Abstract
Background
Ischemic heart disease (IHD) is characterized by lesions in major coronary arteries produced by the atherosclerotic phenomenon. IHD is currently thought to be a complicated disorder, and studies have revealed that, in addition to the usual traditional risk factors, genetic factors also play major roles in its occurrence. Due to the intricate interactions between genetic and environmental risk factors, the link between ACE polymorphisms and other risk variables in IHD is not fully characterized. The purpose of this study was to look at how ACE gene I/D polymorphism and dyslipidemia affect the risk of developing IHD complications in hypertensive patients.
Methods
A hospital-based case–control study of 70 hypertensive IHD patients and 70 age- and sex-matched healthy controls was conducted. Clinical parameters were measured to assess the associated risk factors. Deoxyribonucleic acid (DNA) was isolated from blood samples, and the ACE I/D genotypes were identified using polymerase chain reaction (PCR) and analyzed by agarose gel electrophoresis.
Results
Our analysis showed that the ACE-DD genotype (OR = 2.72, 95% CL = 1.11–6.64; P < 0.05) and D allele (OR = 1.93, 95% CL = 1.18–3.13; P < 0.05) are considerably higher in patients than controls. Our study also identified dyslipidemia, which was found to be considerably greater in patients than controls (OR = 4.69, 95% CL = 1.86–11.82; P < 0.001), indicating that it is a major risk factor for the onset and progression of IHD.
Conclusion
The ACE I/D gene of the DD genotype and the D allele have been linked to an increased risk of developing hypertensive IHD complications. Moreover, dyslipidemia is a risk factor for the onset of ischemic heart disease.
Introduction
Ischemic heart disease (IHD) is one of the leading causes of death globally and is characterized by fat accumulation in the blood vessels, a reduction of the artery cavity, slowed blood flow, and ultimately ischemic heart failure.Citation1 IHD is currently regarded as a complex illness, and new research has demonstrated that genetic and environmental variables play a significant role in the development of the disease in addition to established risk factors such as age, sex, smoking, dyslipidemia, hypertension (HTN), and type 2 diabetes mellitus T2DM.Citation2 Hypertension, a significant risk factor for IHD, is estimated to cause 7.5 million deaths worldwide. It has been demonstrated that blood pressure levels are gradually and favorably associated with the onset of ischemic heart disease.Citation3
Lipids and lipoproteins are becoming increasingly important in clinical practice, mainly because they are associated with IHD in cases of abnormalities known as dyslipidemia.Citation4 Increased blood levels of triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), or total cholesterol (TC) and decreasing blood level of high-density lipoprotein cholesterol (HDL-C) are the hallmarks of dyslipidemia.Citation5 Although estimates suggest that over 50% of adults globally have dyslipidemia, the prevalence of the condition varies depending on the population.Citation6
The ACE, a key element of RAAS, catalyzes the formation of the vasoactive peptide angiotensin II from its substrate, angiotensin I. Angiotensin II plays a critical role in the control of blood pressure, sodium homeostasis, fluid volume, and electrolyte balance.Citation7 Apart from this role, recent evidence suggests that RAAS and its component ACE are involved in the pathogenesis of IHD by promoting the development of HTN and dyslipidemia. The I/D polymorphism of the ACE gene is due to an insertion (I) or deletion (D) of a 287-base pair (bp) Alu sequence in intron 16, giving rise to three genotypes: II, ID, and DD.Citation8 Previous research has indicated that the ACE DD genotype is linked with higher plasma ACE concentrations and increased vasoconstriction, which raises the risk of HTN-related IHD complications. However, this is controversial, as researchers have produced contradictory findings with no link between the DD genotype and IHD.Citation9 Thus, this study aimed to identify the link between the ACE gene I/D polymorphism and hypertensive IHD complications and, further, to examine the effect of dyslipidemia on the onset of IHD in the Ethiopian population.
Materials and Methods
Study Participants
From May to August 2022, a hospital-based matched case-control study was conducted at Debre Tabor Referral Hospital. It has a follow-up medical referral clinic (MRC) for serious chronic conditions like IHD and HTN, where treatment and patient follow-up are provided. All patients who visit MRC were the source population, and patients who are under follow-up for HTN with IHD complications were study subjects. The controls for this investigation were any normotensive healthy volunteers who were available during the study period and matched for age and sex. A total of 140 participants of both sexes were enrolled in the study, including 70 IHD patients with hypertension and 70 healthy control subjects. Patients who are diagnosed with kidney disease, secondary HTN, or a chronic bacterial or viral infection, or who are unable to respond or are not willing to sign informed consent were excluded.
