Abstract
Introduction
Coronary artery disease (CAD) remains a significant global health challenge, with percutaneous coronary intervention (PCI) being a primary revascularization method. In-stent restenosis (ISR) post-PCI, although reduced, continues to impact patient outcomes. Inflammation and platelet activation play key roles in ISR development, emphasizing the need for accurate risk assessment tools. The systemic inflammation aggregation index (AISI) has shown promise in predicting adverse outcomes in various conditions but has not been studied in relation to ISR.
Methods
A retrospective observational study included 1712 patients post-drug-eluting stent (DES) implantation. Data collected encompassed demographics, medical history, medication use, laboratory parameters, and angiographic details. AISI, calculated from specific blood cell counts, was evaluated alongside other variables using machine learning models, including random forest, Xgboost, elastic networks, logistic regression, and multilayer perceptron. The optimal model was selected based on performance metrics and further interpreted using variable importance analysis and the SHAP method.
Results
Our study revealed that ISR occurred in 25.8% of patients, with a range of demographic and clinical factors influencing the risk of its development. The random forest model emerged as the most adept in predicting ISR, and AISI featured prominently among the top variables affecting ISR prediction. Notably, higher AISI values were positively correlated with an elevated probability of ISR occurrence. Comparative evaluation and visual analysis of model performance, the random forest model demonstrates high reliability in predicting ISR, with specific metrics including an AUC of 0.9569, accuracy of 0.911, sensitivity of 0.855, PPV of 0.81, and NPV of 0.948.
Conclusion
AISI demonstrated itself as a significant independent risk factor for ISR following DES implantation, with an escalation in AISI levels indicating a heightened risk of ISR occurrence.
Background
Coronary artery disease (CAD) stands as a formidable contributor to global mortality rates, accompanied by significant economic implications.Citation1 Percutaneous coronary intervention (PCI) has emerged as a primary revascularization strategy amidst the evolving landscape of CAD treatment modalities.Citation2 Advancements from conventional balloon angioplasty to contemporary second-generation drug-eluting stent (DES) implantation have substantially reduced the incidence of in-stent restenosis (ISR) from an initial 40% to the current 3%, significantly enhancing long-term patient outcomes.Citation3 Given the high prevalence of cardiovascular diseases worldwide, ISR remains a significant challenge. ISR can lead to various adverse cardiovascular outcomes, such as recurrent angina, recurrent myocardial infarction, and sudden cardiac death.Citation4 Therefore, early and accurate identification of factors contributing to DES-ISR is crucial for clinical management and prognosis.
The complex mechanisms underpinning ISR encompass a spectrum of factors, spanning dyslipidemia, coagulation irregularities, inflammatory cascades, patient-specific variables, anatomical considerations, procedural intricacies, and stent-related parameters.Citation5–8 Extensive research has underscored the pivotal role of inflammation in instigating and perpetuating ISR.Citation9 Furthermore, the synergistic interplay between inflammation and platelet activation serves as a catalyst for neointimal formation and atherosclerosis,Citation10 intensifying the progression of ISR. Immune cells of innate origin, including lymphocytes, neutrophils, and monocytes, contribute to endothelial inflammation, triggering oxidative stress and cytokine release, thereby fostering ISR pathogenesis.Citation11
Platelet activation emerges as a key player in orchestrating the intricate immune-inflammatory response, elucidating the intimate intersection between the coagulation and immune systems.Citation12 The systemic inflammation aggregation index (AISI) represents a novel prognostic marker derived from lymphocytes, neutrophils, monocytes, and platelets, offering insights into the immune-coagulation status and inflammatory milieu within the body.Citation13 Prior studies have highlighted the independent correlation between AISI levels and adverse outcomes in diverse conditions such as idiopathic pulmonary fibrosis (IPF), viral pneumonia, renal ailments, and hypertension.Citation14–17 However, there is currently no research on the association between AISI and ISR. Therefore, this study aims to investigate whether AISI is independently associated with ISR occurrence. Notably, despite the evident prognostic value of AISI across various health conditions, its potential association with ISR remains unexplored. Hence, this study seeks to illuminate the potential independent linkage between AISI levels and ISR occurrence. In light of the increasing application of machine learning (ML) as an innovative algorithmic tool in medical data analysis, this study aims to leverage the robust predictive abilities of ML algorithms to thoroughly investigate the association between AISI and ISR. The goal is to offer a more precise and dependable predictive approach for ISR.
