHP1, inhibitor of NF-κB (I-κB), PAG, and CSK have been shown to be involved in psoriasis animal models.Citation19
Among the evidence for bacteria to trigger psoriasis is the finding of enhanced TLR2 on psoriatic keratinocytes.Citation273 TLR2 is involved in the recognition of Gram-positive bacteria products including lipoproteinsCitation274 and peptidoglycans.Citation274 Furthermore, among the four known PGRPs with function in antibacterial immunity, polymorphisms of PGRP-3 and PGRP-4 gene on PSORS4 susceptibility site have been associated with psoriasis.Citation275,Citation276 Not only streptococcal peptidoglycans have been found in APCs from psoriasis patients,Citation62 but also CD4+ T cell lines established from psoriasis patients have been shown to respond to streptococcal and staphylococcal peptidoglycans.Citation62
Psoriasis association with other autoimmune diseases
Association of psoriasis with other autoimmune diseases is an ongoing research area. Previous studies have shown that there is a higher frequency of autoimmune diseases among psoriasis patients than observed in the general population potentially stemming from cytokine pathways’ dysregulation.Citation277,Citation278 Based on a retrospective study using MEDLINE data from January 1, 1980 to June 1, 2011, the major autoimmune disorders associated with psoriasis include RA, celiac disease, IBD, especially CD, multiple sclerosis, SLE, and autoimmune thyroid disease.Citation279 However, anecdotal reports of other autoimmune diseases associated with psoriasis include Sjögren’s syndrome (SS) and alopecia areata. In addition, a new meta-analysis of a single mutation of CD226, Gly307Ser (rs763361) has suggested that this modification is associated with an increased risk of developing various autoimmune disorders including psoriasis.Citation280
Rheumatoid arthritis
RA and psoriasis are both chronic inflammatory autoimmune diseases. Even when clinical manifestation of these diseases is diverse, several parallel presentations have been observed. A genetic relationship between RA and psoriasis has been reported. One of the genetic associations is the expression of the RUNX1 gene.Citation281 This transcription factor regulates differentiation of hematopoietic stem cells.Citation282 RUNX1 expression in psoriasis has been suggested to confer defective regulation of a phosphoprotein implicated in regulation of membrane dynamism for synapse formation and T cell activation (SLC9A3R1) or by NAT9, which is a new member of the NAT superfamily.Citation25
Another genetic relationship is expression of the psoriatic susceptibility gene PSORS1C1, which has been shown to be significantly increased in blood cells from RA patients.Citation283 Importantly, this gene may be involved in IL-17 and IL-1β production in RA by increasing gene expression in synovial tissues. Additionally, IL-23R polymorphisms have been shown to control susceptibility to psoriasis as well as RA, which further emphasizes the importance of the Th17 pathway in both diseases.Citation284 Based on a retrospective cohort study, RA has the highest odds ratio with psoriasis among various autoimmune diseases evaluated, which included CD, SS, SLE, and others.Citation278
Another gene that has been implicated in RA and psoriasis, as well as with celiac disease, CD, and SLE is TNFAIP3.Citation285 This gene encodes the intracellular ubiquitin-editing protein A20 (TNFAIP3) which is a negative regulator of TNFα-induced NFκβ signaling and TNFα-induced apoptosis.Citation285 Therefore, there is a clear genetic overlap between these diseases.
Immunological similarities have also been observed between psoriasis and RA. Activated auto-reactive T cells have been implicated to initiate and drive organ-specific inflammation in the synovium, in RA, and in the skin of psoriasis patients. Immune cell infiltration into the synovium and skin in RA and psoriasis respectively, is considered one of the principal characteristics of both diseases.Citation286
In both diseases, an imbalance between Th17 and Tregs has been demonstrated, as well as limited functional capabilities by suppressive Tregs.Citation20,Citation181,Citation287 Key signaling molecules such as TNFα, IL-1β, IL-6, IL-17A, IL-17F, IL-21, and IL-23 have been implicated in pathogenesis of both diseases.Citation20,Citation287 Hence, similar therapeutic approaches have been effectively used to treat both diseases, including methotrexate and neutralizing antibodies for TNFα. However, paradoxical psoriasis onset has been observed in RA patients after treatment with neutralizing antibodies for TNFαCitation44,Citation231,Citation232 as well as with tocilizumab, an anti-IL-6 receptor specific antibody.Citation46,Citation47,Citation49 This paradoxical event is of interest considering both TNFαCitation288 and IL-6Citation289,Citation290 have been shown to be key signals in psoriasis development. Therefore, further studies are required for better understanding of the role of TNFα and IL-6 inhibition in psoriasis.
Celiac disease
Celiac disease is an autoimmune disease triggered by gluten ingestion that affects the small intestine. Association of celiac disease with psoriasis remains controversial. Several small studies support the association by demonstrating significantly higher rates of celiac disease in psoriasis patients compared to controls and by elevated celiac disease-associated antibodies in psoriasis patients that correlate with their disease severity as well as improvement of psoriasis by implementation of a gluten free diet,Citation291–Citation297 although other studies attempting to link celiac disease have shown no association.Citation298
Previous publicationsCitation299,Citation300 present several potential mechanisms regarding a positive association between psoriasis and celiac disease. Firstly, vitamin D deficiency is commonly seen in celiac diseaseCitation301 and is known to predispose patients to psoriasis development.Citation302,Citation303 Secondly, patients with celiac disease who are exposed to gliadin may trigger a CD4+ T cell response and subsequent proinflammatory cytokine cascade involving, for example, IFNγ Citation304 in both peripheral blood and skin, which might trigger or exacerbate psoriasis development.Citation305 Another potential mechanism is through common genetic factors such as the IL-2/IL-21 locus on chromosome 4q27, which has been previously linked with both psoriasisCitation306 and celiac disease.Citation307,Citation308 Since the mechanisms for the positive association between celiac disease and psoriasis are not well understood, more studies to explore this association need to be performed.
Crohn’s disease
CD is a chronic IBD that can affect the entire gastrointestinal tract but commonly affects the terminal ileum and colon. The prevalence of CD is seven cases per 1,000,000 adults in the United States.Citation309 Several genetic and immuno-pathological associations have been observed between CD and psoriasis.
Increased prevalence of psoriasis in CD patients has been previously demonstrated.Citation310–Citation312 Five independent case control studies have shown that psoriasis prevalence is 8.9% in CD patients but only 1.4% in control individuals.Citation313 Furthermore, 10% of CD patients had relatives with psoriasis, while the prevalence among control patients was only 2.9%.Citation313 Also, some studies have shown that asymptomatic bowel inflammation may exist among psoriasis patients.Citation314
Genetic overlap has been demonstrated between psoriasis and CD. Several genes that encode for IL-23-associated molecules including IL23R, IL12B, and TYK2 have been associated with CD and psoriasis.Citation315–Citation318 Furthermore, combined analysis of GWAS for both diseases has identified seven shared susceptibility loci between psoriasis and CD.Citation319
Important immuno-pathologic associations have been demonstrated between psoriasis and CD. First, increased levels of TNFα have been shown in intestinal and other tissues from CD patientsCitation320,Citation321 as well as in psoriatic lesions in psoriasis patients.Citation322 TNFα inhibitors are effective in both diseasesCitation229,Citation322–Citation324 however, paradoxical onset of psoriasis in patients treated with TNFα inhibitors has also been observed.Citation325,Citation326 Second, both diseases present infiltration of T lymphocytes, macrophages, monocytes, DCs, and neutrophils as well as high levels of other cytokines such as IFNγ, IL-12, IL-6, and IL-17 into lesional tissue.Citation20,Citation327
Also, in both psoriasisCitation234 and CD,Citation328 Th17 cells have been reported to be key in establishing chronic inflammation. Imbalance between Tregs and Th17 cells by loss of CD4+CD25high Foxp3+ Tregs function,Citation181,Citation329 as well as increased levels of proinflammatory Th17 cells,Citation234,Citation330 have been associated with aberrant immune response in both diseases. Furthermore, recent studies have demonstrated IL-17 producing Tregs in inflamed intestinal mucosa of CD patientsCitation331 as well as in lesional skin of psoriasis patients,Citation332 indicating a possible re-differentiation of Tregs towards a proinflammatory phenotype to further perpetuate the chronic inflammation stage of these diseases. Recently, a potential protective role for IL-17 in CD and IBD has also been proposed based upon adoptive transfer studies of colitis induction demonstrating exacerbation of disease in the absence of IL-17-producing cells.Citation333
Another cytokine that appears to play an important role in both diseases is IL-6. In psoriasis, high levels of IL-6 have been detected in lesional plaquesCitation289 and are responsible for phosphorylation of STAT3 which renders Teff cells refractory to Tregs inhibition.Citation290 In CD, IL-6 has been shown to be produced by lamina propria macrophages and T cells in the inflamed gut, which also results in IL-6-dependent STAT3 phosphorylation of Teff cells.Citation334 Studies blocking IL-6 signaling have shown beneficial effects in a pilot trial of CD patients;Citation335 however further studies to determine the clinical importance of IL-6 signaling blockade in both diseases need to be completed.