Data Collection Methods
The socio-demographic characteristics of both patients and healthy control subjects were taken through a semi-structured questionnaire. Portable digital scales and portable stadiometers were used to determine body weight and height, respectively. Weight in kilograms divided by the square of height in meters is how body mass index (BMI) is determined. Participants were classified as underweight (BMI <18.5 kg/m2), healthy (18.5–25 kg/m2), overweight (25.0–29.9 kg/m2) or obese (≥30 kg/m2) based on their BMI.Citation10 A digital instrument was used to measure blood pressure in the sitting stance after 5 minutes of rest, and the mean of three readings was used to compute SBP and DBP. Participants were categorized as hypertensive, if mean SBP ≥140mmHg and mean DBP ≥90mmHg or if they used antihypertensive medication; pre-hypertension, SBP 120–139 mmHg or DBP 80–89 mmHg; normal blood pressure, SBP <120 mmHg and DBP <80 mmHg.Citation11
Sample Collection and Laboratory Methods
All participants, including patients and healthy controls, had a blood sample of 5 mL taken from the median cubital vein by laboratory staff under quality control and safety procedures. From the 5 mL sample, 3 mL was retained in the test tube without anticoagulants to allow the blood to clot. The tubes were then spun at 7602 ×g for 5 minutes to extract the serum, which was then collected into new tubes for biochemical tests. Enzymatic analyses of TC, TG, LDL, HDL, creatinine, and glucose were performed on each test in the Debre Tabor Referral Hospital diagnostic laboratory using the Dimension EXL 200 fully automated analyzer. If the fasting plasma glucose concentration is greater than 110 mg/dL, diabetes mellitus has been identified.Citation12 Dyslipidemia can be defined if TC, TG, and LDL levels are above 200 mg/dL, 150 mg/dL, and 1300 mg/dL, respectively, and the HDL level is below 60 mg/dL.Citation13 Kidney disease is diagnosed if the blood creatinine concentration is greater than 1.3 mg/dL.Citation14
Genomic DNA was extracted from the remaining 2 mL samples collected in ethylenediaminetetraacetic acid (EDTA) (anticoagulant) containing tubes of each participant in the University of Gondar molecular biology laboratory. The non-enzymatic salting-out approachCitation15 was used to isolate DNA from EDTA anticoagulated blood from both patients and controls. This blood was then transferred to a sterile 1.5-mL Eppendorf tube. Red blood cells (RBCs) were lysed and eliminated using a buffer solution. Similarly, nuclear lysis buffer solution was used to lyse white blood cells. Then, to precipitate and remove proteins, 6M NaCl of a highly concentrated salt was applied. After freezing with isopropanol and washing with 70% ice-cold ethanol, the DNA was precipitated. Genomic DNA was then dissolved in Tris-EDTA buffer (TE). The purity of extracted genomic DNA was verified utilizing 1% agarose gel electrophoresis (), and the sample was stored at −20 °C till used.Citation16
Direct PCR was used to identify the I/D alleles of the ACE gene polymorphism using specific primers (5′- CTG GAG ACC ACT CCC ATC CTT TCT-3′ and 5′- GAT GTG GCC ATC ACA TTC GTC AGA T-3′, respectively).Citation17 A final volume (25 μL) of PCR mixture was prepared by combining 12.5 μL of master mix (MgCl2, dNTPs, PCR buffer, and Taq polymerase), 1 μL of forward primer, 1μL of reverse primer, 2 μL of each sample, and 8.5 μL of PCR-grade water. The first denaturation step of the PCR amplification was set at 95℃ for 5 minutes. The DNA was then amplified for 35 cycles with denaturation at 94℃ for 30s, annealing at 58℃ for 30s, extension at 72℃ for 1 minute, and a final extension at 72℃ for 5 minutes.Citation18
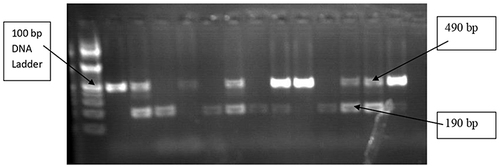
ACE I/D genotypes 490 bp band (II), 190 bp band (DD), and both 490 and 190 bp band (ID) PCR products were electrophoretically separated for 50 minutes at 120 V on a 2% agarose gel (). The PCR-amplified products (12 μ l) were mixed with 3 μ l of loading dye before being injected into the agarose gel wells. DNA ladders, which are molecular weight markers, were electrophoresed along with the DNA fragments to be able to estimate the sizes of fragments of interest, and 3 μ l of 2% Ethidium Bromide was also used for staining. In 1X tris acetate EDTA (TAE) buffer, electrophoresis was performed, and a UV transilluminator was used to see the gel.Citation19
Statistical Analysis
The data were analyzed using STATA version 14.1. The means and standard deviations (x±s) were the measures used to describe quantitative data. A t-test for independent samples was used to compare continuous variables between hypertensive IHD patients and healthy controls. Using the chi-square test, the genotype and allele frequency distributions were compared. The risk associations of ACE gene I/D polymorphisms with hypertensive IHD were assessed using logistic regression at a 95% confidence level (CL). A one-way analysis of variance (ANOVA) was used to compare the association between the ACE genotypes and clinical variables. P values of less than 0.05 were considered statistically significant.
