Abstract
Psoriasis vulgaris is a chronic inflammatory disease that classically affects skin and joints and is associated with numerous comorbidities. There are several clinical subtypes of psoriasis including the uncommon pustular variants, which are subdivided into generalized and localized forms. Generalized forms of pustular psoriasis include acute generalized pustular psoriasis, pustular psoriasis of pregnancy, and infantile and juvenile pustular psoriasis. Localized forms include acrodermatitis continua of Hallopeau and palmoplantar pustular psoriasis. These subtypes vary in their presentations, but all have similar histopathologic characteristics. The immunopathogenesis of each entity remains to be fully elucidated and some debate exists as to whether these inflammatory pustular dermatoses should be classified as entities distinct from psoriasis vulgaris. Due to the rarity of these conditions and the questionable link to the common, plaque-type psoriasis, numerous therapies have shown variable results and most entities remain difficult to treat. With increasing knowledge of the pathogenesis of these variants of pustular psoriasis, the development and use of biologic and other immunomodulatory therapies holds promise for the future of successfully treating pustular variants of psoriasis.
Introduction
Psoriasis vulgaris is a chronic inflammatory condition associated with significant morbidity and mortality.Citation1 Plaque psoriasis is the most common form of psoriasis vulgaris and classically presents as discrete, erythematous plaques with an overlying silvery scale on extensor surfaces. Psoriasis can also involve the nails and scalp or progress to erythroderma. Less common clinical presentations include guttate, inverse, or pustular psoriasis.Citation2
Within the classification of pustular psoriasis, the disease is further subdivided into generalized pustular psoriasis (GPP) and localized pustular psoriasis. GPP includes acute GPP, pustular psoriasis of pregnancy, and infantile/juvenile pustular psoriasis. Localized pustular psoriasis includes palmoplantar psoriasis and acrodermatitis continua of Hallopeau (ACH).Citation2–Citation5
All variants of pustular psoriasis have similar presentations in that they involve an eruption of superficial pustules in a given distribution, typically with an erythematous base or studded on a background of erythema. As such, histopathologic examination of all variants reveals parakeratosis, extensive mononuclear and neutrophilic inflammatory infiltrate in the epidermis, and epidermal edema and hyperplasia.Citation5–Citation10 Spongiform pustules of Kogoj and hyperplasia of suprapapillary capillaries may be seen.Citation5,Citation11–Citation13 Munro’s microabscesses typical of plaque psoriasis are frequently seen as well.Citation13
Generalized pustular psoriasis
Acute GPP (von Zumbusch type)
History
Acute GPP, also known as von Zumbusch type, is a dermatologic disease for which classification is somewhat debated.Citation14 Sixty-five percent of cases of acute GPP occur in patients with a prior diagnosis of psoriasis vulgaris,Citation15,Citation16 and historically, acute GPP has been presented as a variant of plaque psoriasis. However, recent research indicates that acute GPP and plaque psoriasis are genetically distinct conditions without a common pathophysiologic mechanism.Citation17
Epidemiology/presentation
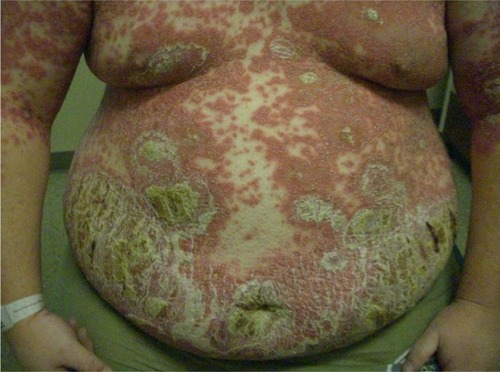
Acute GPP is associated with significant morbidity and in some cases, mortality, especially in the absence of appropriate treatment.Citation16 It is characterized by generalized sterile pustule formation with widespread inflammation and erythema (). The pustules often expand and coalesce, forming lakes of pus.Citation1 Acute GPP is often associated with systemic symptoms such as fever, chills, malaise, anorexia, nausea, and severe pain.Citation1,Citation18 Other physical findings may be present with acute GPP, such as subungual pustules and geographic tongue.Citation1 The systemic nature of the disease is reflected in the laboratory abnormalities found in many patients, including hypocalcemia and hypoalbuminemia, as well as in the possible presence of polyarthralgias and neutrophilic cholangitis-induced cholestasis.Citation19,Citation20
Figure 1 Generalized pustular psoriasis.
The mean age at onset of acute GPP is 40.9 years, with no difference in onset related to the presence or absence of a prior history of psoriasis vulgaris.Citation15 Two separate studies have found a higher incidence of acute GPP in male, with an incidence of 57%–62%.Citation16,Citation20 The majority of studies, however, found a predominance in female, ranging from 53% to 73%.Citation15,Citation19,Citation21,Citation22 Females do tend to have a nonstatistically significant earlier onset compared to male, with a mean age of 39.4 years compared to 44.3 years.Citation15
Pathophysiology
Various precipitating factors have been reported to trigger or flare acute GPP, including corticosteroid use and withdrawal, pregnancy (also termed impetigo herpetiformis), upper respiratory tract infections, stress, nonsteroidal anti-inflammatory drugs, terbinafine, ustekinumab, tumor necrosis factor (TNF)-α inhibitors, and methotrexate.Citation15,Citation23
The use and subsequent withdrawal of systemic corticosteroids is an important cause of an eruption of acute GPP.Citation24 The use of systemic corticosteroids leads to inhibition of the inflammatory system. Acute withdrawal of systemic corticosteroids can lead to an acute inflammatory process acting as a trigger for acute GPP.Citation12 In one retrospective study of 102 patients with acute GPP, systemic glucocorticoids were determined to be a likely trigger for the onset of the disease in 44% of patients.Citation15
Upper respiratory tract infections, and other infections, can also lead to an acute activation of neutrophils, which may act as a trigger for the onset of acute GPP.Citation12 Choon et alCitation15 reported that acute infections have been found to be a trigger or exacerbating factor in 16% of acute GPP patients, with 38.5% of patients exhibiting positive antistreptolysin antibodies. Ustekinumab, a monoclonal antibody against the p40 subunit common to interleukin (IL)-12 and IL-23, which is commonly used for the treatment of plaque psoriasis, can lead to a change in a patient’s psoriasis morphology from plaque psoriasis to pustular psoriasis and can also cause new or worsening acute GPP.Citation25,Citation26 Other drugs, such as terbinafine and methotrexate, may also serve as triggers for acute GPP as a form of drug-specific allergic reactions, which can often be confirmed with patch tests.Citation12,Citation27
GPP may also be strongly associated with certain predisposing genetic factors. In a study of nine Tunisian families with GPP, a genetic mutation in the IL36RN gene leading to an aberrant IL-36 receptor antagonist (IL-36RA) was discovered in all affected patients in the study and was noted to cause an unstable IL-36RA protein with decreased affinity for its receptor.Citation28 The mutation in the IL-36RA protein also led to an increase in proinflammatory cytokines.Citation29 GPP in the setting of this specific genetic mutation has been termed “DITRA” or “deficiency in IL-36RA”.Citation30 The IL36RN gene codes for the IL-36RA molecule. This molecule inhibits the effects of multiple IL-36–associated cytokines, including IL-36α, IL-36β, and IL-36γ, all of which are members of the IL-1 cytokine family.Citation31 These and numerous other cytokines in the IL-1 cytokine family are abundantly expressed in skin.Citation32 When the IL-36R is bound by an agonistic cytokine in the IL-1 family, downstream proinflammatory pathways are activated.Citation30 However, the inhibitory effect of IL-36RA competes with the agonistic IL-36 cytokines for attachment to IL-36R.Citation30 Antagonism of the IL-36R with the IL-36RA results in an anti-inflammatory milieu by the inhibition of the nuclear factor kappa-light-chain-enhancer of activated B cells and mitogen-activated protein kinase signaling pathways and the activation of their downstream inflammatory pathways.Citation30,Citation31,Citation33 Hence, without the anti-inflammatory effect of functional IL-36RA (as is seen in mutations of the IL36RN gene), IL-36 is uninhibited in its ability to enhance the production of proinflammatory mediators, leading to the unopposed effects of IL-36 on nuclear factor kappa-light-chain-enhancer of activated B cells and mitogen-activated protein kinase inflammatory pathways.Citation28,Citation32,Citation34
IL-36R can be located on a number of cells found in the skin, including dendritic cells, keratinocytes, and monocytes.Citation30 IL-36R activation can also stimulate type 1 T-helper cells (TH1 cells) in humans. Activation of IL-36R within these cells leads to activation of inflammation within the skin and development of the clinical features of inflammatory skin conditions, specifically GPP.