Atopic dermatitis
Atopic dermatitis (AD) and psoriasis are among the most common inflammatory skin diseases. However, AD patients suffer from frequent skin infections, while psoriasis patients exhibit relatively few skin infections. There are two forms of AD: extrinsic, characterized by elevated immunoglobulin E levels and eosinophils in peripheral circulation, and intrinsic which only affects ∼20% of patients and presents with normal immunoglobulin E levels and eosinophils numbers.Citation336 Chronic phase AD shares many characteristics with psoriasis including epidermal hyperplasia, altered terminal keratinocyte differentiation and T and DC infiltrates.Citation337 Thus, even though phenotypically both diseases differ, histologically there are similarities between psoriasis and AD. In AD the epidermal differentiation process is disrupted due to primary genetic mutations in the epidermal differentiation complex localized on chromosome 1q21 that leads to deficiencies in epidermal barrier function.Citation337,Citation338 The epidermal differentiation complex contains the FLG gene. FLG is an intracellular protein that promotes epidermal differentiation and hydration by aggregating keratin intermediate filaments within the corneocytes and drawing water into the stratum corneum.Citation338 Loss-of-function mutations in FLG have been associated with AD. In psoriasis, previous studies have shown no association between FLG mutations and early onset of psoriasis in childhood.Citation339 However, a recent publication demonstrated an association between rare mutations such as p.K4022X in the FLG gene with psoriasis in a Chinese population.Citation340 Therefore, a possible correlation between FLG gene mutations and both diseases remains to be further studied. Comparison of genomic overlap between psoriasis and AD revealed that nearly two thirds of the variants exhibit a risk profile for AD that is the opposite of those observed for psoriasis, however, one third revealed an association with the same allele. Thus both divergent and shared patterns occur between these common dermatological diseases.Citation341
Using gene chip microarray, the innate immune response genes in AD and psoriatic skin were compared.Citation342 Decreased levels of HBD-3, HBD-2, iNOS, and IL-8 were observed in AD skin as compared to psoriasis.Citation342 Furthermore, TNFα and IFNγ are decreased in AD skin in comparison to psoriasis, which may be due to the previously mentioned down-regulation of the innate immune response genes.Citation342
AD is characterized by significant barrier disruption as well as increased susceptibility to allergic sensitization and microbial colonization and infections.Citation343,Citation344
Previously the classical belief was that AD was a Th2 cell-mediated disease while psoriasis was a Th1 driven disease, however, the discovery and association of Th17 cells with epidermal activation has been shifting this hypothesis. Th17 cells are associated with several aspects of psoriasis pathogenesis including increased neutrophil chemotaxis and increased production of antimicrobial peptides. In acute AD, Th17 cells have also been demonstrated to be increased in both peripheral blood and skin lesions;Citation345 however, IL-17 production by Th17 cells has been shown to be decreased in chronic AD patients when compared to chronic psoriasis patients.Citation346 Therefore, it is possible that Th17 cells are not activated or may be inhibited by Th2 cytokines in AD.Citation347
Systemic lupus erythematosus
SLE and psoriasis association has been reported as rare. A retrospective study evaluating published work from 1927 to 2007 established that only 22 reports of 111 cases presented coexistence of the two diseases,Citation348 while another report showed 0.6% of 520 SLE patients have concomitant psoriasis.Citation349 An independent separate investigation from 300 psoriatic patients found 17 (5.7%) SLE immunological characteristics.Citation348 Therefore, the exact incidence of SLE in psoriasis remains controversial. The strongest connection between psoriasis and SLE is the discovery that SLE, RA, and psoriasis share dysfunctional binding of the transcription factor RUNX1 to its binding site due to nucleotide polymorphisms.Citation25,Citation350,Citation351 RUNX1 binding on chromosome 2 is altered in many patients with SLE and psoriasis patients have an altered RUNX1 binding site on chromosome 17. Additional genetic similarities between psoriasis and SLE are also captured in polymorphisms associated with the Act 1 encoding gene TRAF3IP2 exhibited by SLE patients.Citation352 Previous work has identified TRAF3IP2 as a genetic susceptibility locus for psoriasis and a connection to IL-17 signaling.Citation353,Citation354 A newly emerging role of Th17 and IL-17 in SLE has also been recently reported.Citation355–Citation357 Finally, dysfunctional Treg control has also been described in both SLE and psoriasisCitation181,Citation289,Citation358,Citation359 suggesting that common immune and genetic dysfunctions may direct similar responses in both SLE and psoriasis.
Sjögren’s syndrome
SS is a chronic, autoimmune disease caused by mononuclear cell infiltrates and progressive damage to the exocrine glands.Citation360 A recent retrospective study demonstrated that psoriasis patients had an increased odds ratio of developing SS.Citation278 Several other case reports have identified SS patients who developed psoriasis, or vice versa.Citation361–Citation366 The cellular determinant(s) driving psoriasis and SS have similar features including activated Teff cells, Th17 cells, and a role for Tregs as well.Citation367 Although an increased risk of developing SS exists for psoriasis patients, no definitive genetic markers have been identified to date that link these respective diseases.
Vitiligo
Vitiligo is a common depigmentation of the skin disorder that affects around 1%–2% of the world population.Citation368 It is an autoimmune disorder caused by the selective destruction of skin melanocytes which results in the formation of white, depigmented skin patches.Citation368 Coexistence of psoriasis and vitiligo used to be considered very rare but this might be an underestimation. Several isolated case reportsCitation369–Citation371 as well as few retrospective studies have shown a possible association between psoriasis and vitiligo.Citation372–Citation375 The biggest retrospective study using data from 2,441 vitiligo patients found that 23% of these patients had one or more autoimmune diseases and that psoriasis was the second most common comorbidity following thyroid-related disorders.Citation375
Vitiligo and psoriasis share several immunological similarities including Th1 and Th17 signaling pathwaysCitation234,Citation376 as well as dysfunction of Tregs.Citation289,Citation290,Citation329,Citation332,Citation377 Also, both diseases have been shown to be associated with NALP1 gene variationsCitation378 as well as phenotype similarities such as Koebner phenomenon,Citation35 neuropeptides, and skin patches.Citation3,Citation368 Furthermore, a recent report demonstrated the simultaneous presence of a family history of CVD with the presence of activated inflammatory pathways and the absence of organ-specific autoantibodies in both psoriasis and vitiligo.
Conclusion and future directions
Psoriasis patients have an increased risk of developing numerous other autoimmune diseases.Citation278 The increased risk for the various other diseases has been associated with an overactive immune response (eg, activated T cells, memory T cells, DCs, neutrophils, Th17, IL-17, TNFα),Citation4,Citation149,Citation158,Citation181,Citation299,Citation357 genetic modifications (eg, RUNX1, SNPs,Citation25,Citation28,Citation281,Citation350 CARD14),Citation26,Citation27 decreased suppressive function (Treg,Citation181,Citation289,Citation290,Citation327,Citation328,Citation332,Citation358,Citation359 IL-10) as well as potential environmental or epigenetic triggers. The autoimmune diseases associated with psoriasis often have a similar pattern of cellular responders suggesting that common signaling pathways may be implicated in the background of each autoimmune response. Despite numerous extensive GWAS studies, definitive causal loci for psoriasis or many of the other autoimmune disorders mentioned are still elusive. However, these studies have demonstrated that several common genes are present in various autoimmune disorders suggesting that convergent molecular pathways may mediate autoimmune response or susceptibility to autoimmunity. Given the striking similarities in the response elements, signaling pathways, cellular mediators, and genetic associations observed in autoimmune diseases associated with psoriasis, elicitation of additional autoimmune disorders in patients with psoriasis may have a low threshold that is readily overcome upon initiation of psoriasis immunopathogenesis. Targeted therapeutics addressing common elements in autoimmune disorders, such as anti-IL-17 therapies or TNFα inhibitors are beginning to cross platforms and be applied in several autoimmune diseases. Further research to refine the common autoimmune elements should lead to the development of more tailored therapies for all autoimmune disorders.
Acknowledgments
was designed and illustrated by David C Soler. Grant numbers and sources of support: National Institutes of Health (P30AR39750), Murdough Family Center for Psoriasis.
Disclosure
The authors have no conflict of interest to disclose.
