Abstract:
Insulinoma is a predominantly benign and rare neuroendocrine tumor. Patients with insulinoma typically present with neurologic symptoms from hypoglycemia, such as confusion, dizziness, and behavioral changes, as well as symptoms from a surge in catecholamine levels, such as palpitations, diaphoresis, and tachycardia. Symptomatic patients usually have glucose levels below 55 mg/dL and are relieved of their symptoms when carbohydrate is administered. The 48-hour test, performed by measuring blood glucose levels of insulin, C peptide, and proinsulin collected every 4–6 hours in fasting patients, accurately confirms the diagnosis of insulinoma in the majority of the patients. Once the diagnosis is confirmed, the next step in management involves identifying the location of the tumor and successfully removing it surgically. In the last two decades, clinicians have moved away from invasive angiography for preoperative localization. A multiphase computed tomography (CT) can be used to localize the lesion and evaluate for metastasis. If CT does not detect the lesion, selective arterial calcium stimulation test is recommended to identify the region of the lesion in the pancreas. Some argue that all preoperative localization techniques are superfluous. The combination of intraoperative ultrasound and operative palpation has led to a nearly 100% success rate. Recently, in select cases, laparoscopic enucleations and resections of insulinomas have been performed with shorter length of stay and faster recovery time. Despite advances in imaging, a little over 10% of insulinoma patients undergo reexploration for missing lesions. Patients who are not candidates for tumor resections or awaiting surgery have had symptomatic relief from diazoxide and somatostatin analogs among various medical therapies. In patients with metastatic insulinoma, progression-free survival and overall survival are reported from newly approved chemotherapeutic agents. Liver-directed therapies, such as ablation and selective radiation, and cytoreductive surgery have also been performed for symptom control and prolonging survival.
Introduction
Insulinomas are rare functional neuroendocrine tumors (NETs) of the pancreas. The challenges of insulinoma diagnosis, localization, and surgical management have seen changes over the last few decades. In addition to describing the past standards of care for insulinoma, this review will elaborate on present-day best practice and recent advances made in the diagnosis and management.
Our current understanding of insulinomas began with the discovery of pancreatic islet cells by Paul Langerhans in 1869.1–Citation3 In 1922, the extraction of insulin, or “isletin”, from a dog’s pancreas by Banting and Best led the way to several studies that examined the physiologic significance of the hormone.Citation2 The following year, in 1923, Harris introduced the clinical possibility of spontaneous “hyperinsulinism”, in patients with blood sugars below 70 mg per 100 cc whose symptoms improved by feeding.Citation2 In 1927, Wilder and colleagues established an association between hyperinsulinism and a functional islet cell tumor. They performed surgical exploration on a patient with hyperinsulinism only to discover an unresectable islet cell carcinoma with hepatic metastasis.Citation2 Two years later, Roscoe Graham was able to resect an islet cell tumor documenting the first surgical cure of hyperinsulinemia.Citation2 In 1935, Whipple and Frantz published a manuscript summarizing the historic advances that defined insulinomas, along with their own observations. This paper, represented the first published account of the diagnostic “Whipple’s Triad”: (1) symptoms of hypoglycemia provoked by fasting; (2) circulating glucose level less than 50 mg/dL at the time symptoms presented; and (3) the relief of symptoms with the administration of glucose.Citation2
Demographics
The incidence of insulinoma is four per million persons each year, and insulinomas often present as a solitary pancreatic tumor.Citation1 The majority of insulinomas are small, measuring less than 2 cm.Citation4,Citation5 Despite its rare occurrence, insulinoma is the most common functional neuroendocrine tumor.Citation6 Although the large majority of insulinomas are sporadic, up to 10% may be associated with hereditary multiple endocrine neoplasia type I (MEN-1).Citation7 MEN-1 is an autosomal-dominant syndrome affecting mainly the parathyroid glands, anterior pituitary, endocrine pancreas, and duodenum, due to inactivation of the MEN1 gene on chromosome 11q13.Citation7