GPP and its important genetic association with IL-36RA deficiency has also been shown to exhibit a gene dosage effect. In individuals found to have only monoallelic mutations in the IL36RN gene, the onset of GPP is significantly delayed.Citation31 This delay is perhaps due to these heterozygous patients requiring longer or more intense exposure to the precipitating factors associated with GPP in order to develop the disease.Citation31 However, despite the evidence of a strong correlation between IL-36RA deficiency and GPP, not all patients with GPP have evidence of IL-36RA mutations.
Another study regarding the various possible genetic mutations associated with GPP was conducted in nine children from six families who presented with neonatal sterile osteomyelitis, periostitis, and generalized pustulosis. This study discovered the condition present in these children was linked to a genetic mutation involving IL1RN, which encodes the IL-1 receptor antagonist. The IL-1 receptor antagonist inhibits IL-1α and IL-1β, which are proinflammatory cytokines. A mutation in the gene ILRN leads to the absence of IL-1 receptor antagonist protein, resulting in uninhibited inflammatory actions of IL-1α and IL-1β.Citation35
Treatment
While the treatment of chronic plaque psoriasis has become relatively effective in the era of biologic therapies, treatment of pustular forms of psoriasis remains an area lacking in research, in part due to the rarity of the disease.Citation36 This dearth of research has led to lack of universally accepted, evidence-based guidelines for treatment and management of pustular forms of psoriasis and off-label use of many medications.Citation37 Evaluation of the various treatment options available has yielded limited guidance on selecting therapies for such patients.Citation18 Therapeutic choices for pustular psoriasis are most often based on severity, extent of involvement, and disease morphology.Citation18
Acute GPP has proven to be, like many psoriasis variants, a difficult disease to treat. Guidelines published in 2012 by the Medical Board of the National Psoriasis Foundation recommend acitretin, cyclosporine, or methotrexate to be the first-line therapies for acute GPP ().Citation18 Severe and extensive disease is likely to most effectively be treated with infliximab or cyclosporine, given the quicker onset of action with these drugs.Citation18 Infliximab, a TNF-α inhibitor, has a uniquely rapid onset of therapeutic effect. One study described the efficacy in nine of ten patients with acute GPP who experienced results within 24 hours to 7 days of the first treatment.Citation38 A similar study showed an improvement in all patients with acute GPP at 2 weeks following initiation of infliximab therapy, with disappearance of pustules in all enrolled patients and continued remission at 30 weeks.Citation38,Citation39
Table 1 Treatment of generalized pustular psoriasis
The recommended second-line therapies include adalimumab, etanercept, psoralen plus ultraviolet-A (PUVA) phototherapy, topical corticosteroids, topical calcipotriene, and topical tacrolimus, or combination therapy for recalcitrant disease (which includes a first-line oral systemic agent plus a biologic agent such as adalimumab or etanercept).Citation18,Citation40–Citation45 The initial use of infliximab to treat severe acute disease, followed by treatment with etanercept has also been reported to be successful.Citation18 The use of systemic steroids is generally avoided when treating pustular psoriasis, given the risk of worsening disease following withdrawal of the medication. If necessary, however, they may be used in circumstances in which other therapeutic options are not available.Citation43
In recent years, additional reports of biologic therapies targeting IL-1, IL-12/23p40, and IL-17A have been reported. Anakinra, an IL-1 receptor antagonist, has been described to successfully treat GPP, and gevokizumab, an IL-1β inhibitor, showed some promise in treating two patients with GPP.Citation46–Citation48 Ustekinumab, a monoclonal antibody targeted against the shared p40 subunit of IL-12 and IL-23, has been used to successfully treat GPP, as well.Citation37 Most recently, the biologic therapy secukinumab, an IL-17A inhibitor, has shown promising ability to provide rapid resolution of symptoms in the treatment of GPP.Citation49
The unique treatment of GPP with granulocyte and monocyte adsorption (GMA) apheresis has also been described. Because the immunopathogenesis of GPP involves activated neutrophils and monocytes, GMA provides an effective treatment that involves removal of activated granulocytes and monocytes from the patient’s circulation, resulting in improvement of acute GPP.Citation14,Citation50,Citation51
Despite reports of successful treatment of acute GPP with various biologic agents, the literature supporting the treatment of acute GPP is weak.Citation18 Further exploration with larger, randomized, controlled studies of biological medications in the treatment of acute GPP are needed in order to determine its efficacy and safety on a larger scale.Citation47
Pustular psoriasis of pregnancy
History
The pustular dermatitis, known today as pustular psoriasis of pregnancy, was first described in 1872 by Von Hebra using the term “impetigo herpetiformis”.Citation52 Later, clinical and histological studies of patients with impetigo herpetiformis led to the discovery of similarities between this entity and pustular psoriasis. However, the classification of this dermatitis as a disease distinct from pustular psoriasis is still debated, and in various studies, it continues to be discussed as impetigo herpetiformis.Citation10,Citation53
Epidemiology/presentation
Pustular psoriasis of pregnancy is a rare autoimmune inflammatory dermatosis observed most commonly in the second half to third trimester of pregnancy. Some publications, however, report the occurrence as early as the first month of pregnancy.Citation10,Citation11,Citation54,Citation55 The true incidence of pustular psoriasis of pregnancy is unknown owing to the paucity of published cases.Citation54
Patients typically present with an eruption of numerous sterile pustules overlying annular or polycyclic erythematous patches, with lesions usually beginning within skin folds and spreading radially.Citation53,Citation56 The face, palms, and soles are largely spared.Citation11 Constitutional symptoms may also be present, including fever, chills, malaise, nausea, diarrhea, dehydration, arthralgias, and even tachycardia and seizures.Citation11,Citation53 Common lab findings include leukocytosis with a neutrophilic predominance, anemia, increased erythrocyte sedimentation rate, as well as hypocalcemia, hypophosphatemia, hypoalbuminemia, and decreased vitamin D levels in some cases.Citation5,Citation11,Citation53,Citation57 In reports of more severe disease, hypocalcemia which led to tetany, delirium, and convulsions was observed.Citation55
The presence of pustular psoriasis of pregnancy has been historically associated with poor neonatal outcomes including placental insufficiency, stillbirth, fetal abnormalities, and early neonatal death, with a correlation between severity and duration of the disease and poor neonatal prognosis.Citation11,Citation58,Citation59 Maternal death has also been reported.Citation52 Given this correlation, patients who present with severe or recalcitrant pustular psoriasis of pregnancy are often counseled regarding early induction of labor.Citation10 Following delivery, many patients have dramatic and rapid resolution of their dermatologic disease.Citation5,Citation10,Citation11 However, recurrence of disease in subsequent pregnancies is common.Citation58,Citation60