References
- ParisiRSymmonsDPGriffithsCEGlobal epidemiology of psoriasis: a systematic review of incidence and prevalenceJ Invest Dermatol2013133237738523014338
- CameronJBVooheesASHistory of PsoriasisLondonSpringer2014
- PereraGKDi MeglioPNestleFOPsoriasisAnnu Rev Pathol2012738542222054142
- RaychaudhuriSKMaverakisERaychaudhuriSPDiagnosis and classification of psoriasisAutoimmun Rev2014134–549049524434359
- GriffithsCEBarkerJNPathogenesis and clinical features of psoriasisLancet2007370958326327117658397
- ChapmanBPMoynihanJThe brain-skin connection: role of psychosocial factors and neuropeptides in psoriasisExpert Rev Clin Immunol20095662362720477685
- FeldmanSRMalakoutiMKooJYSocial impact of the burden of psoriasis: effects on patients and practiceDermatol Online J2014208
- HrehorowESalomonJMatusiakLReichASzepietowskiJCPatients with psoriasis feel stigmatizedActa Derm Venereol2012921677221879243
- de KorteJSprangersMAMombersFMBosJDQuality of life in patients with psoriasis: a systematic literature reviewJ Investig Dermatol Symp Proc200492140147
- KimballABJacobsonCWeissSVreelandMGWuYThe psychosocial burden of psoriasisAm J Clin Dermatol20056638339216343026
- FeldmanSRFleischerABJrReboussinDMThe economic impact of psoriasis increases with psoriasis severityJ Am Acad Dermatol19973745645699344194
- PearceDJLucasJWoodBDeath from psoriasis: representative US dataJ Dermatolog Treat200617530230317092861
- ArmstrongAWGelfandJMGargAOutcomes research in psoriasis and psoriatic arthritis using large databases and research networks: a report from the GRAPPA 2013 Annual MeetingJ Rheumatol20144161233123624882860
- GelfandJMYeungHMetabolic syndrome in patients with psoriatic diseaseJ Rheumatol Suppl201289242822751586
- KimballABGladmanDGelfandJMNational Psoriasis Foundation clinical consensus on psoriasis comorbidities and recommendations for screeningJ Am Acad Dermatol20085861031104218313171
- LoveTJQureshiAAKarlsonEWGelfandJMChoiHKPrevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003–2006Arch Dermatol2011147441942421173301
- GordonKClincal Outcome MeasurementsGordonKRudermanEPsoriasis and Psoriatic Arthritis an Integrated ApproachSpringer Science and Business Media2005125129
- van den BogaardEHTjabringaGSJoostenICrosstalk between keratinocytes and T cells in a 3D microenvironment: a model to study inflammatory skin diseasesJ Invest Dermatol2014134371972724121402
- BowcockAMThe genetics of psoriasis and autoimmunityAnnu Rev Genomics Hum Genet200569312216124855
- LowesMASuarez-FarinasMKruegerJGImmunology of psoriasisAnnu Rev Immunol20143222725524655295
- ElderJTPSORS1: linking genetics and immunologyJ Invest Dermatol200612661205120616702966
- StuartPMalickFNairRPAnalysis of phenotypic variation in psoriasis as a function of age at onset and family historyArch Dermatol Res2002294520721312115023
- MallonENewsonRBunkerCBHLA-Cw6 and the genetic predisposition to psoriasis: a meta-analysis of published serologic studiesJ Invest Dermatol1999113469369510504461
- TomfohrdeJSilvermanABarnesRGene for familial psoriasis susceptibility mapped to the distal end of human chromosome 17qScience19942645162114111458178173
- HelmsCCaoLKruegerJGA putative RUNX1 binding site variant between SLC9A3R1 and NAT9 is associated with susceptibility to psoriasisNat Genet200335434935614608357
- JordanCTCaoLRobersonEDRare and common variants in CARD14, encoding an epidermal regulator of NF-kappaB, in psoriasisAm J Hum Genet201290579680822521419
- JordanCTCaoLRobersonEDPSORS2 is due to mutations in CARD14Am J Hum Genet201290578479522521418
- TsoiLCSpainSLKnightJIdentification of 15 new psoriasis susceptibility loci highlights the role of innate immunityNat Genet201244121341134823143594
- JohnstonAXingXGuzmanAMIL-1F5, -F6, -F8, and -F9: a novel IL-1 family signaling system that is active in psoriasis and promotes keratinocyte antimicrobial peptide expressionJ Immunol201118642613262221242515
- MuhrPZeitvogelJHeitlandIWerfelTWittmannMExpression of interleukin (IL)-1 family members upon stimulation with IL-17 differs in keratinocytes derived from patients with psoriasis and healthy donorsBr J Dermatol2011165118919321410667
- LowesMARussellCBMartinDATowneJEKruegerJGThe IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responsesTrends Immunol201334417418123291100
- KwokPYChenXDetection of single nucleotide polymorphismsCurr Issues Mol Biol200352436012793528
- DuffinKCKruegerGGGenetic variations in cytokines and cytokine receptors associated with psoriasis found by genome-wide associationJ Invest Dermatol2009129482783318830267
- Di MeglioPDi CesareALaggnerUThe IL23R R381Q gene variant protects against immune-mediated diseases by impairing IL-23-induced Th17 effector response in humansPLoS One201162e1716021364948
- SagiLTrauHThe Koebner phenomenonClin Dermatol201129223123621396563
- LadizinskiBLeeKCWilmerEA review of the clinical variants and the management of psoriasisAdv Skin Wound Care201326627128423669329
- KalayciyanAAydemirEHKotogyanAExperimental Koebner phenomenon in patients with psoriasisDermatology2007215211411717684372
- MalhotraSKMehtaVRole of stressful life events in induction or exacerbation of psoriasis and chronic urticariaIndian J Dermatol Venereol Leprol200874659459919171981
- GuptaAKSibbaldRGKnowlesSRLyndeCWShearNHTerbinafine therapy may be associated with the development of psoriasis de novo or its exacerbation: four case reports and a review of drug-induced psoriasisJ Am Acad Dermatol1997365 Pt 28588629146568
- Pierard-FranchimontCPierardGEL’iatrogénie psoriasique. [Drug-related psoriasis]Rev Med Liege2012673139142 French22611830
- TsankovNAngelovaIKazandjievaJDrug-induced psoriasis. Recognition and managementAm J Clin Dermatol20001315916511702297
- TsankovNKVassilevaSVLazarovaAZBerowaNVBotev-ZlatovNOnset of psoriasis coincident with tetracycline therapyAustralas J Dermatol19882921111123269701
- BasavarajKHAshokNMRashmiRPraveenTKThe role of drugs in the induction and/or exacerbation of psoriasisInt J Dermatol201049121351136121091671
- DenadaiRTeixeiraFVSteinwurzFRomitiRSaad-HossneRInduction or exacerbation of psoriatic lesions during anti-TNF-alpha therapy for inflammatory bowel disease: a systematic literature review based on 222 casesJ Crohns Colitis20137751752422960136
- FryLBakerBSTriggering psoriasis: the role of infections and medicationsClin Dermatol200725660661518021899
- GraslandAMaheERaynaudEMaheIPsoriasis onset with tocilizumabJoint Bone Spine201380554154223731639
- LaurentSLe ParcJMClericiTBrebanMMaheEOnset of psoriasis following treatment with tocilizumabBr J Dermatol201016361364136520731650
- SteinwurzFDenadaiRSaad-HossneRInfliximab-induced psoriasis during therapy for Crohn’s diseaseJ Crohns Colitis20126561061622398095
- WendlingDLetho-GyselinckHGuillotXPratiCPsoriasis onset with tocilizumab treatment for rheumatoid arthritisJ Rheumatol201239365722383356
- CohenADKagenMFrigerMHalevySCalcium channel blockers intake and psoriasis: a case-control studyActa Derm Venereol200181534734911800142
- LeeREGaspariAALotzeMTChangAERosenbergSAInterleukin 2 and psoriasisArch Dermatol198812412181118153263840
- WolfRTamirABrennerSPsoriasis related to angiotensin-converting enzyme inhibitorsDermatologica1990181151532203659
- NaldiLPeliLParazziniFCarrelCFPsoriasis Study Group of the Italian Group for Epidemiological Research in DermatologyFamily history of psoriasis, stressful life events, and recent infectious disease are risk factors for a first episode of acute guttate psoriasis: results of a case-control studyJ Am Acad Dermatol200144343343811209111
- NorrlindRA case of psoriasis pustulosa after an angina tonsillarisActa Derm Venereol1954341–212212313157869
- WhyteHJBaughmanRDAcute Guttate Psoriasis and Streptococcal InfectionArch Dermatol19648935035614096349
- SigurdardottirSLThorleifsdottirRHValdimarssonHJohnstonAThe association of sore throat and psoriasis might be explained by histologically distinctive tonsils and increased expression of skin-homing molecules by tonsil T cellsClin Exp Immunol2013174113915123750651
- SigurdardottirSLThorleifsdottirRHValdimarssonHJohnstonAThe role of the palatine tonsils in the pathogenesis and treatment of psoriasisBr J Dermatol2013168223724222901242
- ThorleifsdottirRHSigurdardottirSLSigurgeirssonBImprovement of psoriasis after tonsillectomy is associated with a decrease in the frequency of circulating T cells that recognize streptococcal determinants and homologous skin determinantsJ Immunol2012188105160516522491250
- CampanatiAGanzettiGMartinaEHelicobacter pylori infection in psoriasis: results of a clinical study and review of the literatureInt J Dermatol2015545e109e11425808243
- HalaszCLHelicobacter pylori antibodies in patients with psoriasisArch Dermatol1996132195968546497
- OnsunNArda UlusalHSuOImpact of Helicobacter pylori infection on severity of psoriasis and response to treatmentEur J Dermatol201222111712022063790
- BakerBSLamanJDPowlesAPeptidoglycan and peptidoglycan-specific Th1 cells in psoriatic skin lesionsJ Pathol2006209217418116493599
- BakerBSPowlesAFryLPeptidoglycan: a major aetiological factor for psoriasis?Trends Immunol2006271254555117045843
