Abstract
Purpose
The purpose of the present study was to evaluate the effect of time (season, surgical starting time in the daytime, preoperative waiting time) on patients with gastric cancer.
Methods
A retrospective collection of medical records of patients who underwent gastrectomy at a single clinical center from January 2013 to December 2018 was performed. Medical records were collected, and short-term outcomes and long-term survival were analyzed by different time groups.
Results
A total of 586 patients were included in this study. In terms of surgical starting time, the midday group had a shorter operation time (p=0.017) but more complications (p=0.048) than the non-midday group. No significant difference was found based on the season of gastrectomy. The long preoperative waiting group had a shorter postoperative hospital stay than the short waiting group (p=0.026). No significant difference was found between the short-waiting group and long-waiting group in overall survival for all clinical stages. Age (p=0.040, HR=1.017, 95% CI=1.001–1.033), BMI (p<0.001, HR=0.879, 95% CI=0.844–0.953) and clinical stage (p<0.001, HR=2.053, 95% CI=1.619–2.603) were independent prognostic factors predicting overall survival; however, season of gastrectomy, surgical starting time and preoperative waiting time were not identified as independent prognostic factors.
Conclusion
Surgical starting time at the midday could cause more complications, and surgeons should be careful when the surgical starting time is midday.
Introduction
Gastric cancer is generally acknowledged as one of the most common malignant tumors in the world, with approximately 1 million new cases diagnosed every year.Citation1,Citation2 Radical gastrectomy is still a recommended method for curable gastric cancer.Citation3 There are a multiple number of factors that can affect short-term and long-term outcomes, including BMI, age, pathological tumor stage, and histologically differentiated types.Citation4–Citation6
The workload of surgeons and anesthesiologists can increase the adverse events of surgeries.Citation7 Patients who are anesthetized at different times of the day may experience different clinical outcomes.Citation8 Long working hours of work can cause loss of concentration, lack of sleep and fatigue.Citation7,Citation9 There is a clear correlation between the start time of surgery and adverse events, with the highest adverse events in the afternoon.Citation8 Prior studies reported that the surgery starting time affected the prognosis of patients in general surgery, heart surgery, gynecologic surgery, orthopedic surgery and neurosurgery.Citation10–Citation12
The preoperative waiting time before surgery might also affect the short-term and long-term outcomes. However, as previously described, a waiting time beyond more than 90 days before surgery would not affect overall survival in patients with clinical stages I, II and III.Citation13–Citation15 Previous studies have proven that the birth season may affect the incidence of tumors, and furthermore, tumors diagnosed in winter may affect the prognosis as well.Citation16,Citation17 However, one study reported that the diagnosis season did not affect the prognosis of gastric cancer.Citation18
The effect of preoperative waiting time, surgical staring time in the daytime and season of gastrectomy remain unclear because of limited research. Therefore, the purpose of the present study was to explore the effect of time (season, surgical starting time, preoperative waiting time) on patients with gastric cancer.
Patients and Methods
Patients
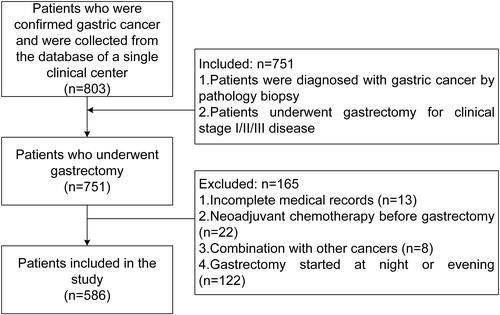
A retrospective collection of medical records of patients who underwent gastrectomy at a single clinical center from January 2013 to December 2018 was performed. This study was approved by the ethics committee (2020–649), and all patients signed informed consent forms. The inclusion criteria were as follows: 1. Patients were diagnosed with gastric cancer by pathology biopsy; and 2. Patients underwent gastrectomy for clinical stage I/II/III disease. The exclusion criteria were as follows: 1. Incomplete medical records; 2. Neoadjuvant chemotherapy before gastrectomy; 3. Combination with other cancers; and 4. Gastrectomy started at night or evening. A total of 751 patients met the inclusion criteria of 803 patients in the database. Of these, 165 patients were excluded. Finally, a total of 586 patients were included in the study ().