Pathophysiology
The pathophysiology of pustular psoriasis of pregnancy is even more elusive than in other forms of pustular psoriasis, which is likely due to the low incidence of the disease.Citation53,Citation54 Female with pustular psoriasis of pregnancy seldom report a personal or family history of psoriasis; but in some cases, resolution of the acute pustular form of the disease results in lingering classic psoriatic lesions.Citation55,Citation57 As mentioned, an association between this disease in pregnant female and hypocalcemia or low serum vitamin D levels may be present, but these lab findings are not evident in all patients and there is no evidence to support causality of these abnormal lab values.Citation55
Literature on the theoretical pathogenesis of pustular psoriasis of pregnancy typically discusses possible triggers associated with development of the disease. Potential triggers include increasing levels of progesterone during the last trimester of pregnancy, hypocalcemia, and lower levels of elafin, an epidermal skin-derived antileukoproteinase, which may all contribute to the production of epidermal pustules.Citation61 Associations with hormonal contraception, increased stress levels, seasonal changes, concurrent bacterial infections, and certain medications have been described.Citation53 There have also been reports of the association between pustular psoriasis of pregnancy and hypothyroidism.Citation62
Another important consideration in defining the theoretical pathogenesis of pustular psoriasis of pregnancy involves specific situations in which patients suffer from an acute eruption or worsening of GPP during pregnancy. Pregnancy has been noted as a precipitating factor for the new development or acute flare of GPP.Citation12,Citation16,Citation25,Citation63–Citation65
Treatment
Patients should be assessed by their obstetrician for potential early induction of labor as the first and best therapeutic option for this disease. In terms of therapeutic options, systemic corticosteroids are widely accepted to be the most commonly used first-line treatment for pustular psoriasis of pregnancy ().Citation54,Citation66 Robinson et alCitation18 developed a treatment algorithm for GPP in pregnancy, which included cyclosporine, infliximab, topical corticosteroids, and topical calcipotriene as the first-line treatment options, in addition to systemic prednisone.Citation43,Citation66–Citation69 Case reports regarding the successful use of cyclosporine in this disease are present in the literature.Citation11 An algorithm for second-line treatment options include PUVA and ultraviolet-B (UVB) therapy following delivery.Citation19,Citation70,Citation71 Related to this treatment regimen are published reports of successful use of retinoid photochemotherapy (involving isotretinoin and PUVA) after unsuccessful attempts with first-line therapies and following delivery of the fetus.Citation57 In cases of severe, recalcitrant pustular psoriasis of pregnancy, use of ustekinumab has also been reported as a successful alternative, despite the lack of fetal safety data and the general categorization of biologics as pregnancy category B drugs.Citation72
Table 2 Treatment of pustular psoriasis of pregnancy
As mentioned, delivery of the fetus often results in rapid resolution of the dermatitis, which should be considered in cases of severe disease despite optimal medical management.Citation5,Citation10,Citation11
Infantile and juvenile pustular psoriasis
While psoriasis is a common disease in children and adolescents, pustular psoriasis in patients younger than 18 years is exceedingly rare.Citation73 There are only a few reports in the literature regarding pustular psoriasis in children, owing to its rarity.Citation74 One report reviewing 12 cases of juvenile pustular psoriasis indicates a 2:1 male predominance.Citation75 The average age of onset ranges from 6.6 to 7.6 years.Citation75,Citation76 The diagnosis is typically made based on clinical and histological features.Citation75 Clinically, juvenile pustular psoriasis can present in a diffuse generalized pustular pattern similar to adult GPP, or more commonly, it can present in a circinate or annular pattern.Citation77 The affected children often have systemic findings including fever, as well as lab abnormalities such as leukocytosis and elevated erythrocyte sedimentation rate.Citation75
The etiology and pathogenesis of juvenile pustular psoriasis are unknown.Citation75 The disease has been reported in several sibling or other familial associations, lending to the theory of at least a partial genetic component to the dermatosis.Citation78 Juvenile pustular psoriasis, like other forms of psoriasis, is often associated with identifiable triggers such as infection, vaccination, and corticosteroid withdrawal.Citation79
Treatment
The rarity of pustular psoriasis in children has also led to a lack of evidence-based treatment literature.Citation74 The Medical Board of the National Psoriasis Foundation has published guidelines on the treatment of pustular psoriasis in children. First-line therapy for this disease is similar to that of pustular psoriasis in adults. Acitretin, cyclosporine, etanercept, or methotrexate can each be used safely as first-line therapeutic options in children with pustular psoriasis ().Citation18 Second-line therapeutic options include adalimumab, infliximab, and UVB phototherapy.Citation18 Juvenile pustular psoriasis has a characteristic tendency to recur frequently, and in some cases, it has been reported to recur on an annual basis.Citation75,Citation76
Table 3 Treatment of infantile and juvenile pustular psoriasis
Localized pustular psoriasis
Palmoplantar pustular psoriasis
History
Palmoplantar pustular psoriasis (PPP), also known as palmoplantar pustulosis, is a form of localized pustular psoriasis on the palms and/or soles. This entity was first described in 1888 by Crocker with the name “dermatitis repens”.Citation80 The classification of palmoplantar pustulosis remains an area of debate. Several authors propose that PPP is a distinct entity, while others believe PPP is a variant of psoriasis.Citation2,Citation81
Epidemiology/presentation
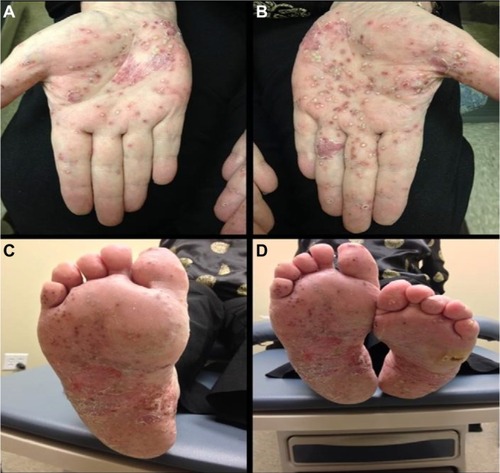
Palmoplantar pustular psoriasis (PPP) presents clinically as sterile pustules with erythema, hyperkeratosis, and scaling on the palms and soles ().Citation82 The disease tends to predominate on the thenar, hypothenar, and central areas of the palms, as well as the corresponding areas of the soles, and it can extend proximally to the patient’s wrists and heels.Citation81,Citation83 The pustular lesions may expand and coalesce before resolving, commonly resulting in a brown discoloration upon resolution.Citation81 PPP affects female more frequently than male.Citation84 A study conducted in Japan comparing the clinical and epidemiological characteristics of PPP and palmoplantar psoriasis found a significant female predominance of 67%.Citation83
Figure 2 Palmoplantar pustular psoriasis.