Insulinoma in patients with MEN-1 have additional challenges not encountered in sporadic cases.Citation8 MEN-1-associated insulinomas tend to occur throughout the pancreas, are almost always multifocal, and develop earlier than sporadic pancreatic endocrine tumors.Citation7 Genetic testing for MEN1 gene should be offered to patients with insulinoma in whom the diagnosis of MEN-1 is considered. Recurrence of insulinoma is also greater among patients with MEN-1 syndrome, 21% at 10 years compared with 5% at 10 years for those without the syndrome.Citation1,Citation8,Citation9 MEN-1 syndrome-associated insulinomas at times continue to be present despite simple enucleation and local resections. Surgical management of insulinomas associated with MEN-1 should be guided by two principles: total removal of gross disease and safe prophylactic pancreatic resection.Citation8 It is therefore essential to address these multifocal lesions by performing distal pancreatectomy to the portal vein, along with enucleations of tumors in the head of the pancreas using intraoperative ultrasound (IOUS)Citation6,Citation8 Such a procedure provides prophylactic resection to minimize recurrence and also prevents endocrine and exocrine pancreatic insufficiency.Citation3
Clinical presentation and diagnosis
Diagnosing insulinoma precisely requires keen clinical observation and laboratory tests. Diverse symptoms are described in patients presenting with this tumor. Unfortunately only 53% of patients are diagnosed within 5 years of experiencing their first symptom.Citation10 Spontaneous hypoglycemia from insulinoma can cause neuroglycopenic symptoms. Insulinomas typically present with neurologic symptoms of confusion, dizziness, and behavioral changes.Citation10–Citation12 In severe cases, patients can present with seizures and coma.Citation10–Citation12 Glucose levels below 55 mg/dL produces a surge in catecholamine levels that subsequently cause palpitations, trembling, diaphoresis, and tachycardia.Citation3,Citation11 All these symptoms are relieved or prevented when the patient consumes glucose rich food, as described by Whipple and Frantz ().Citation2
Table 1 Symptoms of insulinoma and frequency
It is important to understand the mechanism of insulin secretion in order to understand the significance of biochemical assays used to detect insulinomas. Proinsulin secreted from β-cells of the pancreatic islets of Langerhans is cleaved into insulin and C-peptide.Citation1,Citation3,Citation13 Both proinsulin and C-peptide levels in the blood are inappropriately elevated during events of hypoglycemia in a patient with insulinoma. As a result, serum insulin level is also excessively high despite low blood glucose level in these patients.Citation1,Citation3,Citation13 Prescribed insulin does not have C-peptide, and hence, hypoglycemia caused by exogenous insulin administration will show suppressed C peptide levels.Citation1,Citation3,Citation13
Once a high clinical suspicion for insulinoma is confirmed, biochemical tests based on prolonged supervised fasting are conducted to confirm the diagnosis. The 72-hour monitored fast has been the gold standard for diagnosing this tumor for over 80 years.Citation3,Citation9,Citation14 The protocol entails measuring levels of plasma glucose, insulin, C peptide, and proinsulin in the same specimen and repeating the measurements every 6 hours until the plasma glucose level is ≤60 mg/dL. At this point, the interval is reduced to every 1–2 hours, and the fast is terminated either when the plasma glucose level is ≤45 mg/dL or the patient has signs and symptoms of hypoglycemia.Citation1 The absence of signs and symptoms typical of hypoglycemia during a 72-hour fast excludes the diagnosis of a hypoglycemic disorder.Citation1 In addition to a low plasma glucose level, the interpretation of a positive 72-hour fast is suggested by the following parameters: increased levels of insulin (≥6 μU/mL), C peptide (≥0.2 nmol/L), and proinsulin (≥5 pmol/L), and an absence of sulfonylurea in the plasma.Citation1
Despite long-standing and reliable use of the 72-hour monitored fast, recent work at the National Institutes of Health strongly supports the use of a 48-hour fast and measurements of plasma insulin and proinsulin without prolonged fasting or other stimulation or suppression. Additionally, the protocol for the 72-hour test could be different from center to center, whereas the 48-hour test is easily reproducible and cost effective.Citation9 The 48-hour test is conducted by measuring blood glucose levels of insulin, C peptide, and proinsulin collected every 4–6 hours. Sulfonylurea is also measured, as it can cause hypoglycemia and is not present in patients with insulinoma.Citation1 The test is continued in a similar fashion to the 72-hour fast until the patient develops hypoglycemia, defined as plasma glucose below 40 mg/dL, and neuroglycopenic symptoms as mentioned earlier.Citation9 Hypoglycemic patients with inappropriate insulin and C peptide levels, and negative sulfonylurea screen then go on to have localization studies.Citation9 In one study, for 95% of insulinomas, the fast was terminated at 48 hours and very few were required to fast the full 72 hours, when the subtle signs of neuroglycopenia were not picked up.Citation9 It is therefore essential to note that very infrequently, a 72-hour test may be required to evoke evident hypoglycemia.