Definition
The season of gastrectomy was defined according to the location of the clinical center: spring was from March to May, summer was from June to August, autumn was from September to November and winter was from December to February. The preoperative waiting time was defined as the time from pathological diagnosis of gastric cancer to gastrectomy. The surgical starting time was divided into two groups: the midday group (12 to 14 o’clock) and the non-midday group (8 to 12, 14–18 o’clock). Complications were evaluated by Clavien-Dindo classification, and the grades I–V were included in this study.Citation19 TNM stage was based on the UICC tumor lymph node metastasis classification system definition. Overall survival was defined as the time from gastrectomy to death or the end of the study if the patient was still alive.
Data Collection
The baseline information was retrospectively collected, including sex, age, BMI, season of gastrectomy, comorbidity, preoperative waiting time, surgical starting time and preoperative clinical stage. The operation information included surgical procedure, reconstruction method, operation time, estimated blood loss and number of retrieved lymph nodes. The postoperative information included postoperative hospital stay, pathological stage and overall survival time.
Statistical Analysis
The median and range or the mean and standard deviation were used for continuous variables. The preoperative waiting time was divided into a long-waiting group and a short-waiting group according to the median. The surgical starting time was divided into a midday group and a non-midday group. The Mann–Whitney-U test and chi-square test were used to compare the differences between the two groups. The season of gastrectomy was divided into four groups: spring, summer, autumn and winter. The Kruskal–Wallis test and chi-square test were used to compare the differences across the four groups. The Log-rank test and Cox-proportional hazards model were used to analyze overall survival. Data were analyzed using SPSS (version 20.0) statistical software. A bilateral p value of <0.05 was considered statistically significant.
Results
Clinical Characteristics of the Patients
A total of 586 patients were included in this study, and all the patients underwent gastrectomy by two experienced surgeons on the same team in this study. Sex, age, BMI, season of gastrectomy, comorbidity, preoperative waiting time, surgical starting time, preoperative clinical stage, surgical procedure, reconstruction method, operation time, estimated blood loss, number of retrieved lymph nodes, postoperative hospital stay, complications and the pathological stage are shown in .
Table 1 Clinical Characteristics of the Patients
Outcomes Associated with the Surgical Starting Time
The surgical starting time was divided into a midday group and a non-midday group according to the median. The operation time, estimated blood loss, number of retrieved lymph nodes, complications and postoperative hospital stay were compared between the two groups. The midday group had a shorter operation time (p=0.017) but more complications (p=0.048) ().
Table 2 Outcomes of Midday and Non-Midday Groups
Outcomes Associated with Different Seasons
According to the season of gastrectomy, we divided the seasons into four groups: spring, summer, autumn and winter. There was no difference in terms of operation time, estimated blood loss, number of retrieved lymph nodes, complications or postoperative hospital stay (p>0.05) ().
Table 3 Outcomes of Different Season
Outcomes of the Short and Long Preoperative Waiting Time Group
The preoperative waiting time was divided into a short-waiting group and a long-waiting group according to the median. There was no significant difference in operation time, estimated blood loss, number of retrieved lymph nodes or complications (p>0.05). A significant difference was found in the postoperative stay, and the long-waiting group had a shorter postoperative stay than the short-waiting group (p=0.026) ().