Methods
Data collection spanned from January 2018 to December 2022, encompassing patients who underwent DES implantation at the Central Hospital of Enshi Prefecture and followed up with angiography 6–18 months post-procedure. Exclusion criteria comprised patients with cognitive impairment, autoimmune disorders, malignancies, severe infections, hepatic or renal impairment, prior coronary artery bypass grafting, balloon angioplasty, chronic inflammatory conditions, or significant valvular heart disease. Ultimately, 1712 patients were included in this retrospective observational study, which was approved by the Institutional Review Board of the Enshi Tujia and Miao Autonomous Prefecture Central Hospital in accordance with the Helsinki Declaration. Given the retrospective nature of the study, written informed consent was not required. The waiver of patient consent was granted by the Institutional Review Board based on the nature of the study and the assurance of patient data confidentiality in compliance with the Declaration of Helsinki. ISR was defined as luminal narrowing exceeding 50% post-PCI, encompassing the stent segment and adjacent vessel sections within a 5 mm span proximal and distal to the stent.Citation18
Data compilation comprised demographic details (age, sex), lifestyle habits (smoking), medical Backgrounds (hypertension, diabetes, stroke), medication regimens (anticoagulants, diuretics), and assorted laboratory parameters (complete blood count, liver and kidney function tests, glucose levels, lipid profile, and thyroid-stimulating hormone). Angiographic specifics entailed stent quantity, lesion locale, and stenosis severity. The systemic inflammation aggregation index was computed by sequentially multiplying the neutrophil, platelet, and monocyte counts and dividing by the lymphocyte count.Citation19
The machine learning models were developed using a five-fold cross-validation strategy. Patients were randomly divided into training and validation subsets at a 7:3 ratio for each machine learning (ML) model (). Notable models, including Random Forest (RF), XGBoost, neural network, logistic regression, and multilayer perceptron (MLP), were trained and evaluated using metrics such as the area under the receiver operating characteristic curve (AUC), accuracy, sensitivity, specificity, positive predictive value, and negative predictive value. A stacked ensemble learning model was constructed by integrating the predictions of the five machine learning models. A meta-model based on LASSO was applied after parameter tuning to enhance predictive performance. Calibration curves were generated to evaluate model alignment, ensuring accuracy and stability. The top ten contributors to model outcomes were visually identified through random forest analysis determining variable importance.
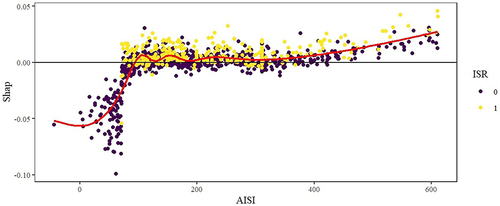
Additionally, the SHAP (SHapley Additive exPlanation) methodology was used to elucidate the Average Individual SHAP Impact (AISI). This involved examining the relationship between variables and SHAP values using scatter plots and smooth curves.
Statistical analyses were conducted using R version 4.3.2. Continuous variables were reported as mean ± standard deviation, while categorical variables were presented as counts and percentages. Statistical comparisons were carried out using the Student’s t-test for continuous variables and chi-square or Fisher’s exact tests for categorical variables, with a significance level set at P < 0.05.
Result
Baseline Information
According to , in a cohort of 1712 patients, ISR was experienced by 443 individuals, representing 25.8% of the study population. Demographic analysis unveiled that male patients, those with a history of stroke, smokers, and individuals with reduced anticoagulant usage exhibited increased susceptibility to ISR. Factors such as lesion location, stenosis severity, and the number of stents implanted also played crucial roles in ISR incidence. Laboratory investigations highlighted significantly elevated white blood cell counts and AISI levels in the ISR group compared to those without stenosis (6.53 vs 6.13; 198.56 vs 170.17). Moreover, total cholesterol and low-density lipoprotein levels were notably higher in the ISR group. Conversely, variables like age, hypertension, diabetes, diuretic use, TBIL, creatinine, glucose, uric acid, PDW, HDL-C, and TSH showed no statistical significance (P > 0.05). To ensure the transparency and integrity of the dataset and validate the model’s suitability across various datasets, we provide basic statistical descriptions of the test and validation sets in Table S1.