Preoperative localization
Once the biochemical diagnosis of insulinoma is confirmed, the next step is preoperative localization. The most effective method of localizing insulinomas is still a matter of controversy as both preoperative and intraoperative approaches have been advocated. Most surgeons value preoperative imaging to evaluate for evidence of metastatic disease. This allows the surgeon to discuss with the patient the extent and type of surgery that is planned.Citation13 Preoperative localization of insulinomas can be noninvasive or invasive ().Citation15 Noninvasive imaging modalities include abdominal ultrasonography, bolus-enhanced helical computed tomography (CT), magnetic resonance imaging (MRI), and somatostatin receptor scintigraphy. Invasive studies are selective angiography, transhepatic portal venous sampling, endoscopic ultrasonography (EUS), and selective arterial calcium stimulation (SACS).Citation15,Citation16 While preoperative localization increases intraoperative success, some have argued that preoperative localization is not necessary.Citation16 They suggest that the combination of surgical exploration and IOUS can identify more than 90% of insulinomas.Citation16
Figure 1 The distribution of the sensitivity rate of preoperative noninvasive and invasive methods in the localization of insulinoma, in all published cases (n=6,222).
Abbreviations: Angio, angiography; CT, computed tomography; EUS, endoscopic ultrasonography; MRI, magnetic resonance imaging; SACS, selective arterial calcium stimulation; THPVS, transhepatic portal venous sampling; US, ultrasound.
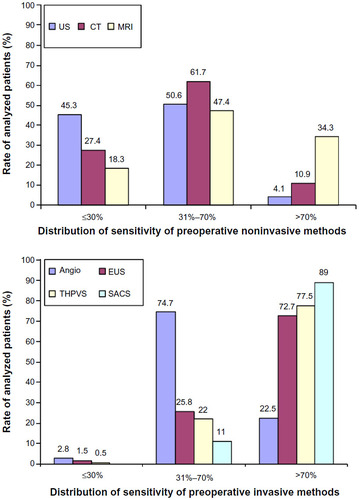
A recent 2014 systematic review of all localization techniques of insulinoma, including preoperative noninvasive and invasive methods in 6,222 published insulinoma cases, evaluated the success of noninvasive and invasive techniques.Citation15 With the use of ultrasonography in 34.4% of cases, insulinomas were correctly localized in only one-third of the cases. In 95% of the studies, ultrasonography had an average sensitivity of less than 70%. MRIs had the highest sensitivity in identifying the small pancreatic insulinomas, but the mean sensitivity remained approximately 45%. MRIs were also applied only 9.5% of the time and lacked good patient compliance. SSTR scintigraphy was performed less than 1% of the time and localized insulinomas only 24.6% of the time. CT demonstrated sensitivity ranging from 2% to 95.3% in various studies and correctly identified insulinomas in only 44% of cases. The average sensitivity of CT was less than 70% in the majority of reports.Citation15 However, with the use of dynamic CT with native, arterial, and portal vein phases, insulinomas were better localized, with increased sensitivities of 94%, 95.3%, and 83%, respectively. Contrast-enhanced CT was also routinely used in most cases to rule out liver metastases.Citation15
A variety of invasive preoperative localization techniques have been used for localization. Selective digital subtraction angiography was considered the gold standard for preoperative localization of insulinoma, with success rate above 90%.Citation3,Citation15 This success has been difficult to match after the 1990s as only 29%–50% of insulinomas were localized using arteriography.Citation5,Citation15 Additional problems with cost, and technical difficulties have also impacted its utility as a first-line study for insulinoma. Moreover, the success, availability, and increased use of the aforementioned noninvasive diagnostic techniques have also contributed to the decreased application of invasive angiography. An invasive localization study that has also fallen out of favor is transhepatic portal venous sampling. Here, a percutaneous and transhepatic catheter is passed into a branch of the portal vein, followed by introduction of the catheter into small veins draining the pancreas.Citation13,Citation15 An elevated insulin level sampled from these veins reveals the location of the insulinoma in the pancreas. Although transhepatic portal venous sampling has a sensitivity of more than 70% and has intraoperative application, the special skills required to perform the procedure with minimal morbidity limits its broad application.
