Abstract
Purpose
This study aimed to determine the risk factors associated with the number of thrombectomy device passes and establish a nomogram for predicting the number of device pass attempts in patients with successful endovascular thrombectomy (EVT).
Methods
We enrolled patients from a signal comprehensive stroke center (CSC) who underwent EVT because of large vessel occlusion stroke. Multivariate logistic regression analysis was used to develop the best-fit nomogram for predicting the number of thrombectomy device passes. The discrimination and calibration of the nomogram were estimated using the area under the receiver operating characteristic curve (AUC-ROC) and a calibration plot with a bootstrap of 1000 resamples. A decision curve analysis (DCA) was used to measure the availability and effect of this predictive tool.
Results
In total, 130 patients (mean age 64.9 ± 11.1 years; 83 males) were included in the final analysis. Age (odds ratio [OR], 1.085; 95% confidence interval [CI], 1.005–1.172; p = 0.036), baseline Alberta Stroke Program Early computed tomography (ASPECTS) score (OR, 0.237; 95% CI, 0.115–0.486; p < 0.001), and homocysteine level (OR, 1.090; 95% CI, 1.028–1.155; p = 0.004) were independent predictors of device pass number and were thus incorporated into the nomogram. The AUC-ROC determined the discrimination ability of the nomogram, which was 0.921 (95% CI, 0.860–0.980), which indicated good predictive power. Moreover, the calibration plot revealed good predictive accuracy of the nomogram. The DCA demonstrated that when the threshold probabilities of the cohort ranged between 5.0% and 98.0%, the use of the nomogram to predict a device pass number > 3 provided greater net benefit than did “treat all” or “treat none” strategies.
Conclusion
The nomogram comprised age, baseline ASPECTS score, and homocysteine level, can predict a device pass number >3 in acute ischemic stroke (AIS) patients who are undergoing EVT.
Introduction
Endovascular thrombectomy (EVT) is the standard of care for acute ischemic stroke (AIS) due to large vessel occlusion (LVO).Citation1–Citation6 The promotion and popularization of EVT for clinical applications have enabled EVT to offer considerable benefits for functional prognosis following ischemic stroke; however, approximately 50% of patients die or are still dependent after 3 months.Citation7 Such adverse results may not be solely attributable to unsuccessful recanalization, given that approximately one-third of patients who undergo early complete recanalization following a stroke do not achieve ideal results.Citation8 The occurrence of such situations may be related to issues with patient screening or intravascular treatment procedures.Citation9
Previously, numerous studies focused on the number of thrombectomy device passes during intravascular treatment procedures and reported that the number of thrombectomy device passes is closely related to the clinical outcome and prognosis of patients with ischemic stroke.Citation9–Citation14 The most significant predictor of good prognosis was successful target-vessel recanalization.Citation11,Citation15 With every additional thrombectomy device pass, the vascular recanalization rate for each individual receiving continuous thrombectomy decreases successively. Three passes may be the optimal number of attempts for target vessels; moreover, four or more attempts may not improve the probability of recanalization and may not be prognostic, while also increasing the risk of associated complications, such as hemorrhagic transformation.Citation16 Therefore, only with fewer consecutive thrombectomy operations to achieve complete recanalization of target vessels can better clinical prognoses and outcomes be obtained.
Developing the optimal EVT strategy is crucial for achieving the best clinical outcome, and predicting the number of thrombectomy device passes may offer benefits for formulating treatment strategies. Therefore, this study aimed to determine the risk factors associated with the number of thrombectomy device passes and establish a nomogram for predicting the number of device pass attempts in patients who have undergone successful EVT (modified thrombolysis for cerebral infarction IIb or III).
