Abstract
Purpose
To develop a simple risk-scoring system to forecast scrub typhus severity.
Patients and methods
Seven years’ retrospective data of patients diagnosed with scrub typhus from two university-affiliated hospitals in the north of Thailand were analyzed. Patients were categorized into three severity groups: nonsevere, severe, and dead. Predictors for severity were analyzed under multivariable ordinal continuation ratio logistic regression. Significant coefficients were transformed into item score and summed to total scores.
Results
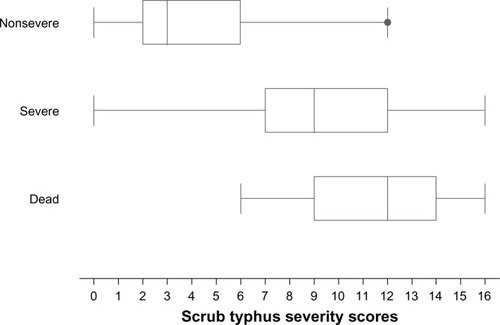
Predictors of scrub typhus severity were age >15 years, (odds ratio [OR] =4.09), pulse rate >100/minute (OR 3.19), crepitation (OR 2.97), serum aspartate aminotransferase >160 IU/L (OR 2.89), serum albumin ≤3.0 g/dL (OR 4.69), and serum creatinine >1.4 mg/dL (OR 8.19). The scores which ranged from 0 to 16, classified patients into three risk levels: non-severe (score ≤5, n=278, 52.8%), severe (score 6–9, n=143, 27.2%), and fatal (score ≥10, n=105, 20.0%). Exact severity classification was obtained in 68.3% of cases. Underestimations of 5.9% and overestimations of 25.8% were clinically acceptable.
Conclusion
The derived scrub typhus severity score classified patients into their severity levels with high levels of prediction, with clinically acceptable under- and overestimations. This classification may assist clinicians in patient prognostication, investigation, and management. The scoring algorithm should be validated by independent data before adoption into routine clinical practice.
Introduction
Scrub typhus is one of the common acute febrile illnesses encountered in tropical countries, including Thailand.Citation1,Citation2 The infection often causes vasculitis and multiple-organ failure.Citation3,Citation4 Patients with such complications usually have poor prognoses that may end with death, especially those with delayed diagnosis and treatment. Systemic complications commonly reported as causes of death include respiratory involvement (15%–36%),Citation5–Citation7 cardiovascular involvement (2%–34%),Citation5,Citation6,Citation8,Citation9 renal involvement (9%–20%),Citation6,Citation9–Citation11 hepatic involvement (4%–31%),Citation8,Citation9 central nervous system involvement (4%–23%),Citation8,Citation12 or multiple-organ involvement (11.9%).Citation13 Mortality is reported in up to 30% of cases.Citation4,Citation14
Clinical characteristics for prognostication of scrub typhus severity and death have been the subject of many studies. These characteristics may include any combinations of the following systems: respiratory system – dyspnea, crepitation, and abnormal chest film; cardiovascular system – septic shock; hepatobiliary system – serum albumin ≤3 g/dL, bilirubin >1.5 mg/dL, and more than twofold increase in aspartate aminotransferase (AST); and kidney system – serum creatinine > 1.4 mg/dL and positive urine albumin.Citation12,Citation15–Citation17 Early detection of these characteristics might be used to assist clinical guidelines for patient management.
Clinical prediction rules to forecast disease severity were developed for a few infectious diseases, such as community-acquired pneumonia.Citation18 However, there have been no studies attempting to develop a clinical risk-scoring system to forecast the severity of scrub typhus.
The present study was conducted to develop a simple clinical risk-scoring algorithm to predict the severity of scrub typhus in patients suspected of infection. The scoring system may assist routine clinical guidelines to improve patient management.
Patients and methods
This study was approved by the research ethics committees of the two hospitals in Chiang Mai and Chiang Rai, and the Faculty of Medicine at Chiang Mai University.
Patients
Retrospective data analysis was conducted at two university-affiliated general hospitals in the north of Thailand during 2004 and 2010. Patients were professionally diagnosed with scrub typhus, based on the exposure history of disease, presenting with acute fever and at least one of the following signs and symptoms: myalgia, headache, conjunctival injection, cough, profuse sweating, maculopapular rash, and lymphadenopathy,Citation14 accompanied by the presence of eschar and/or positive immunochromatographic test for scrub typhus. Patients were classified into three groups: 1) nonsevere – patients without any complications; 2) severe – patients with severe complications; and 3) dead – those who died in hospital from scrub typhus. Patients undergoing any intervention trials during the same period were excluded from data analysis.
Definitions of severe scrub typhus
Severe scrub typhus was operationally defined as patients who presented with involvement of at least one of the following organ systems.