Table 4 Outcomes of Short and Long Preoperative Waiting Time Groups
Overall Survival
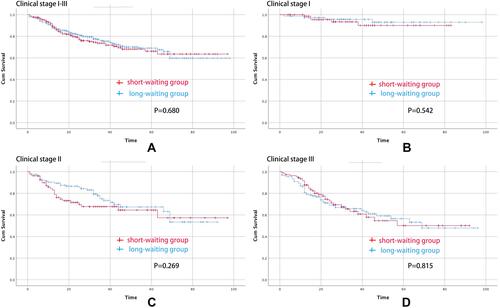
The median follow-up time was 28.5 (1–98) months. There was no significant difference between the short-waiting group and long-waiting group in terms of all-stage overall survival (p=0.680), clinical stage I overall survival (p=0.542), clinical stage II overall survival (p=0.269) or clinical stage III overall survival (p=0.815). Multivariate analyses for the identification of independent prognostic factors for overall survival in all clinical stage gastric cancer were performed. Age (p=0.040, HR=1.017, 95% CI=1.001–1.033), BMI (p<0.001, HR=0.879, 95% CI=0.844–0.953) and clinical stage (p<0.001, HR=2.053, 95% CI=1.619–2.603) were independent prognostic factors for predicting overall survival; however, season of gastrectomy, surgical starting time and preoperative waiting time were not identified as an independent prognostic factors () ().
Table 5 Multivariate Analyses for the Identification of Independent Prognostic Factors for Overall Survival in All Clinical Stage Gastric Cancer
Figure 2 Overall survival curves for patients undergoing gastrectomy. (A) Overall survival curves for the waiting groups among all patients. (B) Overall survival curves for the waiting groups among clinical stage I patients. (C) Overall survival curves for the waiting groups among clinical stage II patients. (D) Overall survival curves for the waiting groups among clinical stage III patients.
Discussion
A total of 586 patients were included in this study. Baseline information was collected, and short-term and long-term outcomes were compared between different time groups. The midday group had a shorter operation time but more complications, and no significant difference was found among the different season groups and preoperative waiting time groups. Furthermore, season of gastrectomy, surgical starting time and preoperative waiting time were not identified as independent prognostic factors.
The starting time of the surgery might affect the concentration of the anesthesiologist and surgeons.Citation8 Previous studies have compared morning and afternoon surgical starting times, but it remains controversial whether morning surgical starting times have advantages over afternoon surgical starting times.Citation10–Citation12 Similarly, surgery during the daytime had fewer complications than at nighttime.Citation20,Citation21 Workload and fatigue may be major contributors to these outcomes. China is a populous country and has the largest number of gastric cancer patients worldwide. The workload of surgeons is heavy, especially in large hospitals and can result in fatigue in surgeons.Citation7,Citation9 In this study, we compared the midday group and the non-midday group, and we found that the midday group had shorter operation time but more complications. In addition to workload and fatigue, the post-lunch sleepiness might account for the outcomes. Napping is common in southern China, and post-lunch sleepiness occurs whether lunch is consumed or not.Citation22,Citation23 There may be reasons why the midday surgical starting group might have more complications, and the careless surgical procedures might cause less surgical time.
Patients experienced a period of waiting time from diagnosis to gastrectomy. Limited hospital capacity, family members’ consideration of whether to undergo surgery and preoperative comorbidity of patients can prolong the preoperative waiting time. However, the patient’s psychological condition is anxious when waiting for gastrectomy. Patients worried about the tumor progression in the case of a long preoperative waiting time, especially during the coronavirus disease 2019 (COVID-19) pandemic, when the preoperative waiting time was prolonged.Citation24 In this study, we found that the long-waiting group had a shorter postoperative hospital stay than the short-waiting group, however, a significant difference was not found in terms of complications and long-term survival. The cause of the shorter postoperative hospital stays of the long-waiting group remained unclear, and adequate preoperative preparation might shorten the postoperative hospital stay. Previous studies reported than a half-year wait time for surgery was not independently associated with the survival of patients with clinical stage I gastric cancer,Citation13 and a preoperative wait time up to 90 days did not affect survival in patients with clinical stage II/III gastric cancer.Citation14 The results in this study were consistent with previous studies. However, delayed gastrectomy is not recommended because of the preoperative waiting time-related psychological distress and displeasure.Citation25,Citation26
The season correlates with the temperature and sunlight.Citation27 Studies on the relationship between seasons and tumors have mainly focused on the birth season and the incidence of tumors.Citation16 Previous studies reported that persons born in spring have a higher risk of skin cancer than those born in summer,Citation28 and for persons born in winter, the risk of lung cancer and squamous cell carcinoma was lower than those born in other seasons.Citation17 However, there have been few reports on the impact of surgery season on patients with tumors. We hypothesized that different seasons might affect the wound healing and short-term complications. However, there was no significant difference in short-term postoperative complications between different surgical seasons in this study, which was also consistent with a previous related study.Citation18 Therefore, our results suggested no association between the season of gastrectomy and short-term outcomes, and furthermore, season was not a risk factor for postoperative complications.