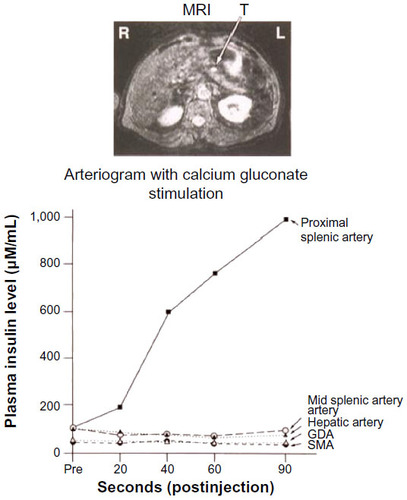
Among the invasive preoperative localization techniques, EUS and SACS have remained effective methods. EUS can detect lesions as small as 5 mm in the head of the pancreas with 92.6% success, but this value drops to 40% as one moves out towards the pancreatic tail.Citation15 EUS was used in approximately 12% of insulinoma cases but achieved, on average, a 73.9% localization success rate.Citation15 Lastly, the SACS test, since its introduction in 1989, has provided another technique for localizing insulinomas (especially those <2 cm in size) to regions of the pancreas, both preoperatively and intraoperatively.Citation17–Citation19 This approach is based on the activity of calcium to stimulate the release of insulin from hyperfunctional β cells in insulinomas, by selectively injecting calcium gluconate into major pancreatic arteries and subsequently measuring insulin levels from blood drawn from the hepatic veins via a second catheter ().Citation17–Citation19 Measurements of insulin concentration in the hepatic vein are taken at 20, 40, and/or 60 seconds after arterial infusion of calcium gluconate.Citation17 A step up in insulin level by twofold indicates the anatomic region of the insulinoma in the pancreas.Citation17 Like EUS, SACS is also operator-dependent and has a reported sensitivity of 84%–94%.Citation15,Citation17,Citation18
Figure 2 Left hepatic vein insulin concentrations after intra-arterial calcium injections.
Abbreviations: GDA, gastroduodenal artery; MRI, magnetic resonance imaging; SMA, superior mesenteric artery; R, right; L, left; T, tumor.