Pathophysiology
Multiple precipitating factors have been found to predispose patients to the induction of palmoplantar psoriasis, including smoking, metal sensitivities, infections, trauma, stress, and medications.Citation7,Citation82,Citation85–Citation88
Smoking is widely known as one of the most important precipitating factors for the development of PPP. Many studies have shown the impressive association between smoking and PPP, citing the prevalence to be between 33% and 95%.Citation7,Citation9,Citation82,Citation83 Smoking has been found to be related to increased levels of IL-17, propagating inflammation in patients with a predisposition for PPP.Citation89,Citation90 Smoking can also influence the expression of nicotinic acetylcholine receptors which can be found in the acrosyringium. Patients with PPP are thought to have an abnormal response to nicotine, resulting in inflammation with smoking.Citation91 The role of smoking in the pathogenesis of PPP is further supported by the findings that cessation of smoking can lead to improvement or near resolution of PPP.Citation85,Citation86,Citation92,Citation93
Sensitivities to metals have also been cited as precipitating factors in PPP.Citation90,Citation94 The most common metal sensitivity described is to nickel and occurs in 3% of patients with PPP.Citation83 Other metal allergies described include chrome and cobalt.Citation85
Infections are another well-recognized precipitating factor for PPP. The most commonly cited infection resulting in an eruption or exacerbation of PPP is tonsillitis.Citation12 According to Takahara, tonsillectomy in patients resulted in subjective improvement of the disease in 94% and objective improvement of the disease in 88% of patients with PPP.Citation95 Other infections associated with PPP include dental infections such as dentoalveolar abscesses, periodontitis, and dental caries, as well as sinusitis.Citation7,Citation96 The theory that focal infections play a central role in the exacerbation of PPP is supported by evidence of improvement or near resolution of skin findings following resolution of the patient’s infection.Citation7,Citation95,Citation96
The Koebner phenomenon can also be associated with new lesions in PPP.Citation83,Citation91,Citation97 In PPP, mechanical stimulation is commonly caused by shoes, resulting in lesions on the soles of patients predisposed to the development of PPP.Citation86
Stressful psychological conditions also impact the onset and worsening of PPP.Citation87 Results from questionnaires provided to patients with PPP showed that 43% of patients with PPP exhibited behaviors consistent with anxiety, worry, and psychosomatic disorders, compared to only 19% of subjects in the control groups.Citation87 Others have shown that up to 46% of patients report worsening of PPP during periods of stress.Citation9
Furthermore, a seasonal influence has also been described for the onset of PPP with the onset and exacerbation worsened by humid and hot conditions.Citation7,Citation87,Citation98
Perhaps the most frustrating precipitating factor noted for PPP is the association with anti-TNF-α inhibitors.Citation99 The importance of TNF-α-inhibitory therapy as a mainstay for treatment in chronic plaque psoriasis is well known. However, treatment of psoriasis with TNF-α inhibitors can cause a paradoxical flare of pustular psoriasis, especially of the palmoplantar morphology.Citation88 This effect has been noted to be a class effect with most TNF-α blocking medications, rather than with specific agents.Citation88 The mechanism behind the relationship between PPP and TNF-α inhibitor therapy remains to be elucidated.
In support of the theory that PPP is a clinical variant of psoriasis, authors have discussed the morphological, clinical, and histological similarities between PPP and psoriasis.Citation83 YamamotoCitation86 discussed the relationship between PPP and psoriasis with evidence of the eruption of plaque-like lesions on the trunk of individuals diagnosed with PPP. Brunasso et alCitation83 supported the same relationship with a retrospective study comparing two groups of patients, one with PPP and the other with psoriasis, and found close similarities between the groups in age of onset, family history of psoriasis, duration of the disease, comorbidities, and smoking status. Eriksson et alCitation9 discussed similarities regarding the histology of the two diseases, specifically regarding neutrophil dysfunction and specific serum studies including eosinophilic cationic protein elevations and other chemokinetic effects in PPP compared to psoriasis vulgaris.