Results
Socio-Demographic and Clinical Characteristics
Distribution by sex and age was similar between hypertensive IHD patient cases and normotensive healthy control groups. Of the total 70 hypertensive IHD participants, 37 (52.86%) were male and 33 (47.14%) were female. Similarly, among the 70 healthy control groups, 36 (51.43%) were males and 34 (48.57%) were females. The mean ages of the cases and control study groups were 59.84 ±14.25 and 57.21 ± 6.66, respectively. SBP, DBP, TC, TG, and LDL-C levels were considerably higher on average in patients compared to controls, but HDL-C levels were lower (p < 0.001). Patients and controls did not differ in terms of family history of HTN and IHD (p > 0.05), nor did they differ in terms of body mass index (BMI), fasting blood glucose (FBG), or blood creatinine (CR) level (p > 0.05) ().
Table 1 Demographic, Clinical and Behavioural Characteristics of the Study Participants in Debre Tabor Referral Hospital, Northwest Ethiopia, 2022
Distribution of ACE Genotypes and Allele Frequencies
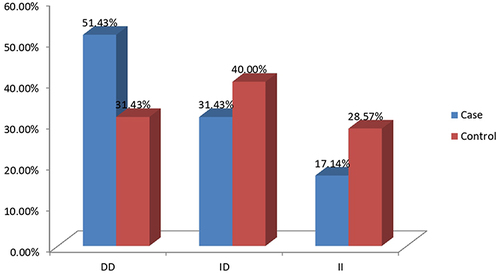
The genotype distribution of ACE I/D gene polymorphisms is given in . In IHD patients, the frequencies of the DD, ID, and II genotypes were respectively 51.43%, 31.43%, and 17.14%; in controls, the rates were 31.43%, 40.00%, and 28.57% (). The homozygous ACE DD genotype was observed to be substantially more common in IHD cases than in controls (odds ratio [OR] = 2.72, 95% CL = 1.11–6.64; P = 0.007).
Table 2 Distribution of ACE Genotypes and Allele Frequencies of the Study Participants in Debre Tabor Referral Hospital, Northwest Ethiopia, 2022
Association Between ACE Genotypes and Clinical Parameters
lists the clinical parameters of hypertensive IHD patients and normotensive healthy controls in relation to ACE ID genotypes. The ACE genotypes (DD, ID, and II) in the study groups were assessed with fasting blood glucose, blood pressure, and lipid profiles. Blood pressure was more strongly correlated with the ACE-DD genotype than the ID and II genotypes for SBP (136.65±15.68 Vs 129.24±16.02 and 125.81±15.55; P < 0.01) and DBP (85.10±11.91 Vs 82.40±8.57 and 80.91±8.14; P < 0.05), respectively. The other clinical parameters were not found to be significant with the genotypes in the study groups (P > 0.05).
Table 3 Association of ACE I/D Genotype with Clinical Characteristics in Debre Tabor Referral Hospital, Northwest Ethiopia, 2022
Association Between Ischemic Heart Disease and Dyslipidemia
The association between IHD and dyslipidemia was analyzed in our study. A comparison of lipid profiles between patients and controls revealed that patients with hypertensive IHD had lower levels of HDL-C and higher levels of TC, TG, and LDL-C than apparently healthy control groups (P < 0.001), and patients had a higher percentage of dyslipidemia 34.28% (n=24) as compared with controls 10% (n=7) ().