Table 1 The Baseline Characteristics of the Subjects Between ISR Group and Non-ISR Group
Model Comparison
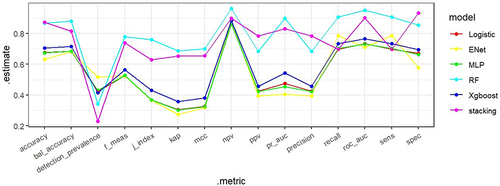
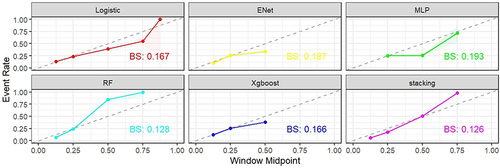
Five distinct machine learning (ML) models were developed, encompassing random forest, Xgboost, LASSO, MLP, and logistic regression. Evaluation of their performance yielded varying AUC values (): 0.9569 (random forest), 0.7358 (Xgboost), 0.7143 (elastic networks), 0.7316 (MLP), and 0.7338 (logistic regression). Notably, the random forest model demonstrated superior predictive capabilities with a high AUC (0.9569) and commendable accuracy (0.911), alongside heightened sensitivity, positive predictive value, and negative predictive value. In contrast, the Xgboost model exhibited relatively poorer performance with lower AUC (0.7358), accuracy (0.705), and positive predictive value. Comparative assessment and visualization of performance metrics among diverse models, showcased in parallel line plots, revealed the robust predictive prowess of the random forest and stacked ensemble models (), with well-fitted calibration curves in comparison to ideal curves, accentuating their high predictive accuracy (). Consequently, the random forest model emerged as the optimal choice, whereas the Xgboost model underperformed in the context of ISR prediction.
Table 2 Performance Evaluation of Five Models
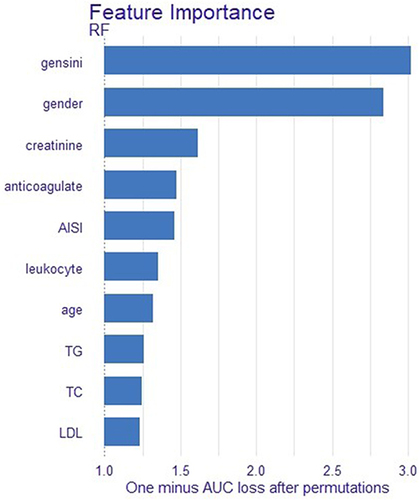
Further insight into the model optimization process was gleaned through variable importance analysis, ranking AISI fifth among the top 10 influential variables (). Subsequently, leveraging the SHAP (Shapley Additive Explanations) method, the impact of AISI on ISR was visually elucidated, elucidating an escalating ISR likelihood with increasing AISI values ().
Discussion
In our retrospective study, after comparing models, we chose the random forest as the optimal model for predicting ISR. The random forest model surpassed others in terms of AUC, sensitivity, and accuracy, showcasing superior predictive performance in our investigation. Further scrutiny of the random forest model unveiled a substantial correlation between ISR and variables such as gensini score, gender, creatinine level, postoperative anticoagulant usage, and AISI. By employing the random forest algorithm, we assessed the predictive impact of each variable on ISR and pinpointed the pivotal factors influencing prediction outcomes. Notably, among the top ten variables, AISI emerged as highly significant, underlining its crucial role in ISR prediction. Moreover, we employed the SHAP method to visually expound on ISR. Through scatter plots and smoothed curves, the relationship between AISI values and SHAP values was elucidated, aiding in clarifying the model’s predictive mechanisms and facilitating decision-making.