- FryLBakerBSPowlesAVPsoriasis – a possible candidate for vaccinationAutoimmun Rev20076528628917412299
- FryLBakerBSPowlesAVFahlenAEngstrandLIs chronic plaque psoriasis triggered by microbiota in the skin?Br J Dermatol20131691475223521130
- KonoMNagataHUmemuraSKawanaSOsamuraRYIn situ expression of corticotropin-releasing hormone (CRH) and proopiomelanocortin (POMC) genes in human skinFASEB J200115122297229911511529
- SlominskiATBotchkarevVChoudhryMCutaneous expression of CRH and CRH-R. Is there a “skin stress response system?”Ann N Y Acad Sci199988528731110816662
- WeiglBAThe significance of stress hormones (glucocorticoids, catecholamines) for eruptions and spontaneous remission phases in psoriasisInt J Dermatol200039967868811044193
- ZbytekBPikulaMSlominskiRMCorticotropin-releasing hormone triggers differentiation in HaCaT keratinocytesBr J Dermatol2005152347448015787816
- KimJEChoDHKimHSExpression of the corticotropin-releasing hormone-proopiomelanocortin axis in the various clinical types of psoriasisExp Dermatol200716210410917222223
- ZbytekBMysliwskiASlominskiACorticotropin-releasing hormone affects cytokine production in human HaCaT keratinocytesLife Sci20027091013102111860150
- AdamzikKMcAleerMAKirbyBAlcohol and psoriasis: sobering thoughtsClin Exp Dermatol201338881982224252076
- HigginsEAlcohol, smoking and psoriasisClin Exp Dermatol200025210711010733631
- ZhuKJZhuCYFanYMAlcohol consumption and psoriatic risk: a meta-analysis of case-control studiesJ Dermatol201239977077322568495
- FarkasAKemenyLPsoriasis and alcohol: is cutaneous ethanol one of the missing links?Br J Dermatol2010162471171619922527
- HayesJKooJPsoriasis: depression, anxiety, smoking, and drinking habitsDermatol Ther201023217418020415825
- NaldiLChatenoudLLinderDCigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control studyJ Invest Dermatol20051251616715982303
- JinYYangSZhangFCombined effects of HLA-Cw6 and cigarette smoking in psoriasis vulgaris: a hospital-based case-control study in ChinaJ Eur Acad Dermatol Venereol200923213213718702622
- YinXYChengHWangWJTNIP1/ANXA6 and CSMD1 variants interacting with cigarette smoking, alcohol intake affect risk of psoriasisJ Dermatol Sci2013702949823541940
- ArmstrongAWHarskampCTArmstrongEJThe association between psoriasis and obesity: a systematic review and meta-analysis of observational studiesNutr Diabetes20122e5423208415
- SterryWStroberBEMenterAInternational Psoriasis CouncilObesity in psoriasis: the metabolic, clinical and therapeutic implications. Report of an interdisciplinary conference and reviewBr J Dermatol2007157464965517627791
- CaoLYSolerDCDebanneSMPsoriasis and cardiovascular risk factors: increased serum myeloperoxidase and corresponding immunocellular overexpression by Cd11b(+) CD68(+) macrophages in skin lesionsAm J Transl Res201361162724349618
- JohnstonAArnadottirSGudjonssonJEObesity in psoriasis: leptin and resistin as mediators of cutaneous inflammationBr J Dermatol2008159234235018547319
- NowlinNSolomonHLetter: Weight loss and psoriasisArch Dermatol1976112101465
- Perez-PerezLAllegueFCaeiroJLZulaicaJMSevere psoriasis, morbid obesity and bariatric surgeryClin Exp Dermatol2009347e421e42219747301
- ZackheimHSFarberEMRapid weight reduction and psoriasisArch Dermatol197110321361404928232
- ChillerKSelkinBAMurakawaGJSkin microflora and bacterial infections of the skinJ Investig Dermatol Symp Proc200163170174
- CogenALNizetVGalloRLSkin microbiota: a source of disease or defence?Br J Dermatol2008158344245518275522
- FredricksDNMicrobial ecology of human skin in health and diseaseJ Investig Dermatol Symp Proc200163167169
- GriceEAKongHHRenaudGA diversity profile of the human skin microbiotaGenome Res20081871043105018502944
- GriceEAKongHHConlanSTopographical and temporal diversity of the human skin microbiomeScience200932459311190119219478181
- FahlenAEngstrandLBakerBSPowlesAFryLComparison of bacterial microbiota in skin biopsies from normal and psoriatic skinArch Dermatol Res20123041152222065152
- GaoZTsengCHStroberBEPeiZBlaserMJSubstantial alterations of the cutaneous bacterial biota in psoriatic lesionsPLoS One200837e271918648509
- PaulinoLCTsengCHBlaserMJAnalysis of Malassezia microbiota in healthy superficial human skin and in psoriatic lesions by multiplex real-time PCRFEMS Yeast Res20088346047118294199
- PaulinoLCTsengCHStroberBEBlaserMJMolecular analysis of fungal microbiota in samples from healthy human skin and psoriatic lesionsJ Clin Microbiol20064482933294116891514
- TomiNSKrankeBAbererEStaphylococcal toxins in patients with psoriasis, atopic dermatitis, and erythroderma, and in healthy control subjectsJ Am Acad Dermatol2005531677215965423
- AlekseyenkoAVPerez-PerezGIDe SouzaACommunity differentiation of the cutaneous microbiota in psoriasisMicrobiome2013113124451201
- DiluvioLVollmerSBesgenPIdentical TCR beta-chain rearrangements in streptococcal angina and skin lesions of patients with psoriasis vulgarisJ Immunol2006176117104711116709873
- BunseTMahrleGSoluble Pityrosporum-derived chemoattractant for polymorphonuclear leukocytes of psoriatic patientsActa Derm Venereol199676110128721481
- ElewskiBDoes pityrosporum ovale have a role in psoriasis?Arch Dermatol19901268111111122383044
- LoberCWBelewPWRosenbergEWBaleGPatch tests with killed sonicated microflora in patients with psoriasisArch Dermatol198211853223256211147
- ProhicAIdentification of Malassezia species isolated from scalp skin of patients with psoriasis and healthy subjectsActa Dermatovenerol Croat2003111101612718790
- BaroniAOrlandoMDonnarummaGToll-like receptor 2 (TLR2) mediates intracellular signalling in human keratinocytes in response to Malassezia furfurArch Dermatol Res2006297728028816283346
- BaroniAPaolettiIRuoccoEPossible role of Malassezia furfur in psoriasis: modulation of TGF-beta1, integrin, and HSP70 expression in human keratinocytes and in the skin of psoriasis-affected patientsJ Cutan Pathol2004311354214675283
- TakahataYSugitaTHirumaMMutoMQuantitative analysis of Malassezia in the scale of patients with psoriasis using a real-time polymerase chain reaction assayBr J Dermatol2007157467067317634085
- ZomorodianKMirhendiHTarazooieBDistribution of Malassezia species in patients with psoriasis and healthy individuals in Tehran, IranJ Cutan Pathol200835111027103118616765
- FarrPMKrauseLBMarksJMShusterSResponse of scalp psoriasis to oral ketoconazoleLancet1985284629219222865422
- RosenbergEWBelewPWImprovement of psoriasis of the scalp with ketoconazoleArch Dermatol198211863703716284060
- Taheri SarvtinMShokohiTHajheydariZYazdaniJHedayatiMTEvaluation of candidal colonization and specific humoral responses against Candida albicans in patients with psoriasisInt J Dermatol20145312e555e56025427068
- WaldmanAGilharADuekLBerdicevskyIIncidence of Candida in psoriasis – a study on the fungal flora of psoriatic patientsMycoses2001443–4778111413927
- MaciasESPereiraFARietkerkWSafaiBSuperantigens in dermatologyJ Am Acad Dermatol201164345547221315950
- MohandasVBallalMDistribution of Candida species in different clinical samples and their virulence: biofilm formation, proteinase and phospholipase production: a study on hospitalized patients in southern IndiaJ Glob Infect Dis2011314821572601
- NemethTMocsaiAThe role of neutrophils in autoimmune diseasesImmunol Lett2012143191922342996
- ToichiETachibanaTFurukawaFRapid improvement of psoriasis vulgaris during drug-induced agranulocytosisJ Am Acad Dermatol2000432 Pt 239139510901732
- SchroderJMMrowietzUChristophersEIdentification of different charged species of a human monocyte derived neutrophil activating peptide (MONAP)Biochem Biophys Res Commun198815212772843282512
- SchroderJMMrowietzUChristophersEPurification and partial biologic characterization of a human lymphocyte-derived peptide with potent neutrophil-stimulating activityJ Immunol198814010353435403283239
- BaggioliniMWalzAKunkelSLNeutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophilsJ Clin Invest1989844104510492677047
- ArnaoutMAWangEAClarkSCSieffCAHuman recombinant granulocyte-macrophage colony-stimulating factor increases cell-to-cell adhesion and surface expression of adhesion-promoting surface glycoproteins on mature granulocytesJ Clin Invest19867825976013090106
- GassonJCWeisbartRHKaufmanSEPurified human granulocyte-macrophage colony-stimulating factor: direct action on neutrophilsScience19842264680133913426390681
- ClarkSCKamenRThe human hematopoietic colony-stimulating factorsScience19872364806122912373296190
- SchonMDenzerDKubitzaRCRuzickaTSchonMPCritical role of neutrophils for the generation of psoriasiform skin lesions in flaky skin miceJ Invest Dermatol2000114597698310771480
- BrinkmannVReichardUGoosmannCNeutrophil extracellular traps kill bacteriaScience200430356631532153515001782
- LinAMRubinCJKhandpurRMast cells and neutrophils release IL-17 through extracellular trap formation in psoriasisJ Immunol2011187149050021606249
- SchubertCChristophersEMast cells and macrophages in early relapsing psoriasisArch Dermatol Res198527753523584026377
- BrodyIMast cell degranulation in the evolution of acute eruptive guttate psoriasis vulgarisJ Invest Dermatol19848254604646512269