Cardiovascular system – presence of any of the following:
○ Systolic blood pressure less than 90 mmHg
○ Abnormal cardiac arrhythmia with no previous history of
§ atrial fibrillation (AF),
§ supra ventricular tachycardia (SVT), or
§ frequent premature ventricular tachycardia (PVC)
○ Myocarditis: elevated creatine kinase MB above baseline
Respiratory system: presence of acute respiratory distress syndrome, defined as PaO2/FiO2 <200 mmHg, with bilateral interstitial infiltration on chest film with normal cardio/thoracic ratio, or no volume overload of central venous pressure from central venous catheter
Central nervous system – presence of any of the following:
○ Glasgow Coma Scale ≤12 without other causes,
○ Seizure without other causes, or
○ Meningoencephalitis
Hematology: platelet count ≤20,000/mm3
Urinary tract: presence of acute renal failure, defined as creatinine ≥2 mg/dL or creatinine change >0.5 mg/dL/day
Gastrointestinal and hepatobiliary tract: presence of hepatitis, defined as elevated AST or alanine aminotransferase (ALT) more than fivefold.
Predictor parameters
Demographic and disease-exposure histories were obtained from medical files recorded at the time of admission: sex, age, underlying diseases (liver cirrhosis, chronic obstructive pulmonary disease, hypertension, diabetes, and human immunodeficiency virus infection), durations of fever.
Signs and symptoms were obtained from chief complaint, history-taking records, and physical examination notes: myalgia, headache, conjunctival injection, cough, maculopapular rash, eschar, lymphadenopathy, abdominal pain, nausea/vomiting, diarrhea, jaundice, hepatomegaly, splenomegaly, seizure, stiff neck, wheezing, crepitation, dyspnea, respiration rate, and presence of fever.
Hemodynamic profiles were obtained from vital-sign record forms: systolic and diastolic blood pressure and pulse rate. Laboratory profiles were obtained from laboratory investigations requested within 24 hours after admission: hematological, biochemistry, urine analyses.
Data analysis
Patient clinical characteristics were compared across the three severity groups by nonparametric trend tests across ordered groups. Strong potential predictors were explored by multivariable ordinal continuation ratio logistic regression (α<0.001). The coefficients of significant predictors were transformed into item scores and summed as scrub typhus severity scores. The scores were classified into risk levels based on inclining of actual risks. Score-categorized risk levels were compared to criterion risk levels to indicate the score performances.
Results
A total of 526 patients with document-confirmed professional diagnosis of scrub typhus were retrieved; 215 cases presented with only eschar, 227 cases had positive immunochromatographic test for scrub typhus, and 84 cases had shown both. These patients were classified as nonsevere scrub typhus (n=357), severe scrub typhus (n=100), and dead of scrub typhus (n=69). The majority of patients were males with similar underlying diseases and durations of fever, but were different according to age ().
Table 1 Patient profiles (n=526)
Clinical signs and symptoms were similar for myalgia, conjunctival injection, cough, abdominal pain, nausea/vomiting, maculopapular rash, eschar, hepatomegaly, splenomegaly, and respiration rate. Those that were different were headache, diarrhea, jaundice, lymphadenopathy, seizure, stiff neck, wheezing, crepitation, dyspnea, body temperature, systolic blood pressure, diastolic blood pressure, and pulse rate ().
Similar laboratory results included hematocrit, hemoglobin, serum globulin, potassium, and chloride. Differences were detected on white blood cell (WBC) count, platelets, neutrophils, lymphocytes, monocytes, total bilirubin, direct bilirubin, albumin, AST, ALT, alkaline phosphatase, blood urea nitrogen creatinine, blood sodium, carbon dioxide, urine albumin levels, and urine sugar (). There were some missing data, as indicated in the corresponding tables.
Table 2 Laboratory profiles (n=526)
Significant predictors
Multivariable significant (P<0.001) predictors for severity were age >15 years (odds ratio [OR] 4.09, 95% confidence interval [CI] 2.26–7.40), pulse rate >100/minute (OR 3.19, 95% CI 1.87–5.43), crepitation (OR 2.97, 95% CI 1.63–5.39), AST >160 IU/L (OR 2.89, 95% CI 1.89–4.43), serum albumin ≤3.0 g/dL (OR 4.69, 95% CI 2.95–7.45), and serum creatinine >1.4 mg/dL (OR 8.19, 95% CI 5.06–13.35) (). Multicollinearity among variables was tested and was insignificant.
Table 3 Significant predictors and assigned item score
The scoring system
Item scores were derived by dividing the coefficient of significant predictors with the smallest coefficient (1.06) and rounding up to the nearest discrete number. The item scores varied from the minimum of 0 to the maximum of 4. Each item scores were summed up to total scrub typhus severity score, which may vary from 0 to 16 ().