Methods and Patients
Study Design and Participants
We retrospectively reviewed our database for LVO patients who received EVT from January 2017 to July 2021 at Gansu Provincial Hospital. AIS patients were included if they had an anterior circulation occlusion onset time of less than 12 h and a posterior circulation occlusion onset time of less than 24 h. Patients treated with bridging therapy, which consisted of intravenous thrombolysis and endovascular therapy, were also included. Exclusion criteria were as follows: 1) patients who declined to provide consent for the study or patients who were unable to provide consent and had no legal representatives; 2) only received intravenous thrombolysis or intra-arterial thrombolysis without mechanical thrombectomy; 3) pregnant or had complications with hematologic malignancies or severe organ failure; 4) allergies to narcotic drugs, heparin, or interventional devices; 5) disability before stroke (defined by a modified Rankin scale (mRS) score of > 2), which would hamper the assessment of functional outcomes; and 6) failure to recanalize the vessel (modified thrombolysis for cerebral infarction 0–IIa).
Data Collection
We retrieved data, which included gender, age, history of smoking and alcohol use (smoking > 1/day for more than 1 year; drinking alcohol three times per week at an average of 50 g/day for more than 1 year), history of stroke/transient ischemic attack (TIA), hypertension, diabetes mellitus and atrial fibrillation, baseline National Institutes of Health Stroke Scale (NIHSS) score, baseline mRS score, baseline Alberta Stroke Program Early computed tomography score (ASPECTS), baseline systolic blood pressure (SBP), and baseline diastolic blood pressure (DBP), Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification, occlusion position (internal carotid artery, middle cerebral artery, anterior cerebral artery, or vertebral basilar artery), procedural features (preoperative intravenous thrombolysis, balloon-guided catheter, distal aspiration, intraoperative arterial thrombolysis, time from onset to admission, and time from onset to puncture), preoperative laboratory indicators (thrombin time [TT], D-dimer [DD], triglycerides, ureophil, low density cholesterol [LDL-C], high-density cholesterol [HDL-C], cholesterol homocysteine, and glucose), ratio of LDL-C level to HLD-C level, and outcome parameters (hemorrhagic transformation and discharge mRS score).
Statistical Analysis
Categorical variables are expressed as numbers (percentages) and continuous variables are expressed as means (standard deviation) or medians (quartile). Differences in baseline characteristics between groups for continuous variables were assessed using Student’s t-test or the Mann–Whitney U-test, according to the normality of distribution, and chi-square or Fisher’s exact tests were used for categorical variables, according to sample size. For the multivariable analysis, we adjusted for all potential confounders, and statistical significance for associations was set at p < 0.1 for the univariate analysis.
The nomogram was created by assigning a graphic preliminary score to each predictor with a point range from 0 to 100, which was summed to generate a total score. This was converted into the logit and then into individual probabilities (from 0% to 100%) of device pass numbers > 3. The discriminative performance of the nomogram was assessed by calculating the area under the receiver operating characteristic curve ((AUC-ROC). Calibration was tested using a calibration plot with a bootstrap of 1000 resamples, which described the degree of fit between the actual and nomogram-predicted device pass numbers > 3.
We also performed decision curve analysis (DCA) to estimate the clinical utility of the nomogram. DCA estimates the net benefit of a model based on the difference between the number of true-positive and false-positive results and is widely used for assessing whether a nomogram-assisted decision improves patient outcomes. Statistical analysis was performed using SPSS software version 25.0 (IBM, New York, NY) and R statistical software version 4.0.4 (R Foundation, Vienna, Austria). A two-tailed p-value < 0.05 was considered statistically significant.
Results
In total, 130 patients were enrolled in this study. Twenty-eight patients were excluded for the following reasons: only intravenous thrombolysis or intra-arterial thrombolysis was performed without mechanical thrombectomy (n = 3), sinus thrombosis (n = 5), incomplete clinical data (n = 6), and unsuccessful EVT (n = 14). Of the 130 patients (mean age 64.9 ± 11.1 years; 83 males) included in the final analysis, 46 (35.4%) had a history of smoking, 77 (59.2%) had a history of alcohol use, 33 (25.4%) had hypertension, 27 (20.8%) had diabetes mellitus, 24 (18.5%) had a history of stroke/TIA, and 38 (29.2%) had atrial fibrillation.
shows the demographics and clinical characteristics of the subgroups based on device pass numbers > 3 or ≤ 3. Compared with patients with a device pass number ≤ 3 during EVT, patients with a device pass number > 3 were older (p < 0.001), had higher baseline NIHSS scores (p < 0.001), had lower baseline ASPECTS scores (p < 0.001), had higher ureophil levels (p = 0.050), had higher LDL-C levels (p = 0.039), had higher HDL-C levels (p = 0.047), had higher homocysteine levels (p = 0.001), and had higher ratio of LDL-C to HDL-C levels (p < 0.001).