- HarvimaITNaukkarinenAHarvimaRJQuantitative enzyme-histochemical analysis of tryptase- and chymase-containing mast cells in psoriatic skinArch Dermatol Res199028274284331706585
- HarvimaITNaukkarinenAPaukkonenKMast cell tryptase and chymase in developing and mature psoriatic lesionsArch Dermatol Res199328541841928342961
- IversenOJLysvandHJacobsenTBerghKLieBA. The psoriasis-associated antigen, pso p27, is expressed by tryptase-positive cells in psoriatic lesionsArch Dermatol Res199528755035057625863
- ToyrySFrakiJTammiRMast cell density in psoriatic skin. The effect of PUVA and corticosteroid therapyArch Dermatol Res198828052822853178285
- KrogstadALLonnrothPLarsonGWallinBGIncreased interstitial histamine concentration in the psoriatic plaqueJ Invest Dermatol199710956326359347790
- PetersenLJHansenUKristensenJKStudies on mast cells and histamine release in psoriasis: the effect of ranitidineActa Derm Venereol19987831901939602224
- AckermannLHarvimaITPelkonenJMast cells in psoriatic skin are strongly positive for interferon-gammaBr J Dermatol1999140462463310233311
- ToruniowaBJablonskaSMast cells in the initial stages of psoriasisArch Dermatol Res198828041891933233011
- StellatoCde PaulisACiccarelliAAnti-inflammatory effect of cyclosporin A on human skin mast cellsJ Invest Dermatol19929858008041373749
- YamamotoTMatsuuchiMIrimajiriJOtoyamaKNishiokaKTopical anthralin for psoriasis vulgaris: evaluation of 70 Japanese patientsJ Dermatol200027748248510935350
- MashikoSBouguermouhSRubioMHuman mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitisJ Allergy Clin Immunol20151362351359.e125792465
- ZabaLCKruegerJGLowesMAResident and “inflammatory” dendritic cells in human skinJ Invest Dermatol2009129230230818685620
- LowesMAChamianFAbelloMVIncrease in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a)Proc Natl Acad Sci U S A200510252190571906216380428
- WagnerEFSchonthalerHBGuinea-ViniegraJTschachlerEPsoriasis: what we have learned from mouse modelsNat Rev Rheumatol201061270471420877306
- JariwalaSPThe role of dendritic cells in the immunopathogenesis of psoriasisArch Dermatol Res2007299835936617680257
- ToebakMJGibbsSBruynzeelDPScheperRJRustemeyerTDendritic cells: biology of the skinContact Dermatitis200960122019125717
- SereKBaekJHOber-BlobaumJTwo distinct types of Langerhans cells populate the skin during steady state and inflammationImmunity201237590591623159228
- RomaniNBrunnerPMStinglGChanging views of the role of Langerhans cellsJ Invest Dermatol20121323 Pt 287288122217741
- GlitznerEKorosecABrunnerPMSpecific roles for dendritic cell subsets during initiation and progression of psoriasisEMBO Mol Med20146101312132725216727
- UlsenheimerAGerlachJTJungMCPlasmacytoid dendritic cells in acute and chronic hepatitis C virus infectionHepatology200541364365115726647
- ReizisBBuninAGhoshHSLewisKLSisirakVPlasmacytoid dendritic cells: recent progress and open questionsAnnu Rev Immunol20112916318321219184
- RobersonEDLiuYRyanCA subset of methylated CpG sites differentiate psoriatic from normal skinJ Invest Dermatol20121323 Pt 158359222071477
- NestleFOConradCTun-KyiAPlasmacytoid predendritic cells initiate psoriasis through interferon-alpha productionJ Exp Med2005202113514315998792
- AlbanesiCScarponiCBosisioDSozzaniSGirolomoniGImmune functions and recruitment of plasmacytoid dendritic cells in psoriasisAutoimmunity201043321521920166874
- ChuCCDi MeglioPNestleFOHarnessing dendritic cells in inflammatory skin diseasesSemin Immunol2011231284121295490
- NestleFOTurkaLANickoloffBJCharacterization of dermal dendritic cells in psoriasis. Autostimulation of T lymphocytes and induction of Th1 type cytokinesJ Clin Invest19949412022098040262
- ZabaLCFuentes-DuculanJSteinmanRMKruegerJGLowesMANormal human dermis contains distinct populations of CD11c+ BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophagesJ Clin Invest200711792517252517786242
- BarralDCBrennerMBCD1 antigen presentation: how it worksNat Rev Immunol200771292994118037897
- ZabaLCFuentes-DuculanJEungdamrongNJPsoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cellsJ Invest Dermatol20091291798818633443
- ZabaLCCardinaleIGilleaudeauPAmelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responsesJ Exp Med2007204133183319418039949
- ZabaLCFuentes-DuculanJEungdamrongNJIdentification of TNF-related apoptosis-inducing ligand and other molecules that distinguish inflammatory from resident dendritic cells in patients with psoriasisJ Allergy Clin Immunol2010125612611268.e920471070
- HanselAGüntherCIngwersenJHuman slan (6-sulfo LacNAc) dendritic cells are inflammatory dermal dendritic cells in psoriasis and drive strong TH17/TH1 T-cell responsesJ Allergy Clin Immunol20111273787794e1e921377044
- ClarkRASkin-resident T cells: the ups and downs of on site immunityJ Invest Dermatol2010130236237019675575
- ClarkRAResident memory T cells in human health and diseaseSci Transl Med20157269269rv1
- ClarkRAChongBMirchandaniNThe vast majority of CLA+ T cells are resident in normal skinJ Immunol200617674431443916547281
- KryczekIBruceATGudjonssonJEInduction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasisJ Immunol200818174733474118802076
- GudjonssonJEJohnstonASigmundsdottirHValdimarssonHImmunopathogenic mechanisms in psoriasisClin Exp Immunol200435118
- GudjonssonJEThorarinssonAMSigurgeirssonBKristinssonKGValdimarssonHStreptococcal throat infections and exacerbation of chronic plaque psoriasis: a prospective studyBr J Dermatol2003149353053414510985
- LeungDYTraversJBGiornoREvidence for a streptococcal superantigen-driven process in acute guttate psoriasisJ Clin Invest1995965210621127593594
- LeungDYTraversJBNorrisDAThe role of superantigens in skin diseaseJ Invest Dermatol19951051 Suppl37S42S7615995
- PrinzJCPsoriasis vulgaris – a sterile antibacterial skin reaction mediated by cross-reactive T cells? An immunological view of the pathophysiology of psoriasisClin Exp Dermatol200126432633211422184
- Suarez-FarinasMFuentes-DuculanJLowesMAKruegerJGResolved psoriasis lesions retain expression of a subset of disease-related genesJ Invest Dermatol2011131239140020861854
- ClarkRAGone but not forgotten: lesional memory in psoriatic skinJ Invest Dermatol2011131228328521228808
- ZhouLIvanovIISpolskiRIL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathwaysNat Immunol20078996797417581537
- ChenZO’SheaJJTh17 cells: a new fate for differentiating helper T cellsImmunol Res20084128710218172584
- BrownellISexy and 17: TH17 effector T cells and psoriasisJ Drugs Dermatol20076885385617763621
- LowesMAKikuchiTFuentes-DuculanJPsoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cellsJ Invest Dermatol200812851207121118200064
- MaHLLiangSLiJIL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammationJ Clin Invest2008118259760718202747
- NogralesKEZabaLCGuttman-YasskyETh17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathwaysBr J Dermatol200815951092110218684158
- MosmannTRCherwinskiHBondMWGiedlinMACoffmanRLTwo types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteinsJ Immunol19861367234823572419430
- Infante-DuarteCHortonHFByrneMCKamradtTMicrobial lipopeptides induce the production of IL-17 in Th cellsJ Immunol2000165116107611511086043
- HarringtonLEHattonRDManganPRInterleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineagesNat Immunol20056111123113216200070
- ParkHLiZYangXOA distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17Nat Immunol20056111133114116200068
- NickoloffBJCracking the cytokine code in psoriasisNat Med200713324224417342112
- ClarkRAKupperTSMisbehaving macrophages in the pathogenesis of psoriasisJ Clin Invest200611682084208716886055
- SugiyamaHGyulaiRToichiEDysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferationJ Immunol2005174116417315611238
- PeternelSKastelanMImmunopathogenesis of psoriasis: focus on natural killer T cellsJ Eur Acad Dermatol Venereol200923101123112719453772
- OttavianiCNasorriFBediniCCD56brightCD16(-) NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammationEur J Immunol200636111812816323244
- LaggnerUDi MeglioPPereraGKIdentification of a novel proinflammatory human skin-homing Vgamma9Vdelta2 T cell subset with a potential role in psoriasisJ Immunol201118752783279321813772
- TakedaHOkuboYKogaMAizawaKLipid analysis of peripheral blood monocytes in psoriatic patients using Fourier-transform infrared microspectroscopyJ Dermatol200128630331111476108
- Bar-EliMGallilyRCohenHAWahbaAMonocyte function in psoriasisJ Invest Dermatol1979732147149379240
- BrunnerPMKoszikFReiningerBInfliximab induces downregulation of the IL-12/IL-23 axis in 6-sulfo-LacNac (slan)+ dendritic cells and macrophagesJ Allergy Clin Immunol2013132511841193e823890755
- GoldenJBGroftSGSquerieMVChronic Psoriatic Skin Inflammation Leads to Increased Monocyte Adhesion and AggregationJ Immunol201519552006201826223654
- RogacevKSCremersBZawadaAMCD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiographyJ Am Coll Cardiol201260161512152022999728
- ZawadaAMRogacevKSRotterBSuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subsetBlood201111812e50e6121803849