Paliogiannis et al proposed the utility of AISI for outcome prognostication in surgical cohorts back in 2018.Citation20 However, pertinent data regarding the impact of AISI post-drug-eluting stent (DES) percutaneous coronary intervention (PCI) on ISR remains scarce. This study pioneers the evaluation of the AISI-ISR link, establishing AISI as an autonomous risk factor for DES-related ISR in acute coronary syndrome patients. Prior literature has hinted at a mechanistic interplay where inflammation triggers early vascular trauma, fostering neointimal hyperplasia and luminal injury, while stent deployment exacerbates local inflammatory reactions.Citation21 The presence of stents as foreign entities can incite immune responses, alongside inducing arterial wall damage.Citation22 Subsequent cascades involving activated platelets, fibrinogen, neutrophils, monocytes, and lymphocytes propel an uncontrolled inflammatory spiral conducive to inflammation.Citation23 The AISI-driven ISR pathway is delineated by heightened neutrophil, platelet, and monocyte counts, coupled with diminished lymphocyte levels. Increased platelet counts denote an inflammatory-thrombotic milieu, whereas reduced lymphocytes signify unbridled inflammation.Citation24,Citation25 Platelet-neutrophil interactions instigate monocyte recruitment, fostering inflammatory factor release and perpetuating inflammation, ultimately fostering neointimal proliferation and atherosclerosis, culminating in ISR genesis.Citation26–28 AISI, inclusive of platelets alongside innate immune cells, offers a holistic panorama of the systemic inflammatory and immune-coagulation milieu, surpassing single-cell ratio markers like NLR, PLR, and SII in capturing a comprehensive snapshot of the inflammatory and immune landscape.
While our study pioneers the AISI-ISR paradigm, limitations exist. It was a single-center retrospective study with a modest sample size, warranting validation in larger-scale multicenter prospective studies. Moreover, the study solely delved into the AISI-ISR nexus sans an exhaustive evaluation of other ISR influences. Future avenues could explore AISI interplay with a broader spectrum of clinical variables to fortify a more exhaustive predictive model.
Conclusion
In summary, this seminal study unveils AISI as a potent inflammatory indicator in forecasting ISR post-coronary stent placement, unraveling potential pathogenic pathways. This discovery equips clinicians with a novel predictive instrument, forging new avenues for ISR prevention and management post-coronary stent implantation.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Ralapanawa U, Sivakanesan R. Epidemiology and the magnitude of coronary artery disease and acute coronary syndrome: a narrative review. J Epidemiol Glob Health. 2021;11(2):169–177. doi:10.2991/jegh.k.201217.001
- Hoole SP, Bambrough P. Recent advances in percutaneous coronary intervention. Heart. 2020;106(18):1380–1386. doi:10.1136/heartjnl-2019-315707
- Giustino G, Colombo A, Camaj A, et al. Coronary In-Stent restenosis: JACC State-of-the-Art review. J Am Coll Cardiol. 2022;80(4):348–372. doi:10.1016/j.jacc.2022.05.017
- Marfella R, Sardu C, D’Onofrio N, et al. SGLT-2 inhibitors and in-stent restenosis-related events after acute myocardial infarction: an observational study in patients with type 2 diabetes. BMC Med. 2023;21(1):71. doi:10.1186/s12916-023-02781-2
- Yang X, Yang Y, Guo J, et al. Targeting the epigenome in in-stent restenosis: from mechanisms to therapy. Mol Ther Nucleic Acids. 2021;23:1136–1160. doi:10.1016/j.omtn.2021.01.024
- Clare J, Ganly J, Bursill CA, Sumer H, Kingshott P, de Haan JB. The mechanisms of restenosis and relevance to next generation stent design. Biomolecules. 2022;12(3). doi:10.3390/biom12030430