Table 4 Association Between Ischemic Heart Disease and Dyslipidemia in Debre Tabor Referral Hospital, Northwest Ethiopia, 2022
Discussion
Ischemic heart disease is a polygenic illness that is characterized by complicated interactions across several pathophysiological processes involving numerous genes and environmental risk factors. Considerable research has looked at how the D allele and IHD are related, and the ACE DD genotype is linked to greater blood ACE activity.Citation20 In our study, the ACE-DD genotype (odds ratio [OR] = 2.72, 95% CL = 1.11–6.64; P = 0.027) and D allele (odds ratio [OR] = 1.93, 95% CL = 1.18–3.13; P = 0.007) showed a stronger association with IHD patients compared to ID/II genotype and I allele, respectively. Compared with other studies, our results are in agreement with a meta-analysis carried out in the Chinese population that included 44 studies and found that patients with the DD genotype were found to be nearly twice as likely to develop IHD compared to the ID and II genotypes (odds ratio [OR] = 1.95, 95% CL = 1.66–2.29; P < 0.001).Citation21 The findings of other investigations conducted in IranCitation22 and PakistanCitation23 did not, however, uncover any links between IHD and the ACE I/D polymorphisms.
The etiology by which the ACE I/D genotype may influence individuals to develop IHD remains incomplete. According to a number of findings, coronary endothelial dysfunction is linked to significant coronary risk factors.Citation24 The pathophysiology of IHD and endothelial dysfunction are both affected by the ACE I/D polymorphism. Increased ACE activity raises angiotensin II, which influences cell development and proliferation by triggering a number of cytokines and growth factors that reduce nitric oxide bioavailability and result in endothelial dysfunction.Citation25 It was previously reported that increased ACE expression in macrophages and smooth muscle cells from coronary artery plaques suggests that ACE activity in lesions is a major cause of IHD progression.Citation26
We also analyzed a number of conventional cardiovascular risk variables in patients with IHD and found that HTN and dyslipidemia significantly influence the occurrence of IHD. These findings agreed with research conducted in Pakistan,Citation23 Egypt,Citation27 and Iran.Citation28 Hypertension and dyslipidemia contribute to IHD through a number of different mechanisms. An increased risk of developing dyslipidemia, atherosclerosis, and IHD may result from abnormal processes such as arterial wall damage, metabolic abnormalities, oxidative stress, and endothelial dysfunction.Citation29
Our study indicated that IHD patients had significantly higher levels of dyslipidemia than did controls (odds ratio [OR] = 4.69, 95% CL = 1.86–11.82; P< 0.001). It is increasingly documented that dyslipidemia has been identified as a major risk factor for HTN-correlated IHD complications. However, it is still uncertain whether there is a complex interplay between genetic predisposition and environmental variables in the emergence of dyslipidemia.Citation30 So, we explored the link between ACE gene I/D polymorphism and dyslipidemia based on TC, TG, LDL-C, and HDL-C levels. According to our findings, the relationship between ACE gene I/D polymorphisms and dyslipidemia in our study group was not significant (P > 0.05) ().
Conclusion
In summary, this study shows an association between the DD genotype and D allele of the ACE gene I/D polymorphisms and the occurrence of hypertensive IHD complications. In order to diagnose HTN early, identify it, and avoid its IHD consequence, the ACE gene I/D polymorphism may be employed as a biomarker. Additionally, IHD was linked to higher SBP, DBP, TC, TG, LDL-C, and lower HDL-C values. These findings further support the key significance of the relationship between HTN and dyslipidemia in the onset of ischemic heart disease.
Abbreviations
ACE, angiotensin-converting enzyme; BMI, body mass index; IHD, ischemic heart disease; DNA, deoxyribonucleic acid; DBP, diastolic blood pressure; EDTA, ethylenediaminetetraacetic acid; FBG, fasting blood glucose; HDL, high-density lipoprotein; HTN, hypertension; I/D, insertion/deletion; LDL, low-density lipoprotein; PCR, polymerase chain reaction; RAAS, renin–angiotensin–aldosterone system; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; T2DM, type 2 diabetes mellitus.
Data Sharing Statement
The corresponding author can provide the data used and/or analyzed during the current study upon request.
Ethics Approval and Consent to Participate
The study protocol was approved by the University of Gondar Institutional Review Board (IRB) for Ethics in Human Research (Ref. VP/RTT/05/1016/2022). Study participants were recruited only after informed written consent was obtained from each of them. All the data were obtained anonymously and treated confidentially. All the procedures for data collection were conducted according to the principles of the Helsinki Declaration.