Table 1 Demographics and Clinical Characteristics of Subgroups Based on a Device Pass Number >3 or ≤3
shows the multivariate logistic regression analysis for the risk factors associated with a device pass number > 3. After adjustment for confounding factors, multivariate regression analysis detected age (odds ratio [OR] 1.085; 95% confidence interval [CI], 1.005–1.172; p = 0.036), baseline ASPECTS score (OR, 0.237; 95% CI, 0.115–0.486; p < 0.001), and homocysteine level (OR, 1.090; 95% CI, 1.028–1.155; p = 0.004) as independent predictors of device pass numbers > 3 for EVT.
Table 2 Multivariate Logistic Regression Analysis for Risk Factors Associated with Device Pass Number >3
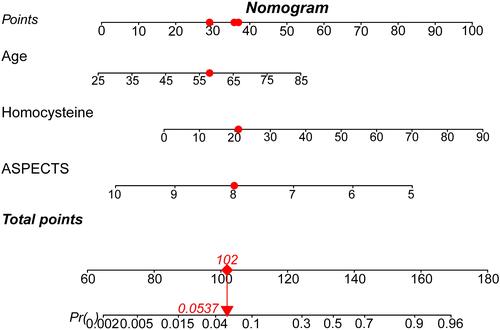
shows the nomogram model for predicting a device pass number > 3 during endovascular therapy. For example, a patient aged 58 years, with a homocysteine level of 21 mmol/L and a baseline ASPECTS score of 8.0 would have a total of 102.0 points (29.0 points for age, 36.0 points for homocysteine, 37.0 points for baseline ASPECTS score). The predicted probability for a device pass number > 3 would be approximately 5.37% for this patient.
Figure 1 Nomogram model for the prediction of a device pass number > 3 during EVT. The nomogram was developed by assigning a graphic initial score to each independent predictor with a point range from 0 to 100, which was then summed to generate a total score and converted into a percentage representing the probability of a device pass number > 3 during EVT. For example, a patient aged 58 years, with a homocysteine level of 21 mmol/L and a baseline ASPECTS score of 8.0 would have a total of 102.0 points (29.0 points for age, 36.0 points for homocysteine, 37.0 points for baseline ASPECTS score). The probability of a predicted device pass number > 3 would be approximately 5.37% for this patient.
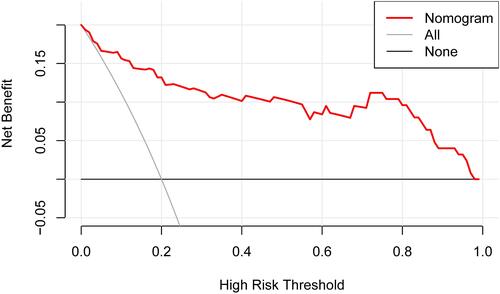
In addition, as shown in , the AUC-ROC of the nomogram was 0.921 (95% CI, 0.860–0.980), which indicated good predictive power. shows the calibration plot to compare the predictions of a device pass number > 3 between the nomogram prediction and actual observations. The calibration plot revealed good predictive accuracy of the nomogram. As shown in , the DCA demonstrated that when the threshold probabilities of the cohort ranged between 5.0% and 98.0%, the use of the nomogram to predict a device pass number > 3 provided greater net benefit than did “treat all” or “treat none” strategies.
Figure 2 Discriminability analysis of the nomogram. The discriminability of this nomogram was 0.921 (95% CI, 0.860–0.980), evaluated using the AUC-ROC, which indicated good predictive power.