Operative localization/management of insulinoma
Once an insulinoma is diagnosed biochemically and localized preoperatively, surgery is the next step. Surgical resection of insulinoma is the gold standard of care and provides the only means for curative treatment of the disease.Citation9,Citation13,Citation15 Patients with the biochemical diagnosis of insulinoma achieve surgical cure ranging from 77% to 100%.Citation9,Citation20 The surgical approach can be open (93.4%) or laparoscopic (6.8%).Citation15 Medical therapy is reserved for patients with malignant insulinoma with unresectable metastasis, comprising approximately 4.4% of the patient population.Citation15 Cytoreductive surgery is also considered in a selected group of patients with metastatic insulinoma.Citation13 Lastly, although few cases of robotic surgery for insulinoma have emerged in the last decade, the advantage of three-dimensional view and enhanced dexterity of its articulated instruments have not translated to better outcomes than those for laparoscopic pancreatic surgery.Citation21 The current surgical mortality rate for insulinoma ranges from 0% to 4%, and the major morbidity rate is less than 20%.Citation9,Citation13
Open approach
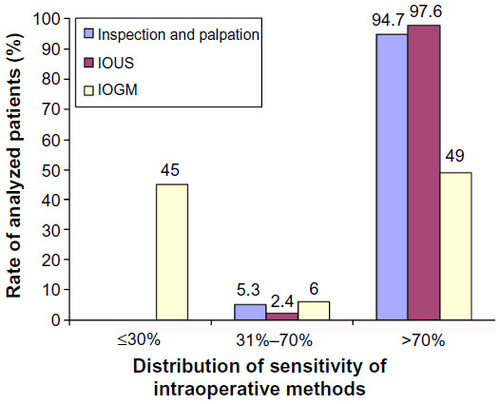
Many consider the combination of IOUS and palpation by an experienced surgeon to be a superior technique of localization, achieving up to a 100% success ().Citation4,Citation9,Citation15,Citation18 Localization with palpation for tumors less than 1 cm in diameter has a sensitivity of 69%, which increases to 79% for tumors ranging from 1.2 to 3 cm.Citation5 Intraoperative blood glucose monitoring has also been used to confirm the removal of all hyperfunctioning islet tissue, based on an increase of 30 mg/dL in blood sugar level in samples taken before and after tumor resection ().Citation15 Despite a reported sensitivity of 87%, intraoperative blood glucose monitoring has been used very infrequently because hypoglycemia during the operation is confounded by continuous infusion of glucose during general anesthesia.Citation15
Figure 3 The distribution of sensitivity rates of intraoperative modalities in the localization of insulinoma, in all published cases (n=6,222).
Abbreviations: IOUS, intraoperative ultrasound; IOGM, intraoperative glucose monitoring.
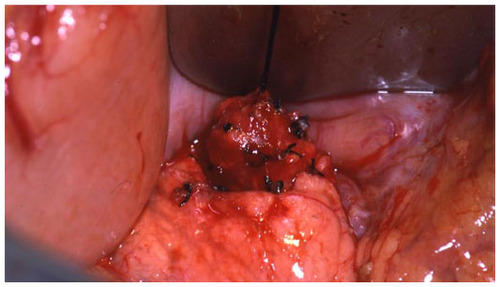
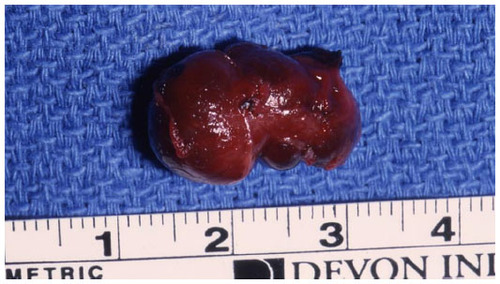
Different techniques for resection of insulinomas have been performed based on the location of the tumor. Enucleation (56%), distal pancreatectomy (31.5%), Whipple procedure (2.9%), subtotal pancreatectomy (2.5%), and, less than 1% of the time, total and central pancreatectomy have been applied.Citation10 The benign nature of most insulinomas allows for enucleation as the procedure of choice when possible.Citation13,Citation22 Although guidelines for deciding which insulinoma lesions should be resected or enucleated are not well established, in general, most surgeons prefer local tumor resection when the lesion is deemed to be too large for safe excision, multifocal in the body or tail of the pancreas, or is too close to the pancreatic duct.Citation22 In regards to tumors in the pancreatic head, enucleations can also be safely performed with the use of IOUS. However, pancreatic head lesions without a well-defined pseudocapsule, of size >4 cm, or that are multifocal or close to the main pancreatic duct should undergo pancreaticoduodenectomy.Citation22 Lastly, for tumors not localized intraoperatively, blind distal pancreatectomies have been performed. This procedure, however, is now discouraged owing to the inaccuracy and lack of therapeutic success of the procedure, its morbidity, and advancements in intraoperative imaging.Citation23