Authors in support of the theory that PPP is a distinct entity from psoriasis vulgaris argue several different points. Many authors discuss the strong genetic evidence of differences between PPP and psoriasis, specifically, the absence of association with PSORS1 locus in PPP, which is common for psoriasis vulgaris, and the presence of IL-36RA mutations in some patients with PPP, which are lacking in patients with psoriasis vulgaris.Citation1,Citation8,Citation17,Citation100,Citation101 Other authors argue that the strong association between smoking and metal intolerance with PPP that is absent in psoriasis vulgaris warrants distinct classification of the disease.Citation85 PPP is also associated with SAPHO syndrome (synovitis, acne, palmoplantar pustulosis, hyperostosis, and osteitis), a syndrome that does not have an association with psoriasis vulgaris.Citation92
The pathophysiology of PPP remains as obscure as its classification. It is widely accepted, however, that the eccrine sweat gland is significant to its pathogenesis. Specifically, the acrosyringium (the most intraepidermal portion of the eccrine sweat gland consisting of the terminal spiral duct) serves as the primary site of inflammation and pustule formation, as supported by evidence of specific chemicals unique to eccrine sweat glands found in the cells lining the vesicles of PPP patients.Citation89,Citation102 Furthermore, recent studies have begun to emphasize the importance of the innate immune system in the development of PPP. Langerhans cells can be found in increased numbers in both lesional and nonlesional skin of PPP patients, supporting an antigen-driven process for inflammation in the disease.Citation98 The inflammation is further driven by the proliferation of inflammatory cytokines such as IL-8 and IL-17.Citation94 IL-17A is highly expressed in the palms and soles of patients with PPP in comparison to healthy subjects,Citation89 while interestingly, IL-12 and IL-23 are not significantly expressed in these areas of patients with PPP. These findings imply a more central role for IL-17A in the inflammatory process for PPP compared to IL-12/IL-23, a difference which could have important implications for treatment.Citation89
Treatment
Palmoplantar forms of psoriasis are notoriously recalcitrant to therapy.Citation36 Uncertainties regarding the pathophysiology of palmoplantar disease lead to lack of a biogenetic understanding for the development of new pharmacological therapies. Like other pustular psoriasis forms, high-quality data regarding the treatment options and efficacies are lacking for PPP. First-line topical therapy involves corticosteroids under occlusion ().Citation18 Adisen et alCitation97 have described successful use of topical corticosteroids, especially under occlusive dressing, for the prevention of new pustule formation in PPP, as well. Second-line topical therapy includes calcipotriene, PUVA, photodynamic therapy, and tacrolimus.Citation18 Topical treatment, however, uncommonly leads to remission of the disease, and progression to systemic treatment is often required.Citation97 First-line systemic therapy for PPP includes acitretin.Citation18
Table 4 Treatment of palmoplantar pustular psoriasis
Second-line systemic therapy involves the use of cyclosporine and biologic agents. The use of biologic agents in the treatment of PPP is becoming more pervasive, given the recalcitrant nature of the disease. The use of anti-TNF-α blocking agents, including adalimumab, etanercept, and infliximab, in PPP has been reported to be successful in case reports and small studies.Citation82,Citation103–Citation105 Morales-Munera et alCitation82 have described the successful use of ustekinumab in five patients with severe, refractory palmoplantar pustulosis, all of whom experienced positive results in 2–3 weeks after the first dose and complete resolution at 20 weeks after therapy. Cases of PPP successfully treated with efalizumab, an anti-leukocyte function-associated antigen-1 monoclonal antibody, have also been reported.Citation88,Citation106 However, efalizumab has since been removed from the market secondary to concerns regarding progressive multifocal leukoencephalopathy.Citation36
Acrodermatitis continua of Hallopeau
History
ACH is another localized form of pustular psoriasis which is historically described using a number of various aliases including acrodermatitis perstans, dermatitis repens, acropustulosis, and pustular acrodermatitis.Citation107,Citation108 The classification of ACH has also been controversial since it was first described, with some authors considering ACH a skin disorder distinct from GPP and others considering ACH to be a variant of the generalized pustular disease.Citation6
Epidemiology/presentation
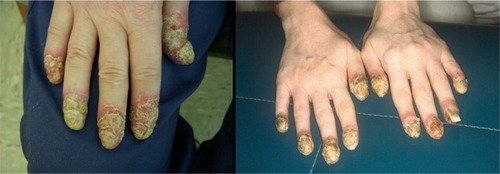
ACH is a chronic form of pustular psoriasis that characteristically involves the digits and most commonly, the fingertips and nails.Citation6 ACH often presents as sterile pustules on the most distal portions of the fingers and toes ().Citation107 It is often painful and extensive with characteristic proximal progression of the sterile pustules leading to onychodystrophy or anonychia.Citation6,Citation107 ACH can have a similar presentation to other pustular disorders of the hands and feet, especially palmoplantar pustulosis. Characteristics that can be utilized to distinguish the various etiologies include ACH’s predisposition for the periungual areas as well as its ability to remain localized to a limited number of digits for many years.Citation109,Citation110 ACH is most common in middle-aged female.Citation111 Further epidemiologic studies have not been conducted owing to the rarity of disease, and as such, most data have been obtained from anecdotal reports.Citation112
Pathophysiology
ACH, like GPP, is also associated with precipitating factors. Those precipitating factors most closely associated with ACH include localized trauma to the distal portion of a digit and localized infections.Citation1,Citation111,Citation113
As mentioned, the classification of ACH has long been debated with ACH possibly being a distinct disease or a variant of GPP. Support for the latter classification is provided both by case reports involving the transition of ACH to GPP and the presence of IL-36RA mutations in patients with ACH, which is the same genetic mutation found in some patients with GPP.Citation6,Citation114
Treatment
Treatment of ACH is primarily based on data from case reports. ACH is notoriously recalcitrant to many therapies.Citation107 Treatments that have been attempted historically included antibiotics and tar.Citation107 Current first-line topical treatment for ACH is corticosteroid use under occlusion (). Second-line topical treatments include calcipotriol, tacrolimus, and fluorouracil, or a combination of these medications.Citation107 However, these medications often do not prevent the occurrence of relapses and progression to systemic treatment is often necessary.Citation107 First-line systemic therapies involve the use of cyclosporine and acitretin, and biologic agents. Biologic agents have more recently become the mainstay of treatment in recalcitrant forms of ACH. Ustekinumab was successfully used to treat a patient with ACH.Citation115 TNF-α inhibitors have proven to be the most efficacious and include the use of infliximab, adalimumab, and etanercept, especially in recalcitrant cases of ACH.Citation107,Citation116 Efalizumab has also historically been used successfully in the treatment of ACH, but efalizumab, as mentioned, is no longer commercially available.Citation36,Citation117 Second-line systemic therapies include systemic corticosteroids, methotrexate, retinoids, PUVA, and narrow- and broad-band UVB.Citation107
Table 5 Treatment of acrodermatitis continua of hallopeau
Evidence-based treatment strategies for ACH are lacking due to the difficulty in diagnosis of ACH and the lack of knowledge regarding the pathophysiology and etiology of the disease. Future management of ACH requires larger controlled studies and further studies on the pathophysiology of the disease, which will likely guide the development of new and effective medications for treatment.
Conclusion
Psoriasis vulgaris is a chronic inflammatory disease that classically affects skin and joints and is associated with numerous comorbidities. There are several clinical subtypes of psoriasis including the uncommon pustular variants, which include acute GPP, pustular psoriasis of pregnancy, infantile and juvenile pustular psoriasis, ACH, and PPP. These variants have similar histopathologic characteristics. However, the immunopathogenesis of each entity remains to be fully elucidated. As such, numerous therapies have shown variable results and most entities remain difficult to treat. In general, topical therapy should be used with lesssevere, more localized forms of the disease. There should be a low threshold to initiate systemic therapies, as most pustular forms of psoriasis are recalcitrant to topical and multiple systemic therapies. Combinations of systemic and topical therapies are encouraged. With increasing knowledge of the pathogenesis of these variants of pustular psoriasis, the development and use of biologic and other immunomodulatory therapies holds promise for the future of successfully treating pustular variants of psoriasis – including the therapies targeting IL-1, IL-17, the p19 unit of IL-23, and potentially IL-36.