- ZawadaAMRogacevKSSchirmerSHMonocyte heterogeneity in human cardiovascular diseaseImmunobiology2012217121273128422898391
- van den OordJJde Wolf-PeetersCEpithelium-lining macrophages in psoriasisBr J Dermatol199413055895947515638
- VestergaardCJustHBaumgartner NielsenJThestrup-PedersenKDeleuranMExpression of CCR2 on monocytes and macrophages in chronically inflamed skin in atopic dermatitis and psoriasisActa Derm Venereol200484535335815370700
- StratisAPasparakisMRupecRAPathogenic role for skin macrophages in a mouse model of keratinocyte-induced psoriasis-like skin inflammationJ Clin Invest200611682094210416886058
- WangHPetersTKessDActivated macrophages are essential in a murine model for T cell-mediated chronic psoriasiform skin inflammationJ Clin Invest200611682105211416886059
- KennedyBCShimatoSAndersonRCBruceJNDefining the mechanisms of CD8 T-cell tumor toleranceImmunotherapy2011312326
- NagarajSGabrilovichDIRegulation of suppressive function of myeloid-derived suppressor cells by CD4+ T cellsSemin Cancer Biol201222428228822313876
- SolitoSPintonLDamuzzoVMandruzzatoSHighlights on molecular mechanisms of MDSC-mediated immune suppression: paving the way for new working hypothesesImmunol Invest2012416–772273723017143
- HoechstBOrmandyLABallmaierMA new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cellsGastroenterology2008135123424318485901
- GabrilovichDIBronteVChenSHThe terminology issue for myeloid-derived suppressor cellsCancer Res2007671425 author reply 42617210725
- OstaninDVBhattacharyaDMyeloid-derived suppressor cells in the inflammatory bowel diseasesInflamm Bowel Dis201319112468247723811636
- XiQLiYDaiJChenWHigh frequency of mononuclear myeloid- derived suppressor cells is associated with exacerbation of inflammatory bowel diseaseImmunol Invest201544327928725775229
- MenterAThe status of biologic therapies in the treatment of moderate to severe psoriasisCutis2009844 Suppl142419916298
- LawsPMYoungHSTopical treatment of psoriasisExpert Opin Pharmacother201011121999200920569091
- MasonARMasonJCorkMDooleyGEdwardsGTopical treatments for chronic plaque psoriasisCochrane Database Syst Rev20092CD00502819370616
- NastAKoppIBAugustinMS3-Leitlinie zur Therapie der Psoriasis vulgaris. [S3-Guidelines for the therapy of psoriasis vulgaris]J Dtsch Dermatol Ges20064Suppl 2S1S126 German17187649
- RaboobeeNAboobakerJJordaanHFGuideline on the management of psoriasis in South AfricaS Afr Med J2010104 Pt 2257282
- ZeichnerJALebwohlMPotential complications associated with the use of biologic agents for psoriasisDermatol Clin2007252207213vii17430757
- YamashitaMKatsumataMIwashimaMT cell receptor-induced calcineurin activation regulates T helper type 2 cell development by modifying the interleukin 4 receptor signaling complexJ Exp Med2000191111869187910839803
- FedermanDGFroelichCWKirsnerRSTopical psoriasis therapyAm Fam Physician199959495796296410068717
- WeinsteinGDTazarotene gel: efficacy and safety in plaque psoriasisJ Am Acad Dermatol1997372 Pt 3S33S389270554
- ChandraratnaRATazarotene: the first receptor-selective topical retinoid for the treatment of psoriasisJ Am Acad Dermatol1997372 Pt 3S12S179270551
- Esgleyes-RibotTChandraratnaRALew-KayaDASeftonJDuvicMResponse of psoriasis to a new topical retinoid, AGN 190168J Am Acad Dermatol19943045815907512583
- MenterAGottliebAFeldmanSRGuidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologicsJ Am Acad Dermatol200858582685018423260
- PathiranaDOrmerodADSaiagPEuropean S3-guidelines on the systemic treatment of psoriasis vulgarisJ Eur Acad Dermatol Venereol200923Suppl 2170
- ZanolliMThe modern paradigm of phototherapyClin Dermatol200321539840614678720
- GollnickHPOral retinoids – efficacy and toxicity in psoriasisBr J Dermatol1996135Suppl 49617
- SbidianEMazaAMontaudiéHEfficacy and safety of oral retinoids in different psoriasis subtypes: a systematic literature reviewJ Eur Acad Dermatol Venereol201125Suppl 2283321388456
- BelgeKBruckJGhoreschiKAdvances in treating psoriasisF1000Prime Rep20146424592316
- CarreteroGPuigLDehesaLGuidelines on the use of methotrexate in psoriasisActas Dermosifiliogr20101017600613
- MontaudieHSbidianEPaulCMethotrexate in psoriasis: a systematic review of treatment modalities, incidence, risk factors and monitoring of liver toxicityJ Eur Acad Dermatol Venereol201125Suppl 21218
- HeydendaelVMSpulsPIOpmeerBCMethotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasisN Engl J Med2003349765866512917302
- BantleJPBoudreauRJFerrisTFSuppression of plasma renin activity by cyclosporineAm J Med198783159643300326
- CurtisJJLukeRGJonesPDiethelmAGHypertension in cyclosporine-treated renal transplant recipients is sodium dependentAm J Med19888521341383041828
- FellstromBCyclosporine nephrotoxicityTransplant Proc2004362 Suppl220S223S15041341
- JullienDPrinzJCLangleyRGT-cell modulation for the treatment of chronic plaque psoriasis with efalizumab (Raptiva): mechanisms of actionDermatology2004208429730615178911
- BonnekohBBöckelmannRPommerAJThe CD11a binding site of efalizumab in psoriatic skin tissue as analyzed by Multi-Epitope Ligand Cartography robot technology. Introduction of a novel biological drug-binding biochip assaySkin Pharmacol Physiol20072029611117167274
- MenterATyringSKGordonKAdalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trialJ Am Acad Dermatol200858110611517936411
- ChaudhariURomanoPMulcahyLDEfficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trialLancet200135792711842184711410193
- MeasePJGoffeBSMetzJEtanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trialLancet2000356922738539010972371
- CollamerANBattafaranoDFPsoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesisSemin Arthritis Rheum201040323324020580412
- KoJMGottliebABKerbleskiJFInduction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 casesJ Dermatolog Treat200920210010818923992
- ScheinfeldNA comprehensive review and evaluation of the side effects of the tumor necrosis factor alpha blockers etanercept, infliximab and adalimumabJ Dermatolog Treat200415528029415370396
- Di CesareADi MeglioPNestleFOThe IL-23/Th17 axis in the immunopathogenesis of psoriasisJ Invest Dermatol200912961339135019322214
- LeonardiCLKimballABPappKAEfficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1)Lancet200837196251665167418486739
- GordonKBPappKALangleyRGLong-term safety experience of ustekinumab in patients with moderate to severe psoriasis (Part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trialsJ Am Acad Dermatol201266574275121978572
- HarperEGGuoCRizzoHTh17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesisJ Invest Dermatol200912992175218319295614
- ZhangLYangXQChengJHuiRSGaoTWIncreased Th17 cells are accompanied by FoxP3(+) Treg cell accumulation and correlated with psoriasis disease severityClin Immunol2010135110811720006553
- PappKALangleyRGSigurgeirssonBEfficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging studyBr J Dermatol2013168241242123106107
- LeonardiCMathesonRZachariaeCAnti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasisN Engl J Med2012366131190119922455413
- PappKALeonardiCMenterABrodalumab, an anti-interleukin-17-receptor antibody for psoriasisN Engl J Med2012366131181118922455412
- TausendWDowningCTyringSSystematic review of interleukin-12, interleukin-17, and interleukin-23 pathway inhibitors for the treatment of moderate-to-severe chronic plaque psoriasis: ustekinumab, briakinumab, tildrakizumab, guselkumab, secukinumab, ixekizumab, and brodalumabJ Cutan Med Surg201418315616924800703
- ChangelianPSFlanaganMEBallDJPrevention of organ allograft rejection by a specific Janus kinase 3 inhibitorScience2003302564687587814593182
- GhoreschiKLaurenceAO’SheaJJJanus kinases in immune cell signalingImmunol Rev2009228127328719290934
- FridmanJSScherlePACollinsRSelective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050J Immunol201018495298530720363976
- GhoreschiKJessonMILiXModulation of innate and adaptive immune responses by tofacitinib (CP-690,550)J Immunol201118674234424321383241
- TraynorKFDA approves tofacitinib for rheumatoid arthritisAm J Health Syst Pharm201269242120
- PappKAMenterAStroberBEfficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a Phase 2b randomized placebo-controlled dose-ranging studyBr J Dermatol2012167366867722924949
- PortsWCKhanSLanSA randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasisBr J Dermatol2013169113714523387374
- StrimbuKTavelJAWhat are biomarkers?Curr Opin HIV AIDS20105646346620978388
- FantuzziGAdipose tissue, adipokines, and inflammationJ Allergy Clin Immunol2005115591191915867843
- TakemuraYWalshKOuchiNAdiponectin and cardiovascular inflammatory responsesCurr Atheroscler Rep20079323824318241619
- TilgHMoschenARRole of adiponectin and PBEF/visfatin as regulators of inflammation: involvement in obesity-associated diseasesClin Sci (Lond)2008114427528818194136
- CoimbraSOliveiraHNeuparthMJMetabolic syndrome in psoriasis patients. Residual inflammation and pro-inflammatory IL17 signaling reduce length of remission. A follow-up studyBr J Dermatol Epub201574