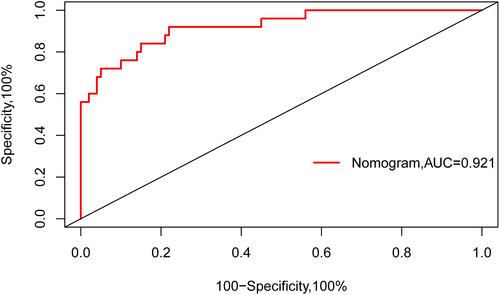
Figure 3 Calibration plot of the nomogram. Calibration plot comparing the predictions of a device pass number > 3 between the nomogram prediction and actual observations. The calibration plot of the nomogram revealed good predictive accuracy.
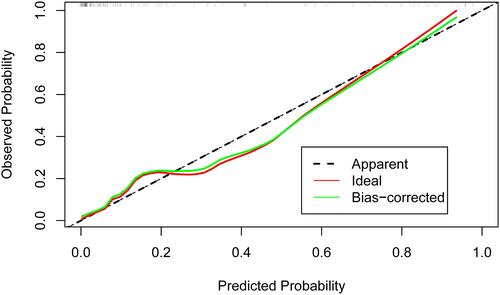
Figure 4 DCA of the nomogram. The x-axis indicates the threshold probability, and the y-axis indicates the net benefit. The DCA demonstrated that when the threshold probabilities of the cohort ranged between 5.0% and 98.0%, the use of the nomogram to predict a device pass number > 3 provided greater net benefit than did “treat all” or “treat none” strategies.
Discussion
Before thrombectomy was available, numerous nomogramic studies were conducted for intravenous tissue plasminogen activator (IV tPA).Citation17 A nomogram is a graphical statistical tool that involves developing a continuous variable scoring system and calculating accurate probabilistic risk outcomes for each patient. The instrument is an important part of modern medical decision-making and is used widely across various professional fields.Citation18–Citation21
In this study, we developed an accurate nomogram prediction model for predicting the number of thrombectomy device passes in patients undergoing EVT. The nomogram prediction model was based on age, baseline ASPECTS score, and homocysteine level in patients with AIS before undergoing EVT. As shown in and , our nomogram demonstrated good discrimination and calibration. Furthermore, the DCA indicated high availability and effect of the predictive tool.
It has previously been reported that older age and a lower baseline ASPECTS score are associated with EVT patients undergoing more device passes.Citation11,Citation12,Citation22–Citation24 In line with this, our nomogram showed that baseline ASPECTS score and age were important predictors of the number of thrombectomy device passes during EVT. In addition, the nomogram indicated that a high homocysteine level at admission was a significant predictor. A meta-analysis that included 13 case-control studies reported that plasma homocysteine level was higher in all subtypes of ischemia stroke than in healthy controls.Citation25 Homocysteine as a risk factor for cardiovascular disease has been reported as early as 1969, and recently, lower homocysteine levels have been shown to reduce the risk of stroke.Citation26–Citation28 Hyperhomocysteinemia adversely affects vascular smooth muscle cells, which leads to their proliferation and contributes to endothelial dysfunction due to oxidative stress, DNA damage, apoptosis, and the promotion of coagulation. Thus, the toxicity of homocysteine is thought to be one of the causes of vascular changes and atherosclerosis.Citation29–Citation31 These dysfunctional processes can seriously damage blood vessels and affect thrombosis, which impacts the number of thrombectomy procedures needed. Several characteristics, such as baseline NIHSS score, occlusion position, ureophil, and low-density lipoprotein, were found to not be associated with device pass number after adjusting for potential confounding factors, which may be attributed to differences in sample size, study population, and study methodology.
To the best of our knowledge, this is the first study to investigate factors related to the number of thrombectomy device passes and develop a nomogram model based on these factors, which provided the most favorable factors for formulating an optimal thrombectomy strategy. In addition, the nomogram was based on variables that can be easily acquired and are used in real-world settings. The data of recent studies on the frequency of thrombectomy are primarily from CSCs in developed areas, and the situation in less developed areas is rarely reported. This study reflects the real situation regarding EVT for AIS patients in CSCs in less developed areas.