Some limitations existed in this study as well. First, this was a retrospective single-center study with 586 patients, and the number of data samples was limited. Second, the median preoperative waiting time was 10 days, which was relatively shorter than that in previous studies. Third, patients with neoadjuvant chemotherapy before gastrectomy were excluded from this study because of the prolonged preoperative waiting time. Finally, for the purpose of comparing surgical starting time in the midday group with that in the non-midday group in the daytime, we excluded gastrectomy started at night and evening; however, this might introduce some selection bias.
Conclusion
Surgical starting time at the midday could cause more complications, and a longer preoperative waiting time could shorten the postoperative hospital stay; therefore, surgeons should be careful when surgical starting time at the midday.
Author Contributions
All authors contributed to data collection and analysis, drafting or revising the manuscript, have agreed on the journal to which the manuscript will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors are grateful to Dr. Zhuozhi Shen, Chongqing Center for Disease Control and Prevention, for the substantial work in the statistical methods.
Disclosure
The authors declare no conflicts of interest.
Additional information
Funding
References
- Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014;40(3):250–260. doi:10.1111/apt.12814
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi:10.1002/ijc.29210
- Peng D, Cheng YX, Zhang W. Does Roux-en-Y construction really bring benefit of type 2 diabetes mellitus remission after gastrectomy in patients with gastric cancer? A systematic review and meta-analysis. Diabetes Ther. 2020;11:2863–2872. doi:10.1007/s13300-020-00934-7
- Tang X, Zhang M, He Q, et al. Histological differentiated/undifferentiated mixed type should not be considered as a non-curative factor of endoscopic resection for patients with early gastric cancer. Front Oncol. 2020;10:1743. doi:10.3389/fonc.2020.01743
- Wada T, Kunisaki C, Ono HA, et al. Implications of BMI for the prognosis of gastric cancer among the Japanese population. Dig Surg. 2015;32(6):480–486. doi:10.1159/000440654
- Gao Z, Ni J, Ding H, et al. A nomogram for prediction of stage III/IV gastric cancer outcome after surgery: a multicenter population-based study. Cancer Med. 2020;9(15):5490–5499. doi:10.1002/cam4.3215
- Gawande AA, Zinner MJ, Studdert DM, et al. Analysis of errors reported by surgeons at three teaching hospitals. Surgery. 2003;133(6):614–621. doi:10.1067/msy.2003.169
- Wright MC, Phillips BB, Mark JB, et al. Time of day effects on the incidence of anesthetic adverse events. Qual Saf Health Care. 2006;15:258–263. doi:10.1136/qshc.2005.017566
- Goitein L, Shanafelt TD, Wipf JE, et al. The effects of work-hour limitations on resident well-being, patient care, and education in an internal medicine residency program. Arch Intern Med. 2005;165(22):2601–2606. doi:10.1001/archinte.165.22.2601
- Badiyan SN, Ferraro DJ, Yaddanapudi S, et al. Impact of time of day on outcomes after stereotactic radiosurgery for non-small cell lung cancer brain metastases. Cancer. 2013;119(19):3563–3569. doi:10.1002/cncr.28237
- Ishiyama Y, Ishida F, Ooae S, et al. Surgical starting time in the morning versus the afternoon: propensity score matched analysis of operative outcomes following laparoscopic colectomy for colorectal cancer. Surg Endosc. 2019;33(6):1769–1776. doi:10.1007/s00464-018-6449-9