Discrimination
The mean severity scores in the nonsevere, severe, and dead groups were 4.2±2.5, 8.9±3.1, and 11.4±2.8 ( and ).
Table 4 Score-classified severity levels, criterion-classified severity levels, and risk-estimation validity
Clinical predictions
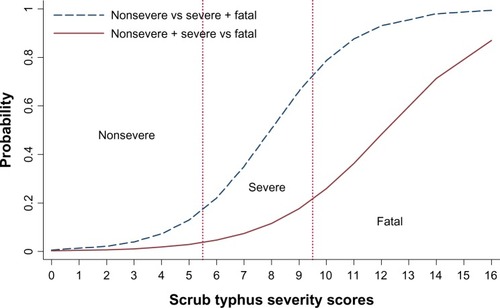
The scores discriminated patients with severe or fatal scrub typhus from those with nonsevere scrub typhus with highlevel validity (area under receiver operating characteristic curve (AuROC) 0.9148, 95% CI 0.8886–0.9410). The corresponding values for discriminating the fatal from the severe and nonsevere groups were lower (AuROC 0.9122, 95% CI 0.8823–0.9420) ().
By classifying the severity scores into three risk levels – nonsevere (scores ≤ 5), severe (scores 6–9), and most severe or fatal (scores ≥10) – the scores classified 266 out of 357 patients correctly in the nonsevere group, with underestimation by one level in twelve cases and no instances of underestimation by two levels. In the severe group, the score classified correctly 43 out of 100 patients, with 19 cases underestimated and 81 cases overestimated. In the dead group, classification was correct in 50 out of 69 cases, with overestimation by one level in 45 cases and overestimation by two levels in ten cases ().
Discussion
Although patients with early and nonsevere scrub typhus usually respond very well to prompt treatment, patients with complications have a poorer prognosis, and the disease may well be fatal for them. Researchers have attempted to develop scoring systems to predict infectious diseases or their severity to help clinical management. Examples were a dengue fever scoring system to differentiate dengue fever from rickettsial and Q fever, which was developed in Taiwan,Citation19 decision-tree algorithms to differentiate and prognosticate dengue hemorrhagic fever,Citation20 and a prediction rule to evaluate the risk for community-acquired pneumonia to help admission decisions.Citation18 These prediction rules used parameters of epidemiologic, demographic, clinical signs and symptoms, or laboratory characteristics. They all shared similar limitations of relatively large numbers of parameters, complicated laboratory tests beyond routine investigations, or tests that may be unavailable in many settings. These restrictions limited clinical application in routine practice.
For scrub typhus, there have been no studies attempting to develop a clinical algorithm to forecast disease severity to assist clinical diagnosis, evaluation, or management. The present study proposed a simple scoring system based on routinely clinical parameters available in most health care settings.
Predictors proposed in this study were more or less similar to what have been proposed in previous studies, such as serum albumin ≤3 g/dLCitation21 and serum creatinine >1.4 mg/dL.Citation17 Some parameters were similar but different in categorization, such as the patient ageCitation21,Citation22 and AST level.Citation17 Some parameters were reported but were not significant in the present study, such as WBC count,Citation17 platelets,Citation17 and bilirubin.Citation17
The present scrub typhus severity score, which ranged from 0 to 16 could be classified into three levels to simulate three levels of disease severities. We proposed the following interpretations and guidelines.
Patients scoring 0–5 were categorized as “nonsevere scrub typhus”. These patients may be managed as outpatients. Antirickettsial agents like doxycycline, chloramphenicol, or azithromycin should be prescribedCitation23,Citation24 and scheduled for follow-up.
Patients scoring 6–9 were categorized as “severe scrub typhus”. These patients are at higher risk of complications, and should be admitted to hospital for close observation. Further investigation and additional intervention may be needed. Patients encountered in lower-level hospitals might be referred to better-equipped hospitals.
Patients scoring 10 or above were classified as “fatal scrub typhus”. These patients experienced the highest risk of death and should be fully investigated for system abnormalities or other life-threatening clinical risks, and should be admitted to an intensive care unit for close monitoring.
An overestimation (25.8%) in the nonsevere group may have resulted in overzealous case management, but this may be considered beneficial for patients. Underestimation was observed in 3.6% of patients with severe scrub typhus. However, the same antirickettsial agents were routinely prescribed in these patients, and the clinical risk should therefore be considered acceptable.
The diagnosis of scrub typhus in the present study was defined as suspected cases based on World Health Organization criteria. This is the most practical definition used in poor, developing countries, where definite diagnosis like indirect immunofluorescence antibodies was hardly available in routine practice. We (the authors) believe that this definition, although not definite, is more relevant in the real world.