Acknowledgments
We would like to thank Professor Alan Menter for providing the clinical images for the figures presented and for his continued mentorship.
Disclosure
Dr Bobbak Mansouri has sat on an advisory board and received an honorarium from Celgene. All other authors have no conflicts of interest in this work.
References
- NaldiLGambiniDThe clinical spectrum of psoriasisClin Dermatol200725651051818021886
- GriffithsCEChristophersEBarkerJNA classification of psoriasis vulgaris according to phenotypeBr J Dermatol2007156225826217223864
- GriffithsCEMCampRDRBarkerJNWNPsoriasis Rook’s Textbook of DermatologyBlackwell Publishing Inc200817311800
- RaychaudhuriSKMaverakisERaychaudhuriSPDiagnosis and classification of psoriasisAutoimmun Rev2014134–549049524434359
- OosterlingRJNobregaREDu BoeuffJAVan Der MeerJBImpetigo herpetiformis or generalized pustular psoriasis?Arch Dermatol19781141015271529718194
- AbbasOItaniSGhosnSAcrodermatitis continua of Hallopeau is a clinical phenotype of DITRA: evidence that it is a variant of pustular psoriasisDermatol201322612831
- AkiyamaTSeishimaMWatanabeHNakataniAMoriSKitajimaYThe relationships of onset and exacerbation of pustulosis palmaris et plantaris to smoking and focal infectionsJ Dermatol199522129309348648000
- AsumalahtiKAmeenMSuomelaSGenetic analysis of PSORS1 distinguishes guttate psoriasis and palmoplantar pustulosisJ Invest Dermatol2003120462763212648227
- ErikssonMOHagforsenELundinIPMichaelssonGPalmoplantar pustulosis: a clinical and immunohistological studyBr J Dermatol199813833903989580788
- LotemMKatzenelsonVRotemAHodMSandbankMImpetigo herpetiformis: a variant of pustular psoriasis or a separate entity?J Am Acad Dermatol1989202 Pt 23383412644321
- Bangale-DaflapurkarSDanveAPustular psoriasis of pregnancy successfully treated with cyclosporineAm J Ther Epub2015910
- IizukaHTakahashiHIshida-YamamotoAPathophysiology of generalized pustular psoriasisArch Dermatolog Res2003295Suppl 1S55S59
- KardaunSHKuiperHFidlerVJonkmanMFThe histopathological spectrum of acute generalized exanthematous pustulosis (AGEP) and its differentiation from generalized pustular psoriasisJ Cutan Pathol201037121220122920738458
- VarmanKMNamiasNSchulmanCIPizanoLRAcute generalized pustular psoriasis, von Zumbusch type, treated in the burn unit. a review of clinical features and new therapeuticsBurns2014404e35e3924491419
- ChoonSELaiNMMohammadNANanuNMTeyKEChewSFClinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, MalaysiaInt J Dermatol201453667668423967807
- Borges-CostaJoSilvaRGonçalvesLFilipePSoares de AlmeidaLMarques GomesMClinical and laboratory features in acute generalized pustular psoriasis: a retrospective study of 34 patientsAm J Clin Dermatol201112427127621495733
- Setta-KaffetziNNavariniAAPatelVMRare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypesJ Invest Dermatol201313351366136923303454
- RobinsonAVan VoorheesASHsuSKormanNJLebwohlMGBeboBFJrKalbRETreatment of pustular psoriasis: from the Medical Board of the National Psoriasis FoundationJ Am Acad Dermatol201267227928822609220
- ViguierMAllezMZagdanskiAMHigh frequency of cholestasis in generalized pustular psoriasis: evidence for neutrophilic involvement of the biliary tractHepatology200440245245815368450
- ZelicksonBDMullerSAGeneralized pustular psoriasis. a review of 63 casesArch Dermatol19911279133913451892402
- AugeyFRenaudierPNicolasJFGeneralized pustular psoriasis (Zumbusch): a French epidemiological surveyEur J Dermatol200616666967317229609
- OzawaAOhkidoMHarukiYTreatments of generalized pustular psoriasis: a multicenter study in JapanJ Dermatol199926314114910209919
- GoirizRDaudenEPerez-GalaSGuhlGGarcia-DiezAFlare and change of psoriasis morphology during the course of treatment with tumour necrosis factor blockersClin Exp Dermatol200732217617917176269
- BrennerMMolinSRuebsamKWeisenseelPRuzickaTPrinzJCGeneralized pustular psoriasis induced by systemic glucocorticosteroids: four cases and recommendations for treatmentBr J Dermatol2009161496496619681858
- WenkKSClarosJMEhrlichAFlare of pustular psoriasis after initiating ustekinumab therapyJ Dermatolog Treat201223321221421254881
- GregoriouSKazakosCChristofidouEKontochristopoulosGVakisGRigopoulosDPustular psoriasis development after initial ustekinumab administration in chronic plaque psoriasisEur J Dermatol201121110410521233062
- OzturkGTurkBGKaracaNGeneralized pustular eruptions due to terbinafineCutan Ocul Toxicol2012311818421888496
- MarrakchiSGuiguePRenshawBRInterleukin-36-receptor antagonist deficiency and generalized pustular psoriasisN Engl J Med2011365762062821848462
- MahilSKCaponFBarkerJNGenetics of psoriasisDermatolog Clin2015331111
- DietrichDGabayCInflammation: IL-36 has proinflammatory effects in skin but not in jointsNat Rev Rheumatol2014101163964025201385
- HussainSBerkiDMChoonSEIL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasisJ Allergy Clin Immunol2015135410671070.e106925458002
- OnoufriadisASimpson MichaelAPink AndrewEMutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasisAm J Hum Genet201189343243721839423
- CaponFIL36RN mutations in generalized pustular psoriasis: just the tip of the iceberg?J Invest Dermatol2013133112503250424129779
- LowesMASuarez-FarinasMKruegerJGImmunology of psoriasisAnn Rev Immunol20143222725524655295
- AksentijevichIMastersSLFergusonPJAn autoinflammatory disease with deficiency of the interleukin-1–receptor antagonistNEngl J Med20093602324262437
- MansouriBPatelMMenterABiological therapies for psoriasisExp Opin Biol Ther2013131217151730
- DaudenESantiago-et-Sanchez-MateosDSotomayor-LopezEGarcia-DiezAUstekinumab: effective in a patient with severe recalcitrant generalized pustular psoriasisBr J Dermatol201016361346134720716216
- LevinECDebbanehMKooJLiaoWBiologic therapy in erythrodermic and pustular psoriasisJ Drugs Dermatol201413334235424595581