- Manjunatha K, Behr M, Vogt F, Reese S. A multiphysics modeling approach for in-stent restenosis: theoretical aspects and finite element implementation. Comput Biol Med. 2022;150:106166. doi:10.1016/j.compbiomed.2022.106166
- Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56(23):1897–1907. doi:10.1016/j.jacc.2010.07.028
- Drachman DE, Simon DI. Inflammation as a mechanism and therapeutic target for in-stent restenosis. Curr Atheroscler Rep. 2005;7(1):44–49. doi:10.1007/s11883-005-0074-5
- Kleinedler JJ, Orchard EA, Foley JD, Rogers LK, Hebert VY, Dugas TR. A dietary approach to increase in-stent stenosis and face validity of a rat model for arterial angioplasty and stenting. Atherosclerosis. 2011;219(2):484–491. doi:10.1016/j.atherosclerosis.2011.09.021
- Gisterå A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol. 2017;13(6):368–380. doi:10.1038/nrneph.2017.51
- McFadyen JD, Kaplan ZS. Platelets are not just for clots. Transfus Med Rev. 2015;29(2):110–119. doi:10.1016/j.tmrv.2014.11.006
- Chen Y, Yu J, Shi L, et al. Systemic inflammation markers associated with bone mineral density in perimenopausal and postmenopausal women. J Inflamm Res. 2023;16:297–309. doi:10.2147/jir.S385220
- Xiu J, Lin X, Chen Q, et al. The aggregate index of systemic inflammation (AISI): a novel predictor for hypertension. Front Cardiovasc Med. 2023;10:1163900. doi:10.3389/fcvm.2023.1163900
- Yang Y, Xu Y, Liu S, Lu P, Zhou H, Yang M. The systemic inflammation indexes predict all-cause mortality in peritoneal dialysis patients. Ren Fail. 2023;45(1):2160348. doi:10.1080/0886022x.2022.2160348
- Zinellu A, Paliogiannis P, Mangoni AA. Aggregate Index of Systemic Inflammation (AISI), disease severity, and mortality in COVID-19: a systematic review and meta-analysis. J Clin Med. 2023;12(14). doi:10.3390/jcm12144584
- Zinellu A, Collu C, Nasser M, et al. The Aggregate Index of Systemic Inflammation (AISI): a novel prognostic biomarker in idiopathic pulmonary fibrosis. J Clin Med. 2021;10(18). doi:10.3390/jcm10184134
- Nakamura D, Dohi T, Ishihara T, et al. Predictors and outcomes of neoatherosclerosis in patients with in-stent restenosis. EuroIntervention. 2021;17(6):489–496. doi:10.4244/eij-d-20-00539
- Tejada B, Joehanes R, Hwang SJ, et al. Systemic inflammation is associated with cardiometabolic risk factors and clinical outcomes. J Inflamm Res. 2022;15:6891–6903. doi:10.2147/jir.S382620
- Paliogiannis P, Ginesu GC, Tanda C, et al. Inflammatory cell indexes as preoperative predictors of hospital stay in open elective thoracic surgery. ANZ J Surg. 2018;88(6):616–620. doi:10.1111/ans.14557
- Wu B, Mottola G, Schaller M, Upchurch GR Jr, Conte MS. Resolution of vascular injury: specialized lipid mediators and their evolving therapeutic implications. Mol Aspects Med. 2017;58:72–82. doi:10.1016/j.mam.2017.07.005
- Cook S, Wenaweser P, Togni M, et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation. 2007;115(18):2426–2434. doi:10.1161/circulationaha.106.658237
- Kuznetsova T, Prange KHM, Glass CK, de Winther MPJ. Transcriptional and epigenetic regulation of macrophages in atherosclerosis. Nat Rev Cardiol. 2020;17(4):216–228. doi:10.1038/s41569-019-0265-3
- van der Meijden PEJ, Heemskerk JWM. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol. 2019;16(3):166–179. doi:10.1038/s41569-018-0110-0
- Jin Q, Fu L, Yang H, et al. Peripheral lymphocyte count defines the clinical phenotypes and prognosis in patients with anti-MDA5-positive dermatomyositis. J Intern Med. 2023;293(4):494–507. doi:10.1111/joim.13607
- Denorme F, Campbell RA. Procoagulant platelets: novel players in thromboinflammation. Am J Physiol Cell Physiol. 2022;323(4):C951–C958. doi:10.1152/ajpcell.00252.2022
- Imazio M, Nidorf M. Colchicine and the heart. Eur Heart J. 2021;42(28):2745–2760. doi:10.1093/eurheartj/ehab221
- Lievens D, von Hundelshausen P. Platelets in atherosclerosis. Thromb Haemost. 2011;106(5):827–838. doi:10.1160/th11-08-0592