The present scoring system, like any other clinical prediction rules, should be validated by independent data before application to routine clinical practice.
Conclusion
The derived scrub typhus severity score classified patients into their severity levels with high levels of prediction, with clinically acceptable under- and overestimations. This classification may assist clinicians in patient prognostication, investigation, and management. Like other clinical prediction rules, the present scoring algorithm needs to be validated by independent data before being adopted into routine clinical practice.
Acknowledgments
The authors wish to thank the authorities of the two hospitals for their support. The study was partially funded by a grant from The Graduate School of Chiang Mai University, Chiang Mai, Thailand.
Disclosure
The authors report no conflicts of interest in this work.
References
- LeelarasameeAChupaprawanCChenchittikulMUdompanthuratSEtiologies of acute undifferentiated febrile illness in ThailandJ Med Assoc Thai200487546447215222513
- SuttinontCLosuwanalukKNiwatayakulKCauses of acute, undifferentiated, febrile illness in rural Thailand: results of a prospective observational studyAnn Trop Med Parasitol2006100436337016762116
- MoronCGPopovVLFengHMWearDWalkerDHIdentification of the target cells of Orientiatsutsugamushi in human cases of scrub typhusMod Pathol200114875275911504834
- WattGKantipongPOrientia tsutsugamushi and scrub typhusRuoultDParolaPRickettsial DiseasesNew YorkInformation Healthcare2007236256
- JeongYJKimSWookYDLeeJWKimKILeeSHScrub typhus: clinical, pathologic, and imaging findingsRadiographics200727116117217235005
- TsayRWChangFYSerious complications in scrub typhusJ Microbiol Immunol Infect199831424024410496165
- WangCCLiuSFLiuJWChungYHSuMCLinMCAcute respiratory distress syndrome in scrub typhusAm J Trop Med Hyg20077661148115217556627
- ChantaCTriratanapaKRatanasirichupPMahapromWHepatic dysfunction in pediatric scrub typhus: role of liver function test in diagnosis and marker of disease severityJ Med Assoc Thai200790112366236918181321
- KumarMKrishnamurthySDelhikumarCGNarayananPBiswalNSrinivasanSScrub typhus in children at a tertiary hospital in southern India: clinical profile and complicationsJ Infect Public Health201251828822341847
- RathiNRathiARickettsial infections: Indian perspectiveIndian Pediatr201047215716420228429
- YenTHChangCTLinJLJiangJRLeeKFScrub typhus: a frequently overlooked cause of acute renal failureRen Fail200325339741012803503
- JimWTChiuNCChanWTClinical manifestations, laboratory findings and complications of pediatric scrub typhus in eastern TaiwanPediatr Neonatol20095039610119579755
- SuputtamongkolYSuttinontCNiwatayakulKEpidemiology and clinical aspects of rickettsioses in ThailandAnn NY Acad Sci2009116617217919538278
- World Health OrganizationWHO recommended surveillance standards Available from: http://www.who.int/csr/resources/publications/surveillance/whocdscsrisr992.pdfAccessed October 5, 2011
- LeeCSHwangJHLeeHBKwonKSRisk factors leading to fatal outcome in scrub typhus patientsAm J Trop Med Hyg200981348448819706919
- ThapLCSupanaranondWTreeprasertsukSKitvatanachaiSChinprasatsakSPhonratBSeptic shock secondary to scrub typhus: characteristics and complicationsSoutheast Asian J Trop Med Public Health200233478078612757226
- VargheseGMAbrahamOCMathaiDScrub typhus among hospitalised patients with febrile illness in South India: magnitude and clinical predictorsJ Infect2006521566016368461
- FineMJAubleTEYealyDMA prediction rule to identify low-risk patients with community-acquired pneumoniaN Engl J Med199733642432508995086
- ChangKLuPLKoWCDengue fever scoring system: new strategy for the early detection of acute dengue virus infection in TaiwanJ Formos Med Assoc20091081187988519933032
- TannerLSchreiberMLowJGHDecision tree algorithms predict the diagnosis and outcome of dengue fever in the early phase of illnessPLoS Negl Trop Dis200823e19618335069
- KimDMKimSWChoiSHYunNRClinical and laboratory findings associated with severe scrub typhusBMC Infect Dis20101010820433689
- WuKMWuZWPengGQWuJLLeeSYRadiologic pulmonary findings, clinical manifestations and serious complications in scrub typhus: experiences from a teaching hospital in eastern TaiwanInt J Gerontol200934223232
- ChrispalABooruguHGopinathKGScrub typhus: an unrecognized threat in South India – clinical profile and predictors of mortalityTrop Doct201040312913320360426
- SuputtamongkolYScrub typhusPoster presented at: 13th International Congress on Infectious DiseasesJune 19–22, 2008Kuala Lumpur, Malaysia