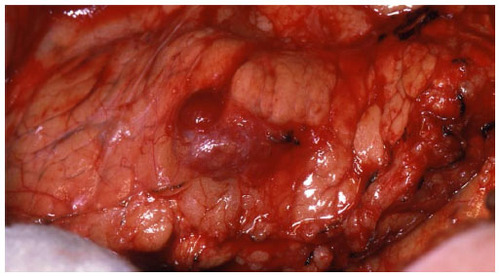
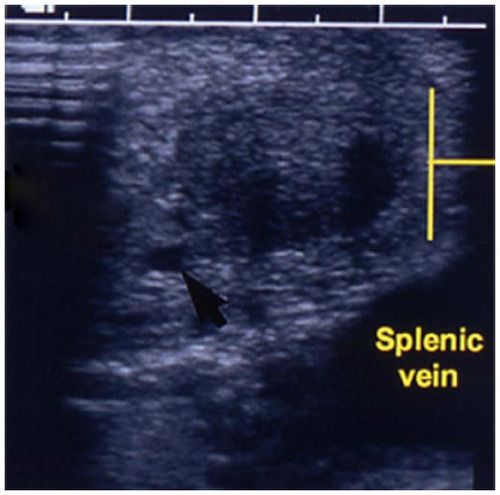
The operative steps of open insulinoma surgery involve the following key maneuvers (–): After laparotomy, the abdomen is explored for evidence of metastasis. The lesser sac is then entered through the gastrocolic ligament, exposing the anterior surface of the pancreas. Next, a Kocher maneuver is performed to mobilize the duodenum and the head of the pancreas. This allows bimanual palpation of the head of the pancreas. At this point, using IOUS to determine the location of the tumor(s) and their relationship to the pancreatic duct and vessels is critical.Citation20 With the guidance of IOUS, insulinomas can be safely enucleated without entering the pancreatic ducts, thereby avoiding the possibility of postoperative pancreatic fistula. Tumors should be removed intact to prevent local recurrence.Citation13
Figure 5 IOUS placed on insulinoma in to identify the location of the mass in relation to vascular structures and pancreatic duct.
Abbreviation: IOUS, intraoperative ultrasound.
Laparoscopic approach
As the application of minimally invasive surgery advances in all fields of surgery, its utility in insulinoma management has also emerged. Successful laparoscopic surgery for insulinoma has been reported since 1995.Citation24 Since then, several publications have demonstrated the procedure to be safe with the added benefit of shorter length of stay and faster recovery time.Citation24,Citation25 Insulinomas are suitable to laparoscopic excision, given their predominantly benign, intrapancreatic, and solitary nature.Citation24 Laparoscopic approach is best suited when the tumor is located on the surface of the pancreas and is further away from the main pancreatic duct.Citation25 Insulinomas that are localized deep in the body or tail of the pancreas and that have close relationship with the pancreatic duct should undergo distal pancreatectomy.Citation25 The application of laparoscopy in insulinoma resection is difficult when there are multiple tumors, tumors in the head or uncinate process, and lesions in the dorsal aspect of the pancreas, due to technical limitations of the approach. Therefore, tumor detection depends solely on laparoscopic ultrasonography, which achieves 86% to 90% localization.Citation24,Citation26
Missing insulinomas and surgical complications
Despite advances in preoperative and intraoperative localization, approximately 13% of patients require reexploration.Citation4 Morbidity rates increase from 21%–25% at the primary operation to 50%–58% after reoperation.Citation4,Citation27 At times, insulinomas are missed because there are multiple tumors, and these subjects should raise strong suspicion of MEN-I syndrome.Citation27
Surgical complications include pancreatic fistula, pseudocyst, intra-abdominal abscess, pancreatitis, hemorrhage, and diabetes.Citation9 Laparoscopic procedures carry similar rates of complication.Citation25 Pancreatic fistulas are the most common complication for patients with insulinomas because of the soft texture of the gland.Citation25 The majority of these complications are managed with conservative drainage and parenteral nutrition, with or without the addition of somatostatin analogs.