- ToriiHNakagawaHLong-term study of infliximab in Japanese patients with plaque psoriasis, psoriatic arthritis, pustular psoriasis and psoriatic erythrodermaJ Dermatol201138432133421544940
- JordanJBieberTWilsmann-TheisDAdalimumab: safe and successful in severe pustular psoriasisJ Eur Acad Dermatol Venereol200923559259318761550
- HonigsmannHGschnaitFKonradKWolffKPhotochemotherapy for pustular psoriasis (von Zumbusch)Br J Dermatol1977972119126911672
- EspositoMMazzottaACascielloCChimentiSEtanercept at different dosages in the treatment of generalized pustular psoriasis: a case seriesDermatology2008216435536018285687
- UmezawaYOzawaAKawasimaTTherapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severityArch Dermatol Res2003295Suppl 1S43S5412677432
- NagaoKIshikoAYokoyamaTTanikawaAAmagaiMA case of generalized pustular psoriasis treated with topical tacrolimusArch Dermatol200313991219
- Rodriguez GarciaFFagundo GonzalezECabrera-PazRGeneralized pustular psoriasis successfully treated with topical tacrolimusBr J Dermatol2005152358758815787845
- ViguierMGuiguePPagesCSmahiABachelezHSuccessful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutationsAnn Int Med20101531666720621920
- HuffmeierUWatzoldMMohrJSchonMPMossnerRSuccessful therapy with anakinra in a patient with generalized pustular psoriasis carrying IL36RN mutationsBr J Dermatol2014170120220423909475
- MansouriBRichardsLMenterATreatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumabBr J Dermatol2015173123924125495649
- BohnerARoennebergSEyerichKEberleinBBiedermannTAcute generalized pustular psoriasis treated with the IL-17A antibody secukinumabJAMA Dermatol2016152448248426650014
- FurusawaKHasegawaTIkedaSImmunosuppressant and infliximab-resistant generalized pustular psoriasis successfully treated with granulocyte and monocyte adsorption apheresisTher Apher Dial201216437938022817129
- ShukuyaRHasegawaTNiwaYOkumaKIkedaSGranulocyte and monocyte adsorption apheresis for generalized pustular psoriasisJ Dermatol201138121130113422007872
- HebraFVUeber einzelne, wahrend der schwangerschaft am wochenbette und bei urinalkrankheiten der frauzen zu beobachtende hautkrankheiten22Wein Med Wochenschr1872
- KondoRNAraujoFMPereiraAMLopesVCMartinsLMPustular psoriasis of pregnancy (impetigo herpetiformis)–case reportAn Bras Dermatol2013886 Suppl 118618924346915
- RothMMPregnancy dermatoses: diagnosis, management, and controversiesAm J Clin Dermatol2011121254121110524
- KroumpouzosGCohenLMDermatoses of pregnancyJ Am Acad Dermatol2001451119 quiz 19–2211423829
- GreenMGBraggJRosenmanKSPomeranzMKPustular psoriasis of pregnancy in a patient whose dermatosis showed features of acute generalized exanthematous pustulosisInt J Dermatol200948329930319261022
- Breier-MalyJOrtelBBreierFSchmidtJBHonigsmannHGeneralized pustular psoriasis of pregnancy (impetigo herpetiformis)Dermatology19991981616410026404
- OumeishOYFarrajSEBatainehASSome aspects of impetigo herpetiformisArch Dermatol198211821031057059209
- BeveridgeGWHarknessRALivingstoneJRImpetigo herpetiformis in two successive pregnanciesBr J Dermatol19667821061124286099
- GligoraMKolacioZHormonal treatment of impetigo herpetiformisBr J Dermatol198210722537104221
- KuijpersALSchalkwijkJRuloHFPeperkampJJvan de KerkhofPCde JongEMExtremely low levels of epidermal skin-derived antileucoproteinase/elafin in a patient with impetigo herpetiformisBr J Dermatol199713711231299274639
- KatsambasAStavropoulosPGKatsiboulasVKostakisPPanayiotopoulosAChristofidouEPetridisAImpetigo herpetiformis during the puerperiumDermatology1999198440040210449943
- KorberAMossnerRRennerRMutations in IL36RN in patients with generalized pustular psoriasisJ Invest Dermatol2013133112634263723648549
- LiXChenMFuXMutation analysis of the IL36RN gene in Chinese patients with generalized pustular psoriasis with/without psoriasis vulgarisJ Dermatol Sci201476213213825212972
- ChangSEKimHHChoiJHSungKJMoonKCKohJKImpetigo herpetiformis followed by generalized pustular psoriasis: more evidence of same disease entityInt J Dermatol200342975475512956698
- EdmondsEVMorrisSDShortKBewleySJEadyRAPustular psoriasis of pregnancy treated with ciclosporin and high-dose prednisoloneClin Exp Dermatol200530670971016197395
- FinchTMTanCYPustular psoriasis exacerbated by pregnancy and controlled by cyclosporin ABr J Dermatol20001423582584
- AdachiAKomineMHiranoTTsudaHKarakawaMMurataSOhtsukiMCase of generalized pustular psoriasis exacerbated during pregnancy, successfully treated with infliximabJ Dermatol Epub2016430
- ShethNGreenblattDTAclandKBarkerJTeixeiraFGeneralized pustular psoriasis of pregnancy treated with infliximabClin Exp Dermatol200934452152219196309
- LohrischIHeilmannSHausteinUFImpetigo herpetiformis and PUVA-treatment (author’s transl)Dermatol Monatschr19791659648652
- VunYYJonesBAl-MudhafferMEganCGeneralized pustular psoriasis of pregnancy treated with narrowband UVB and topical steroidsJ Am Acad Dermatol2006542 SupplS28S3016427987
- AndrulonisRFerrisLKTreatment of severe psoriasis with ustekinumab during pregnancyJ Drugs Dermatol20121110124023134993
- FarberEMNallLChildhood psoriasisCutis199964530931410582153
- FialovaJVojackovaNVanousovaDHercogovaJJuvenile generalized pustular psoriasis treated with etanerceptDermatol Ther201427210510824703268
- XiaoTLiBHeCDChenHDJuvenile generalized pustular psoriasisJ Dermatol200734857357617683391
- ZaraaIFazaaBZeglaouiFPustular psoriasis in childhood in 15 casesTunis Med200482767968315552027
- WollinaUFunfstuckVJuvenile generalized circinate pustular psoriasis treated with oral cyclosporin AEur J Dermatol200111211711911275806
- HublerWRJrFamilial juvenile generalized pustular psoriasisArch Dermatol19841209117411786476855
- KaramfilovTWollinaUJuvenile generalized pustular psoriasisActa Derm Venereol19987832209602231
- CrockerHRDiseases of the SkinLondonH.K. Lewis1888
- FarleyEMasrourSMcKeyJMenterAPalmoplantar psoriasis: a phenotypical and clinical review with introduction of a new quality-of-life assessment toolJ Am Acad Dermatol20096061024103119467374