- KaurSZilmerKKairaneCKalsMZilmerMClear differences in adiponectin level and glutathione redox status revealed in obese and normal-weight patients with psoriasisBr J Dermatol200815961364136718652586
- TakahashiHTakahashiIHonmaMIshida-YamamotoAIizukaHPrevalence of metabolic syndrome in Japanese psoriasis patientsJ Dermatol Sci201057214314420005080
- BoehnckeSThaciDBeschmannHPsoriasis patients show signs of insulin resistanceBr J Dermatol200715761249125117916217
- NakajimaHNakajimaKTarutaniMMorishigeRSanoSKinetics of circulating Th17 cytokines and adipokines in psoriasis patientsArch Dermatol Res2011303645145521681565
- BokarewaMNagaevIDahlbergLSmithUTarkowskiAResistin, an adipokine with potent proinflammatory propertiesJ Immunol200517495789579515843582
- BoehnckeSSalgoRGarbaravicieneJEffective continuous systemic therapy of severe plaque-type psoriasis is accompanied by amelioration of biomarkers of cardiovascular risk: results of a prospective longitudinal observational studyJ Eur Acad Dermatol Venereol201125101187119321241371
- TakahashiHTsujiHHonmaMIshida-YamamotoAIizukaHIncreased plasma resistin and decreased omentin levels in Japanese patients with psoriasisArch Dermatol Res2013305211311623291856
- CoimbraSOliveiraHReisFCirculating levels of adiponectin, oxidized LDL and C-reactive protein in Portuguese patients with psoriasis vulgaris, according to body mass index, severity and duration of the diseaseJ Dermatol Sci200955320220419576730
- CoimbraSOliveiraHReisFC-reactive protein and leucocyte activation in psoriasis vulgaris according to severity and therapyJ Eur Acad Dermatol Venereol201024778979620002653
- del RinconIDWilliamsKSternMPFreemanGLEscalanteAHigh incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factorsArthritis Rheum200144122737274511762933
- RhewEYRamsey-GoldmanRPremature atherosclerotic disease in systemic lupus erythematosus – role of inflammatory mechanismsAutoimmun Rev20065210110516431336
- AinaliCValeyevNPereraGTranscriptome classification reveals molecular subtypes in psoriasisBMC Genomics20121347222971201
- ValeyevNVHundhausenCUmezawaYA systems model for immune cell interactions unravels the mechanism of inflammation in human skinPLoS Comput Biol2010612e100102421152006
- VyseTJToddJAGenetic analysis of autoimmune diseaseCell19968533113188616887
- FryLBakerBSPowlesAVEngstrandLPsoriasis is not an autoimmune disease?Exp Dermatol201524424124425348334
- BesgenPTrommlerPVollmerSPrinzJCEzrin, maspin, peroxiredoxin 2, and heat shock protein 27: potential targets of a streptococcal-induced autoimmune response in psoriasisJ Immunol201018495392540220363977
- ValdimarssonHThorleifsdottirRHSigurdardottirSLGudjonssonJEJohnstonAPsoriasis – as an autoimmune disease caused by molecular mimicryTrends Immunol2009301049450119781993
- BehrMADivangahiMLalandeJDWhat’s in a name? The (mis) labelling of Crohn’s as an autoimmune diseaseLancet2010376973620220320638565
- BakerBSOvigneJMPowlesAVCorcoranSFryLNormal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasisBr J Dermatol2003148467067912752123
- LienESellatiTJYoshimuraAToll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial productsJ Biol Chem199927447334193342510559223
- KainuKKivinenKZucchelliMAssociation of psoriasis to PGLYRP and SPRR genes at PSORS4 locus on 1q shows heterogeneity between Finnish, Swedish and Irish familiesExp Dermatol200918210911518643845
- SunCMathurPDupuisJPeptidoglycan recognition proteins Pglyrp3 and Pglyrp4 are encoded from the epidermal differentiation complex and are candidate genes for the Psors4 locus on chromosome 1q21Hum Genet20061191–211312516362825
- MakredesMRobinsonDJrBalaMKimballABThe burden of autoimmune disease: a comparison of prevalence ratios in patients with psoriatic arthritis and psoriasisJ Am Acad Dermatol200961340541019700012
- WuJJNguyenTUPoonKYHerrintonLJThe association of psoriasis with autoimmune diseasesJ Am Acad Dermatol201267592493022664308
- HsuLNArmstrongAWPsoriasis and autoimmune disorders: a review of the literatureJ Am Acad Dermatol20126751076107923062896
- QiuZXZhangKQiuXSZhouMLiWMCD226 Gly307Ser association with multiple autoimmune diseases: a meta-analysisHum Immunol201374224925523073294
- Alarcon-RiquelmeMERole of RUNX in autoimmune diseases linking rheumatoid arthritis, psoriasis and lupusArthritis Res Ther20046416917315225361
- OkudaTNishimuraMNakaoMFujitaYRUNX1/AML1: a central player in hematopoiesisInt J Hematol200174325225711721959
- SunHXiaYWangLWangYChangXPSORS1C1 may be involved in rheumatoid arthritisImmunol Lett20131531–291423769905
- GregersenPKOlssonLMRecent advances in the genetics of autoimmune diseaseAnnu Rev Immunol20092736339119302045
- VereeckeLBeyaertRvan LooGThe ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathologyTrends Immunol200930838339119643665
- HueberWPatelDDDryjaTEffects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitisSci Transl Med201025252ra72
- McInnesIBSchettGThe pathogenesis of rheumatoid arthritisN Engl J Med2011365232205221922150039
- VictorFCGottliebABTNF-alpha and apoptosis: implications for the pathogenesis and treatment of psoriasisJ Drugs Dermatol20021326427512851985
- GoodmanWALevineADMassariJVIL-6 signaling in psoriasis prevents immune suppression by regulatory T cellsJ Immunol200918353170317619648274
- GoodmanWAYoungABMcCormickTSCooperKDLevineADStat3 phosphorylation mediates resistance of primary human T cells to regulatory T cell suppressionJ Immunol201118663336334521307288
- OjettiVAguilar SanchezJGuerrieroCHigh prevalence of celiac disease in psoriasisAm J Gastroenterol200398112574257514638373
- BirkenfeldSDreiherJWeitzmanDCohenADCoeliac disease associated with psoriasisBr J Dermatol200916161331133419785615
- SinghSSonkarGKUsha SinghSCeliac disease-associated antibodies in patients with psoriasis and correlation with HLA Cw6J Clin Lab Anal201024426927220626025
- MontesuMADessì-FulgheriCPattaroCAssociation between psoriasis and coeliac disease? A case-control studyActa Derm Venereol2011911929321103827
- Damasiewicz-BodzekAWielkoszynskiTSerologic markers of celiac disease in psoriatic patientsJ Eur Acad Dermatol Venereol20082291055106118384553
- MichaelssonGGerdénBHagforsenEPsoriasis patients with antibodies to gliadin can be improved by a gluten-free dietBr J Dermatol20001421445110651693
- MichaelssonGGerdénBOttossonMPatients with psoriasis often have increased serum levels of IgA antibodies to gliadinBr J Dermatol199312966676738286249
- KiaKFNairRPIkeRWPrevalence of antigliadin antibodies in patients with psoriasis is not elevated compared with controlsAm J Clin Dermatol20078530130517902732
- DenhamJMHillIDCeliac disease and autoimmunity: review and controversiesCurr Allergy Asthma Rep201313434735323681421
- LudvigssonJFLindelofBZingoneFCiacciCPsoriasis in a nationwide cohort study of patients with celiac diseaseJ Invest Dermatol2011131102010201621654830
- CellierCFlobertCCormierCRouxCSchmitzJSevere osteopenia in symptom-free adults with a childhood diagnosis of coeliac diseaseLancet2000355920680610711931
- HeinGAbendrothKMullerAWesselGStudies on psoriatic osteopathyClin Rheumatol199110113172065500
- HolickMFVitamin D: A millenium perspectiveJ Cell Biochem200388229630712520530
- NilsenEMJahnsenFLLundinKEGluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac diseaseGastroenterology199811535515639721152
- MonteleoneGPalloneFMacDonaldTTChimentiSCostanzoAPsoriasis: from pathogenesis to novel therapeutic approachesClin Sci (Lond)2011120111120846119
- LiuYHelmsCLiaoWA genome-wide association study of psoriasis and psoriatic arthritis identifies new disease lociPLoS Genet200843e100004118369459
- van HeelDAFrankeLHuntKAA genome-wide association study for celiac disease identifies risk variants in the region harboring IL2 and IL21Nat Genet200739782782917558408
- AdamovicSAmundsenSSLieBAAssociation study of IL2/IL21 and FcgRIIa: significant association with the IL2/IL21 region in Scandinavian coeliac disease familiesGenes Immun20089436436718418394
- KappelmanMDRifas-ShimanSLKleinmanKThe prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United StatesClin Gastroenterol Hepatol20075121424142917904915
- YatesVMWatkinsonGKelmanAFurther evidence for an association between psoriasis, Crohn’s disease and ulcerative colitisBr J Dermatol198210633233307066192
- BernsteinCNWajdaABlanchardJFThe clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based studyGastroenterology2005129382783616143122
- WengXLiuLBarcellosLFAllisonJEHerrintonLJClustering of inflammatory bowel disease with immune mediated diseases among members of a northern california-managed care organizationAm J Gastroenterol200710271429143517437504
- LeeFIBellarySVFrancisCIncreased occurrence of psoriasis in patients with Crohn’s disease and their relativesAm J Gastroenterol19908589629632375323
- ScarpaRMangusoFD’ArienzoAMicroscopic inflammatory changes in colon of patients with both active psoriasis and psoriatic arthritis without bowel symptomsJ Rheumatol20002751241124610813294
- FergusonLRHanDYFraserAGIL23R and IL12B SNPs and Haplotypes Strongly Associate with Crohn’s Disease Risk in a New Zealand PopulationGastroenterol Res Pract2010201053946121253534