Author Contributions
Both authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable or all aspects of the work.
Disclosure
The authors report no conflicts of interest.
Acknowledgments
We would like to give our heartfelt thanks to the Debre Tabor Referral Hospital diagnostic laboratory and the University of Gondar molecular biology laboratory staff for the support we needed to do this study.
References
- Rajabi R. ACE gene Polymorphism in coronary artery disease in West Bank, Palestine. Hebron Univ Res J. 2020;9(1):0–10.
- Amara A, Mrad M, Sayeh A, et al. The effect of ACE I / D polymorphisms alone and with concomitant risk factors on coronary artery disease. Clin Appl Thromb Hemost. 2018. doi:10.1177/1076029616679505
- Fang J, Ayala C, Luncheon C, Ritchey M, Loustalot F. Use of outpatient cardiac rehabilitation among heart attack survivors — 20 states and the district of Columbia, 2013 and Four States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(33):869–873. doi:10.15585/mmwr.mm6633a1
- Hedayatnia M, Asadi Z, Zare-Feyzabadi R, et al. Dyslipidemia and cardiovascular disease risk among the MASHAD study population. Lipids Health Dis. 2020;19(1):1–11. doi:10.1186/s12944-020-01204-y
- Shim YS, Baek JW, Kang MJ, Oh YJ, Yang S, Hwang IT. Reference values for the triglyceride to high-density lipoprotein cholesterol ratio and non-high-density lipoprotein cholesterol in Korean children and adolescents: the Korean national health and nutrition examination surveys 2007–2013. J Atheroscler Thromb. 2016;23(12):1334–1344. doi:10.5551/jat.35634
- Riad M, Adhikari P, Bhattarai S, et al. Risk assessment using the association between renin-angiotensin genes polymorphisms and coronary artery disease. Cureus. 2021;13(3):3–8. doi:10.7759/cureus.14083
- Raza ST, Abbas S, Siddiqi Z, Mahdi F. Association between ACE (rs4646994), FABP2 (rs1799883), MTHFR (rs1801133), FTO (rs9939609) genes polymorphism and type 2 diabetes with dyslipidemia. Ijmcm. 2017;6(2):121–130.
- Mustafa AI, Ibrahim SE, Gohary YM, Al-Husseini NF, Fawzy E, El-Shimi OS. Association between angiotensin-converting enzyme gene insertion deletion polymorphism and androgenetic alopecia susceptibility among Egyptian patients: a preliminary case-controlled study. J Cosmet Dermatol. 2022;21(6):2629–2634. doi:10.1111/jocd.14434
- Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Circulation. 2015;131. doi:10.1161/CIR.0000000000000207
- Tamiru T, Berhane N, Sendeku W, Menegesha D. Association of angiotensin converting enzyme (ACE) gene polymorphisms and risk of diabetic 2 among patients visiting Bahirdar Felegehiwot Referral Hospital North West, Ethiopia. Mol Biol. 2018;7(2). doi:10.4172/2168-9547.1000207
- Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3). doi:10.1542/peds.2017-1904
- Keutmann S, Zylla S, Dahl M, et al. Measurement uncertainty impacts diagnosis of diabetes mellitus: reliable minimal difference of plasma glucose results. Diabetes Ther. 2020;11(1):293–303. doi:10.1007/s13300-019-00740-w
- Gadekar T, Dudeja P, Basu I, Vashisht S, Mukherji S. Correlation of visceral body fat with waist–Hip ratio, waist circumference and body mass index in healthy adults: a cross sectional study. Med J Armed Forces India. 2020;76(1):41–46. doi:10.1016/j.mjafi.2017.12.001
- Helmersson-Karlqvist J, Ridefelt P, Boija EE, Nordin G. Lower creatinine concentration values and lower inter-laboratory variation among Swedish hospital laboratories in 2014 compared to 1996: results from the Equalis external quality assessment program. Clin Chem Lab Med. 2019;57(6):838–844. doi:10.1515/cclm-2018-0670
- Birhan TA. Association of angiotensin-converting enzyme gene insertion/deletion polymorphisms with risk of hypertension among the Ethiopian population. PLoS One. 2022;17(11):e0276021.