Medical management of insulinoma
Insulinoma patients who are awaiting surgery or who are not surgical candidates can be managed with medical therapy and dietary modification to avoid prolonged fasting. The initial drug of choice for patients with insulinoma is diazoxide, a nondiuretic benzothiadiazine derivative. Diazoxide was primarily introduced in the 1950s for the treatment of hypertension; however, its side effects of hyperglycemia has made the drug applicable for the management of insulinoma.Citation28 Diazoxide inhibits insulin release from β cells via stimulation of α-adrenergic receptors and also inhibits cyclic adenosine monophosphate phosphodiesterase, which enhances gylcogenolysis.Citation13,Citation29 A dose of 150–200 mg in two or three divided doses per day can be titrated to a maximum dose of 400 mg per day.Citation13,Citation29 Symptomatic control has been achieved in half the patients on diazoxide.Citation6,Citation13,Citation28 The side effects of diazoxide are hirsutism, edema, gastrointestinal discomfort, weight gain, and nausea, yet, most patients tolerate it well.Citation6,Citation28
Somatostatin analogs octreotide and lanreotide have also provided another class of agents that are useful in the symptomatic management of insulinoma in patients with receptors for the drug. Natural somatostatin has a very short half-life of 2 minutes.Citation30 Long-acting release octreotide and lanreotide both have high affinity towards SSTR2 and SSTR5.Citation30 These receptors are found in varying degrees on insulinomas.Citation31 In about half the patients with insulinoma, octreotide and lanreotide binds these receptors and lower plasma insulin levels.Citation30,Citation31 The short-acting formulation octreotide can be dispensed in quantities of 50 μg subcutaneously two or three times daily, increased up to 1,500 μg daily.Citation13 Long-acting release octreotide is administered intramuscularly with a dose of up to 30 mg every 28 days, and 120 mg of lanreotide is injected into the deep subcutaneous tissue every 28 days.Citation30,Citation32 It is important to note that somatostatin analogs also act on other receptors involved in the regulation of growth hormone and glucagon secretion from alpha cells, thereby causing worsening hypoglycemia in some insulinoma patients.Citation30 The side effects of these somatostatin analogs are mainly gastrointestinal disturbances, such as nausea, emesis, diarrhea, constipation, abdominal pain, malabsorption, and cholelithiasis.Citation13
Recent studies have also focused on the antiproliferative and growth stabilization of somatostatin analogs on malignant NETs.Citation30,Citation32 Two studies, the placebo-controlled, double-blind, prospective randomized study on the effect of long-acting release octreotide in control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID) and the controlled study of lanreotide antiproliferative response in neuroendocrine tumors (CLARINET), have demonstrated promising prolonged progression-free survival among SSTR-positive NETs.Citation32 The somatostatin analog mechanism of decreasing or arresting NETs growth has been attributed to the inhibition of cell proliferation of normal and tumor cells via induction of G1 cell cycle arrest and apoptosis.Citation30,Citation33 Although the success of these somatostatin analogs were mostly for small-bowel NETs and some pancreatic neuroendocrine tumors (PNETs), their antiproliferative-specific effects on insulinomas alone are yet to be defined. With regards to malignant insulinoma, establishing the presence of SSTR2 receptors in the primary tumor and in metastases may be valuable in avoiding severe hypoglycemia in patients without this receptor.Citation33 Limited studies with variable outcomes have identified other SSTR subtypes involved in cell proliferation in malignant insulinomas, such as SSTR5 messenger (m)RNA expression, which could potentially be targeted for therapeutic options.Citation33
Other agents that have been used for the medical treatment of insulinoma with various degrees of outcomes include phenytoin, verapamil, propranolol, glucocorticoids, and lastly glucagon.Citation9
Management of malignant insulinoma