- Morales-MuneraCVilarrasaEPuigLEfficacy of ustekinumab in refractory palmoplantar pustular psoriasisBr J Dermatol2013168482082423210683
- BrunassoAMPuntoniMAbererWDelfinoCFancelliLMassoneCClinical and epidemiological comparison of patients affected by palmoplantar plaque psoriasis and palmoplantar pustulosis: a case series studyBr J Dermatol201316861243125123301847
- HagforsenEAwderMLefvertAKNordlindKMichaelssonGPalmoplantar pustulosis: an autoimmune disease precipitated by smoking?Acta Derm Venereol200282534134612430732
- AmmouryAEl SayedFDhaybiRBazexJPalmoplantar pustulosis should not be considered as a variant of psoriasisJ Eur Acad Dermatol Venereol200822339239318269621
- YamamotoTExtra-palmoplantar lesions associated with palmoplantar pustulosisJ Eur Acad Dermatol Venereol200923111227123219453807
- Saez-RodriguezMNoda-CabreraAAlvarez-TejeraSThe role of psychological factors in palmoplantar pustulosisJ Eur Acad Dermatol Venereol200216432532712224686
- BrunassoAMLaimerMMassoneCParadoxical reactions to targeted biological treatments: a way to treat and trigger?Acta Derm Venereol201090218318520169304
- BissonnetteRNigenSLangleyRGLyndeCWTanJFuentes-DuculanJKruegerJGIncreased expression of IL-17A and limited involvement of IL-23 in patients with palmo-plantar (PP) pustular psoriasis or PP pustulosis; results from a randomised controlled trialJ Eur Acad Dermatol Venereol201428101298130524112799
- MurakamiMHagforsenEMorhennVIshida-YamamotoAIizukaHPatients with palmoplantar pustulosis have increased IL-17 and IL-22 levels both in the lesion and serumExp Dermatol2011201084584721732985
- HagforsenEEdvinssonMNordlindKMichaelssonGExpression of nicotinic receptors in the skin of patients with palmoplantar pustulosisBr J Dermatol2002146338339111952537
- de WaalACvan de KerkhofPCPustulosis palmoplantaris is a disease distinct from psoriasisJ Dermatol Treat2011222102105
- MichaelssonGGustafssonKHagforsenEThe psoriasis variant palmoplantar pustulosis can be improved after cessation of smokingJ Am Acad Dermatol200654473773816546609
- KimDYKimJYKimTGA comparison of inflammatory mediator expression between palmoplantar pustulosis and pompholyxJ Eur Acad Dermatol Venereol201327121559156523802874
- TakaharaMClinical outcome of tonsillectomy for palmoplantar pustulosis and etiological relationship between palmoplantar pustulosis and tonsilsAdv Otorhinolaryngol201172868821865698
- KikuchiNYamamotoTDental infection as a triggering factor in palmoplantar pustulosisActa Derm Venereol201393672172223462950
- AdisenETekinOGulekonAGurerMAA retrospective analysis of treatment responses of palmoplantar psoriasis in 114 patientsJ Eur Acad Dermatol Venereol200923781481919470063
- HagforsenEHedstrandHNybergFMichaelssonGNovel findings of langerhans cells and interleukin-17 expression in relation to the acrosyringium and pustule in palmoplantar pustulosisBr J Dermatol2010163357257920426778
- ShmidtEWetterDAFergusonSBPittelkowMRPsoriasis and palmoplantar pustulosis associated with tumor necrosis factor-alpha inhibitors: the Mayo Clinic experience, 1998 to 2010J Am Acad Dermatol2012675e179e18521752492
- SugiuraKTakemotoAYamaguchiMThe majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonistJ Invest Dermatol2013133112514252123698098
- Renert-YuvalYHorevLBabaySTamsSRamotYZlotogorskiAMolho-PessachVIL36RN mutation causing generalized pustular psoriasis in a Palestinian patientInt J Dermatol201453786686824898045
- MurakamiMOhtakeTHoribeYIshida-YamamotoAMorhennVBGalloRLIizukaHAcrosyringium is the main site of the vesicle/pustule formation in palmoplantar pustulosisJ Invest Dermatol201013082010201620393482
- YawalkarNHungerRESuccessful treatment of recalcitrant palmoplantar pustular psoriasis with sequential use of infliximab and adalimumabDermatology20092181798318974629
- KascheAPfabFHeinRAthanasiadisGIOllertMRingJEberlein-KönigBSevere psoriasis pustulosa palmaris et plantaris (Barber-Konigsbeck) treated successfully with soluble tumour necrosis factor receptor fusion protein (etanercept)J Eur Acad Dermatol Venereol200721225525717243968
- AhmadKRogersSThree years’ experience with infliximab in recalcitrant psoriasisClin Exp Dermatol200631563063316901301
- ColsmanACarrascosaJMFerrandizCSimonJCSuccessful treatment of recalcitrant palmoplantar psoriasis with efalizumabJ Eur Acad Dermatol Venereol200822911311134
- SehgalVNVermaPSharmaSSrivastavaGAggarwalAKRasoolFChatterjeeKAcrodermatitis continua of Hallopeau: evolution of treatment optionsInt J Dermatol201150101195121121950286
- CymermanRMCohenDETreatment of acrodermatitis continua of hallopeau with ustekinumab as monotherapyJAMA Dermatol2016152334634826560053
- WilliamDJamesTBElstonDirkAndrews’ Diseases of the Skin Clinical Dermatology10th edCanadaSaunders Elsevier2006
- RoenigkHPsoriasis3rd edNew YorkMarcel Dekker Inc1998
- YerushalmiJGrunwaldMHHallel-HalevyDAvinoachIHalevySChronic pustular eruption of the thumbs. Diagnosis: acrodermatitis continua of Hallopeau (ACH)Arch Dermatol20001367925930
- PuigLBarcoDVilarrasaEAlomarATreatment of acrodermatitis continua of Hallopeau with TNF-blocking agents: case report and reviewDermatology2010220215415820110631
- RosenbergBEStroberBEAcrodermatitis continuaDermatol Online J20041039
- RanughaPSKumariRThappaDMAcrodermatitis continua of hallopeau evolving into generalised pustular psoriasisIndian J Dermatol2013582161
- AbbasMHolfeldKDesjardinsDZimmerJPustular psoriasis complicated with acute generalized exanthematous pustulosisJ Dermatol Case Rep201482424525024776
- Di CostanzoLNapolitanoMPatrunoCCantelliMBalatoNAcrodermatitis continua of Hallopeau (ACH): two cases successfully treated with adalimumabJ Dermatol Treat2014256489494
- BalatoNGalloLBalatoALa BellaSAyalaFAcrodermatitis continua of Hallopeau responding to efalizumab therapyJ Eur Acad Dermatol Venereol200923111329133019250321