- CargillMSchrodiSJChangMA large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genesAm J Hum Genet200780227329017236132
- NairRPRuetherAStuartPEPolymorphisms of the IL12B and IL23R genes are associated with psoriasisJ Invest Dermatol200812871653166118219280
- Genetic Analysis of Psoriasis Consortium and the Wellcome Trust Case Control Consortium 2StrangeACaponFA genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1Nat Genet2010421198599020953190
- EllinghausDEllinghausENairRPCombined analysis of genome-wide association studies for Crohn disease and psoriasis identifies seven shared susceptibility lociAm J Hum Genet201290463664722482804
- BraeggerCPNichollsSMurchSHStephensSMacDonaldTTTumour necrosis factor alpha in stool as a marker of intestinal inflammationLancet1992339878589911345871
- MurchSHBraeggerCPWalker-SmithJAMacDonaldTTLocation of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel diseaseGut19933412170517098031350
- OhCJDasKMGottliebABTreatment with anti-tumor necrosis factor alpha (TNF-alpha) monoclonal antibody dramatically decreases the clinical activity of psoriasis lesionsJ Am Acad Dermatol2000425 Pt 182983010775863
- PlevySELandersCJPrehnJA role for TNF-alpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn’s diseaseJ Immunol199715912627662829550432
- TarganSRHanauerSBvan DeventerSJA short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study GroupN Engl J Med199733715102910359321530
- AngelucciECoccoAViscidoAVerniaPCaprilliRAnother paradox in Crohn’s disease: new onset of psoriasis in a patient receiving tumor necrosis factor-alpha antagonistInflamm Bowel Dis20071381059106117387676
- FiorinoGAllezMMalesciADaneseSReview article: anti TNF-alpha induced psoriasis in patients with inflammatory bowel diseaseAliment Pharmacol Ther200929992192719210297
- BaumgartDCSandbornWJCrohn’s diseaseLancet201238098531590160522914295
- BrandSCrohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s diseaseGut20095881152116719592695
- Eastaff-LeungNMabarrackNBarbourACumminsABarrySFoxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel diseaseJ Clin Immunol2010301808919936899
- GalvezJRole of Th17 Cells in the Pathogenesis of Human IBDISRN Inflamm2014201492846125101191
- HovhannisyanZTreatmanJLittmanDRMayerLCharacterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseasesGastroenterology2011140395796521147109
- BovenschenHJvan de KerkhofPCvan ErpPEFoxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A- producing cells and are found in lesional skinJ Invest Dermatol201113191853186021654831
- O’ConnorWJrKamanakaMBoothCJA protective function for interleukin 17A in T cell-mediated intestinal inflammationNat Immunol200910660360919448631
- AtreyaRMudterJFinottoSBlockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivoNat Med20006558358810802717
- ItoHTakazoeMFukudaYA pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s diseaseGastroenterology2004126498999615057738
- TokuraYExtrinsic and intrinsic types of atopic dermatitisJ Dermatol Sci20105811720207111
- Guttman-YasskyENogralesKEKruegerJGContrasting pathogenesis of atopic dermatitis and psoriasis – part I: clinical and pathologic conceptsJ Allergy Clin Immunol201112751110111821388665
- GittlerJKKruegerJGGuttman-YasskyEAtopic dermatitis results in intrinsic barrier and immune abnormalities: implications for contact dermatitisJ Allergy Clin Immunol2013131230031322939651
- WingeMCSunesonJLysellJLack of association between filaggrin gene mutations and onset of psoriasis in childhoodJ Eur Acad Dermatol Venereol2013271e124e12722182180
- HuZXiongZXuXLoss-of-function mutations in filaggrin gene associate with psoriasis vulgaris in Chinese populationHum Genet201213171269127422407025
- WeidingerSWillis-OwenSAKamataniYA genome-wide association study of atopic dermatitis identifies loci with overlapping effects on asthma and psoriasisHum Mol Genet201322234841485623886662
- NomuraIGolevaEHowellMDCytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genesJ Immunol200317163262326912960356
- BoguniewiczMLeungDYRecent insights into atopic dermatitis and implications for management of infectious complicationsJ Allergy Clin Immunol2010125141320109729
- CorkMJDanbySGVasilopoulosYEpidermal barrier dysfunction in atopic dermatitisJ Invest Dermatol200912981892190819494826
- TodaMLeungDYMoletSPolarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesionsJ Allergy Clin Immunol2003111487588112704372
- Guttman-YasskyELowesMAFuentes-DuculanJLow expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasisJ Immunol2008181107420742718981165
- Guttman-YasskyENogralesKEKruegerJGContrasting pathogenesis of atopic dermatitis and psoriasis – part II: immune cell subsets and therapeutic conceptsJ Allergy Clin Immunol201112761420143221419481
- JanjumratsangPPhainupongDChanjanakijskulSRoongphibulsopitPPositive direct immunofluorescence and autoantibody profiles in psoriasis patientsJ Dermatol200835850851318789071
- DuboisELWierzchowieckiMCoxMBWeinerJMDuration and death in systemic lupus erythematosus. An analysis of 249 casesJAMA197422712139914024406022
- ProkuninaLCastillejo-LópezCObergFA regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humansNat Genet200232466666912402038
- TokuhiroSYamadaRChangXAn intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid arthritisNat Genet200335434134814608356
- PerriconeCCiccacciCCeccarelliFTRAF3IP2 gene and systemic lupus erythematosus: association with disease susceptibility and pericarditis developmentImmunogenetics2013651070370923836313
- HuffmeierUUebeSEkiciABCommon variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasisNat Genet2010421199699920953186
- WuLWangCBoissonBThe differential regulation of human ACT1 isoforms by Hsp90 in IL-17 signalingJ Immunol201419341590159925024377
- ChenXQYuYCDengHHPlasma IL-17A is increased in new-onset SLE patients and associated with disease activityJ Clin Immunol201030222122520107878
- WenZXuLXuWInterleukin-17 expression positively correlates with disease severity of lupus nephritis by increasing anti-double-stranded DNA antibody production in a lupus model induced by activated lymphocyte derived DNAPLoS One201383e5816123472149
- YuBGuanMPengYCopy number variations of interleukin-17F, interleukin-21, and interleukin-22 are associated with systemic lupus erythematosusArthritis Rheum201163113487349222038405
- BarretoMFerreiraRCLourençoLLow frequency of CD4+CD25+ Treg in SLE patients: a heritable trait associated with CTLA4 and TGFbeta gene variantsBMC Immunol200910519173720
- FranzBFritzschingBRiehlALow number of regulatory T cells in skin lesions of patients with cutaneous lupus erythematosusArthritis Rheum20075661910192017530636
- PeriYAgmon-LevinNTheodorEShoenfeldYSjogren’s syndrome, the old and the newBest Pract Res Clin Rheumatol201226110511722424197
- HirayamaKShiokawaSMiyazakiYPrimary Sjogren’s syndrome complicated by sarcoidosis and psoriasis vulgarisMod Rheumatol200111435635924383785
- NogitaTAramotoYTerajimaSThe coexistence of psoriasis vulgaris, Sjogren’s syndrome, and Hashimoto’s thyroiditisJ Dermatol19921953023051644955
- Rodriguez de la SernaACasas GassoFDiaz LopezCGieli FerrerCAssociation of Sjogren’s syndrome with psoriatic arthritisCan Med Assoc J19841311113291332
- TanakaHMizutaniHOkadaHShimizuMPrimary Sjogren’s syndrome and psoriasis vulgaris in a case of OKT4 epitope deficiencyJ Dermatol19952242622667541811
- WatanabeMShinoharaMKatayamaIAssociation of psoriasis vulgaris with Sjogren’s syndromeJ Dermatol19982553493509640893
- WhaleyKChisholmDMWilliamsonJSjogren’s syndrome in psoriatic arthritis, ankylosing spondylitis and Reiter’s syndromeActa Rheumatol Scand19711721051145579064
- ItoiSTanemuraATaniMImmunohistochemical Analysis of Interleukin-17 Producing T Helper Cells and Regulatory T Cells Infiltration in Annular Erythema Associated with Sjogren’s SyndromeAnn Dermatol201426220320824882975
- HugginsRHSchwartzRAJannigerCVitiligoActa Dermatovenerol Alp Pannonica Adriat200514413714214413516435042
- Bakar-DertliogluSUcakHCicekDBitirenMCoexistence of vitiligo and psoriasis: three case reportsTurk J Pediatr2012541777922397050
- OisoNOtaTKawaraSKawadaAPustular psoriasis and vitiligo in a patient with Turner syndromeJ Dermatol2007341072772917908149
- ParkJMKimHJBaeBGParkYKA case of concurrent vitiligo and psoriasisAnn Dermatol200921333033320523818
- PercivalleSPiccinnoRCaccialanzaMConcurrence of vitiligo and psoriasis: a simple coincidence?Clin Exp Dermatol2009341909119076803
- PowellFCDickenCHVitiligo and psoriasisJ Am Acad Dermatol198381136137
- SandhuKKaurIKumarBPsoriasis and vitiligoJ Am Acad Dermatol200451114915015243545
- ShethVMGuoYQureshiAAComorbidities associated with vitiligo: a ten-year retrospective studyDermatology2013227431131524107643
- BassiounyDAShakerORole of interleukin-17 in the pathogenesis of vitiligoClin Exp Dermatol201136329229721198791
- BasakPYAdilogluAKCeyhanAMTasTAkkayaVBThe role of helper and regulatory T cells in the pathogenesis of vitiligoJ Am Acad Dermatol200960225626019022528
- JinYMaillouxCMGowanKNALP1 in vitiligo-associated multiple autoimmune diseaseN Engl J Med2007356121216122517377159