- Assali AR, Brosh D, Vaknin-Assa H, et al. The impact of circadian variation on outcomes in emergency acute anterior myocardial infarction percutaneous coronary intervention. Catheter Cardiovasc Interv. 2006;67(2):221–226. doi:10.1002/ccd.20608
- Fujiya K, Irino T, Furukawa K, et al. Safety of prolonged wait time for gastrectomy in clinical stage I gastric cancer. Eur J Surg Oncol. 2019;45(10):1964–1968. doi:10.1016/j.ejso.2019.06.006
- Furukawa K, Irino T, Makuuchi R, et al. Impact of preoperative wait time on survival in patients with clinical stage II/III gastric cancer. Gastric Cancer. 2019;22(4):864–872. doi:10.1007/s10120-018-00910-y
- Brenkman HJF, Visser E, van Rossum PN, et al. Association between waiting time from diagnosis to treatment and survival in patients with curable gastric cancer: a population-based study in the Netherlands. Ann Surg Oncol. 2017;24:1761–1769. doi:10.1245/s10434-017-5820-8
- Xiong W, Hao Y, Han L, et al. Associations between birth season and the anatomic subsites of gastric cancer in Beijing, China. Chronobiol Int. 2020;37(11):1636–1643. doi:10.1080/07420528.2020.1792481
- Hao Y, Yan L, Ke E, et al. Birth in winter can reduce the risk of lung cancer: a retrospective study of the birth season of patients with lung cancer in Beijing area, China. Chronobiol Int. 2017;34(4):511–518. doi:10.1080/07420528.2017.1305964
- Unal D, Oguz A, Acmaz B, et al. Lack of any association between season of diagnosis and survival of gastric cancer cases in Kayseri, Turkey. Asian Pac J Cancer Prev. 2014;15(4):1763–1766. doi:10.7314/APJCP.2014.15.4.1763
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi:10.1097/SLA.0b013e3181b13ca2
- Becker F, Voß T, Mohr A, et al. Impact of nighttime procedures on outcomes after liver transplantation. PLoS One. 2019;14(7):e0220124. doi:10.1371/journal.pone.0220124
- Heller JA, Kothari R, Lin HM, et al. Surgery start time does not impact outcome in elective cardiac surgery. J Cardiothorac Vasc Anesth. 2017;31(1):32–36. doi:10.1053/j.jvca.2016.08.015
- Aeschbach D, Cajochen C, Tobler I, et al. Sleep in a sitting position: effect of triazolam on sleep stages and EEG power spectra. Psychopharmacology. 1994;114(2):209–214. doi:10.1007/BF02244838
- Liao JQ, Xu QZ, Yuan ZY, et al. A Study on the popularity and reasons of napping in China. Chin Ergon. 2000. doi:10.1080/001401300404698
- Li YX, He CZ, Liu YC, et al. The impact of COVID-19 on gastric cancer surgery: a single-center retrospective study. BMC Surg. 2020;20:222. doi:10.1186/s12893-020-00885-7
- Gray RE, Fitch MI, Phillips C, et al. Presurgery experiences of prostate cancer patients and their spouses. Cancer Pract. 1999;7(3):130–135. doi:10.1046/j.1523-5394.1999.07308.x
- Robinson KM, Christensen KB, Ottesen B, et al. Diagnostic delay, quality of life and patient satisfaction among women diagnosed with endometrial or ovarian cancer: a nationwide Danish study. Qual Life Res. 2012;21(9):1519–1525. doi:10.1007/s11136-011-0077-3
- Robsahm TE, Tretli S, Dahlback A, et al. Vitamin D3 from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway). Cancer Causes Control. 2004;15:149–158. doi:10.1023/B:CACO.0000019494.34403.09
- La RF, Liso A, Bianconi F, et al. Seasonal variation in the month of birth in patients with skin cancer. Br J Cancer. 2014;111(9):1810–1813. doi:10.1038/bjc.2014.522