Limitations
Several limitations of this study should be addressed. First, we only included patients in Asia, which may limit the generalizability of the results. Second, missing data may affect the accuracy of the model. Third, because of the limited sample size, the number of device passes was not divided in a more detailed manner, which may limit application scenarios of the nomogram.
Conclusion
The nomogram, which included age, baseline ASPECTS score, and homocysteine level, can be used to predict the probability of a device pass number > 3 in AIS patients undergoing EVT. Future studies will require external validation of our nomogram in different populations.
Abbreviations
EVT, endovascular thrombectomy; AIS, acute ischemic stroke; LVO, large vessel occlusion; TIA, transient ischemic attack; NIHSS, National Institutes of Health Stroke Scale; ASPECTS, Alberta Stroke Program Early computed tomography score; mRS, modified Rankin scale; SBP, systolic blood pressure; DBP, diastolic blood pressure; TT, thrombin time; DD, d-dimer; LDL-C, low-density cholesterol; HDL-C, high-density cholesterol; AUC-ROC, area under the receiver operating characteristic curve; DCA, decision curve analysis; TOAST, Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification.
Ethical Approval
The research was conducted in accordance with the provisions of the Helsinki Declaration, which was approved by the Ethics Committee of Gansu Provincial Hospital. Because of the retrospective nature of this study, informed written consent of patients was waived. Patient data were anonymized and de-identified prior to analysis.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all the following areas: drafting, revising, or critically reviewing the article; giving final approval of the version to be published; agreeing on the journal to which the article will be submitted; and agreeing to be accountable for all aspects of the work.
Acknowledgments
We thank all study participants and data collectors for their participation and cooperation. We would also like to thank the Cerebrovascular Disease Center of Gansu Provincial Hospital for its comprehensive cooperation and data support. We would like to thank Futian Tang (from Lanzhou University, China) for their advice on the English language version and revision of the final manuscript.
Disclosure
The authors declare that they have no competing interests in relation to this work.
Additional information
Funding
References
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418.
- Emmer BJ, Beumer D, Steyerberg EW, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20. doi:10.1056/NEJMoa1411587
- Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–2306. doi:10.1056/NEJMoa1503780
- Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–1018. doi:10.1056/NEJMoa1414792
- Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–1030. doi:10.1056/NEJMoa1414905
- Lee JS, Hong JM, Lee KS, et al. Endovascular therapy of cerebral arterial occlusions: intracranial atherosclerosis versus embolism. J Stroke Cerebrovasc Dis. 2015;24(9):2074–2080. doi:10.1016/j.jstrokecerebrovasdis.2015.05.003
- Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731. doi:10.1016/S0140-6736(16)00163-X
- Fransen PS, Berkhemer OA, Lingsma HF, et al. Time to reperfusion and treatment effect for acute ischemic stroke: a randomized clinical trial. JAMA Neurol. 2016;73(2):190–196. doi:10.1001/jamaneurol.2015.3886
- García-Tornel Á, Requena M, Rubiera M, et al. When to stop. Stroke. 2019;50(7):1781–1788. doi:10.1161/STROKEAHA.119.025088
- Kang D, Kim BM, Heo JH, et al. Effects of first pass recanalization on outcomes of contact aspiration thrombectomy. J Neurointerv Surg. 2020;12(5):466–470. doi:10.1136/neurintsurg-2019-015221
- Filioglo A, Cohen JE, Honig A, et al. More than five stentriever passes: real benefit or futile recanalization? Neuroradiology. 2020;62(10):1335–1340. doi:10.1007/s00234-020-02469-x