- Al-Hassani OMH. Detection of AGT gene polymorphism in patient with hypertension in Mosul City. Iraqi J Biotechnol. 2019;18(2):64–69.
- Borai IH, Hassan NS, Shaker OG, et al. Synergistic effect of ACE and AGT genes in coronary artery disease. Beni-Suef Univ J Basic Appl Sci. 2018;7(1):111–117. doi:10.1016/j.bjbas.2017.09.003
- Ghanie A, Partan RU, Indrajaya T, Ali Z, Saleh MI, Hidayat R. The effect of angiotensin-converting enzyme gene polymorphisms in the coronary slow flow phenomenon at south sumatra, Indonesia population. Open Access Maced J Med Sci. 2020;8(A):225–230. doi:10.3889/oamjms.2020.3802
- Mocan O, Radulescu D, Buzdugan E, Cozma A, Leucuta DC, Procopciuc LM. Association between M235T-AGT and I/D-ACE polymorphisms and carotid atheromatosis in hypertensive patients: a cross-sectional study. In Vivo. 2020;34(5):2811–2819. doi:10.21873/invivo.12107
- Hemeed RN, Al-Tu’ma FJ, Al-Koofee DAF, Al-Mayali AH, Ghasemian A. Relationship of angiotensin converting enzyme (I/D) polymorphism (rs4646994) and ischemic heart disease in Iraqi patients with type 2 diabetes mellitus. Rom J Diabetes Nutr Metab Dis. 2020;27(4):372–380. doi:10.46389/rjd-2020-1054
- Xue B, He L. Meta-analysis on the association of genetic polymorphisms of the angiotensin-converting enzyme and coronary artery disease in the Chinese population. Rev Assoc Med Bras. 2019;65(6):930. doi:10.1590/1806-9282.65.6.930
- Sakhteh M, Poopak B, Amirizadeh N, Shamshiri A, Bagheri A, Faranoush M. Polymorphism and synergism of angiotensin-converting enzyme (ACE) and plasminogen activator inhibitor-1 (PAI-1) genes in coronary artery disease. J Renin Angiotensin Aldosterone Syst. 2015;16(4):1168–1174. doi:10.1177/1470320314561247
- Shahid SU, Shabana S, Cooper JA, Rehman A, Humphries SE. Association of ACE and NOS3 gene polymorphism with blood pressure in a case control study of coronary artery disease in Punjab, Pakistan. Pak J Zool. 2016;48(4):1125–1132.
- Vladeanu MC, Bojan I, Bojan A, et al. Angiotensin-converting enzyme gene D-allele and the severity of coronary artery disease. Exp Ther Med. 2020:3407–3411. doi:10.3892/etm.2020.8978
- Mokretar K, Velinov H, Postadzhiyan A, Apostolova M. Association of polymorphisms in endothelial nitric oxide synthesis and renin-angiotensin-aldosterone system with developing of coronary artery disease in Bulgarian patients. Genet Test Mol Biomarkers. 2016;20(2):67–73. doi:10.1089/gtmb.2015.0195
- Nouryazdan N, Adibhesami G, Birjandi M, Heydari R, Yalameha B, Shahsavari G. Study of angiotensin-converting enzyme insertion/deletion polymorphism, enzyme activity and oxidized low density lipoprotein in Western Iranians with atherosclerosis: a case-control study. BMC Cardiovasc Disord. 2019;19(1):1–9. doi:10.1186/s12872-019-1158-4
- Mohamed N, Fawzy L, Hasan A. Angiotensin converting enzyme gene polymorphism in dyslipidemia and hypertension. J Recent Adv Med. 2020. doi:10.21608/jram.2020.33184.1064
- Najafipour H, Shokoohi M, Yousefzadeh G, et al. Prevalence of dyslipidemia and its association with other coronary artery disease risk factors among urban population in Southeast of Iran: results of the Kerman coronary artery disease risk factors study (KERCADRS). J Diabetes Metab Disord. 2016;15(1):1–8. doi:10.1186/s40200-016-0268-0
- Guney AI, Ergec D, Kirac D, et al. Effects of ACE polymorphisms and other risk factors on the severity of coronary artery disease. Genet Mol Res. 2013;12(4):6895–6906. doi:10.4238/2013.December.19.8
- Abd Alamir M, Goyfman M, Chaus A, et al. The correlation of dyslipidemia with the extent of coronary artery disease in the multiethnic study of atherosclerosis. J Lipids. 2018;2018:1–9. doi:10.1155/2018/5607349