- Bourcier R, Saleme S, Labreuche J, et al. More than three passes of stent retriever is an independent predictor of parenchymal hematoma in acute ischemic stroke. J Neurointerv Surg. 2019;11(7):625–629. doi:10.1136/neurintsurg-2018-014380
- Kharouba R, Gavriliuc P, Yaghmour NE, et al. Number of stentriever passes and outcome after thrombectomy in stroke.J Neuroradiology. 2019;46(5):327–330. doi:10.1016/j.neurad.2019.03.014
- Baek J, Kim BM, Heo JH, et al. Number of stent retriever passes associated with futile recanalization in acute stroke. Stroke. 2018;49(9):2088–2095. doi:10.1161/STROKEAHA.118.021320
- Tonetti DA, Desai SM, Casillo S, et al. Successful reperfusion, rather than number of passes, predicts clinical outcome after mechanical thrombectomy. J Neurointerv Surg. 2020;12(6):548–551. doi:10.1136/neurintsurg-2019-015330
- Loh Y, Jahan R, Lieberskind DS, et al. Recanalization rates decrease with increasing thrombectomy attempts. Am J Neuroradiol. 2010;31(5):935–939. doi:10.3174/ajnr.A1958
- Yeo LLL, Chien S, Lin J, et al. Derivation and validation of a scoring system for intravenous tissue plasminogen activator use in Asian patients. J Stroke Cerebrovasc Dis. 2017;26(8):1695–1703. doi:10.1016/j.jstrokecerebrovasdis.2017.03.033
- Shen C, Wang L, Yang X, et al. Construction of an immune-associated genes based prognostic signature in bladder cancer. Artif Cells Nanomed Biotechnol. 2021;49(1):108–119. doi:10.1080/21691401.2020.1865994
- Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med. 2005;353(5):468–475. doi:10.1056/NEJMoa044154
- Cappellari M, Mangiafico S, Saia V, et al. IER-SICH nomogram to predict symptomatic intracerebral hemorrhage after thrombectomy for stroke. Stroke. 2019;50(4):909–916. doi:10.1161/STROKEAHA.118.023316
- Weiser MR, Hsu M, Bauer PS, et al. Clinical calculator based on molecular and clinicopathologic characteristics predicts recurrence following resection of stage I–III colon cancer. J Clin Oncol. 2021;39(8):911–919. doi:10.1200/JCO.20.02553
- Ducroux C, Piotin M, Gory B, et al. First pass effect with contact aspiration and stent retrievers in the Aspiration Versus Stent Retriever (ASTER) trial. J Neurointerv Surg. 2020;12(4):386–391. doi:10.1136/neurintsurg-2019-015215
- Ben Hassen W, Tordjman M, Boulouis G, et al. Benefit of first-pass complete reperfusion in thrombectomy is mediated by limited infarct growth. Eur J Neurol. 2021;28(1):124–131. doi:10.1111/ene.14490
- Flottmann F, Brekenfeld C, Broocks G, et al. Good clinical outcome decreases with number of retrieval attempts in stroke thrombectomy: beyond the first-pass effect. Stroke. 2021;52(2):482–490. doi:10.1161/STROKEAHA.120.029830
- Zhang T, Jiang Y, Zhang S, et al. The association between homocysteine and ischemic stroke subtypes in Chinese: a meta-analysis. Medicine. 2020;99(12):e19467. doi:10.1097/MD.0000000000019467
- Martí-Carvajal AJ, Solà I, Lathyris D, Dayer M. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2017;8(8):CD006612.
- Eikelboom JW, Lonn E, Genest JJ, Hankey G, Yusuf S. Homocyst(e)ine and cardiovascular disease: a critical review of the epidemiologic evidence. Ann Intern Med. 1999;131(5):363–375. doi:10.7326/0003-4819-131-5-199909070-00008
- McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56(1):111–128.
- Liu W, Zhang L, Li S, et al. A Mendelian Randomization Study of plasma homocysteine levels and cerebrovascular and neurodegenerative diseases. Front Genet. 2021;12:653032. doi:10.3389/fgene.2021.653032
- Currò M, Gugliandolo A, Gangemi C, et al. Toxic effects of mildly elevated homocysteine concentrations in neuronal-like cells. Neurochem Res. 2014;39(8):1485–1495. doi:10.1007/s11064-014-1338-7
- Mujumdar VS, Hayden MR, Tyagi SC. Homocyst(e)ine induces calcium second messenger in vascular smooth muscle cells. J Cell Physiol. 2000;183(1):28–36. doi:10.1002/(SICI)1097-4652(200004)183:1<28::AID-JCP4>3.0.CO;2-O