Malignant or metastatic insulinoma, spreading primarily to lymph node or liver, is a rare condition accounting for only 5%–12% of reported cases of insulinoma.Citation14,Citation34 These patients have a poor prognosis, with a median survival period of approximately 2 years.Citation34 The initial surgery for tumor removal or diagnosis is the most important factor in the management of malignant insulinoma.Citation34 Patients with a reasonable performance status, minimal extrahepatic disease, and resectable primary tumor are candidates for cytoreductive surgery. Unfortunately, curative cytoreductive surgery is effective in less than 10% of all patients with metastatic insulinoma.Citation13
Medical interventions, namely diazoxide, hepatic embolization, chemotherapy (streptozocin, doxorubicin, and 5-flurouracil), peptide-receptor radionuclide therapy, and radiofrequency ablation, have been mainly used for disease palliation.Citation9,Citation34
The chemotherapeutic agents everolimus and sunitinib have been recently approved for the management of advanced insulinoma, with promising progression-free survival and overall survival.Citation35,Citation36 Everolimus inhibits mTOR, a serine-threonine kinase that stimulates cell growth, proliferation, and angiogenesis, thereby inhibiting a pathway implicated with tumor proliferation of PNETs.Citation36 Patients randomized to receive everolimus at a dose of 10 mg daily showed a median progression-free survival of 11 months compared with 4.6 months with placebo.Citation36 Adverse effects were mostly stomatitis, rash, diarrhea, and fatigue, as well as anemia and hyperglycemia.Citation36 Similarly, sunitinib, a multitargeted receptor tyrosine kinase inhibitor, has shown delayed tumor growth in PNETs by inhibiting VEGF and PDGF receptors (PDGFRs).Citation35 Patients with PNETs who were randomized to therapy with a dose of 37.5 mg of sunitinib daily had a median progression-free survival of 11.4 months compared with 5.5 months with placebo.Citation35 The study was discontinued early due to more serious adverse events and death encountered in the placebo group. The most frequent side effects of sunitinib observed were diarrhea, nausea, vomiting, asthenia, and fatigue.Citation35
Conclusion
Insulinoma is a very rare neuroendocrine tumor that has a unique presentation at the time of diagnosis. Patients with insulinoma develop symptoms such as confusion, dizziness, and palpitations that are relieved by consuming carbohydrate. Although it is predominantly a benign tumor, many biochemical tests and imaging modalities have been applied to properly diagnose and localize insulinomas. The 48-hour test can be used to accurately diagnose insulinoma in the majority of insulinoma patients, with very few having to complete the full 72-hour test. Preoperative CT scan is helpful in ruling out metastasis. Following the diagnosis of insulinoma, the definitive treatment of the tumor is surgery. IOUS, in conjunction with palpation, can correctly locate insulinoma. In the cases of missing insulinomas, SACS is a helpful tool to identify the anatomic region of the lesion in the pancreas. The medical management of insulinoma patients who are not surgical candidates or with malignant insulinomas has also seen many advances. In addition to the many available agents for symptomatic control of the disease, newly approved agents, such as sunitinib and everolimus, have had encouraging results in progression-free survival.
Disclosure
The authors report no conflicts of interest in this work.
References
- Service FJ. Hypoglycemic disorders. N Engl J Med. 1995;332(17):1144–1152.
- Whipple AO, Frantz VK. Adenoma of islet cells with hyperinsulinism: a review. Ann Surg. 1935;101(6):1299–1335.
- Grant CS. Insulinoma. Baillieres Clin Gastroenterol. 1996;10(4):645–671.
- Richards ML, Gauger PG, Thompson NW, Kloos RG, Giordano TJ. Pitfalls in the surgical treatment of insulinoma. Surgery. 2002;132(6):1040–1049; discussion 1049.
- Boukhman MB, Karam JM, Shaver J, et al. Localization of insulinomas. Arch Surg. 1999;134(8):818–822; discussion 822–823.
- Boukhman MP, Karam JH, Shaver J, Siperstein AE, Duh QY, Clark OH. Insulinoma – experience from 1950 to 1995. West J Med. 1998;169(2):98–104.
- Callender GG, Rich TA, Perrier ND. Multiple endocrine neoplasia syndromes. Surg Clin N Am. 2008;88(4):863–895.
- O’Riordain DS, O’Brien T, van Heerden JA, Service FJ, Grant CS. Surgical management of insulinoma associated with multiple endocrine neoplasia type I. World J Surg. 1994;18(4):488–493; discussion 493–494.
- Shin JJ, Gorden P, Libutti SK. Insulinoma: pathophysiology, localization and management. Future Oncol. 2010;6(2):229–237.
- Dizon AM, Kowalyk S, Hoogwerf BJ. Neuroglycopenic and other symptoms in patients with insulinomas. Am J Med. 1999;106(3):307–310.
- Kennedy EP, Brody JR, Yeo CJ. Neoplasms of the endocrine pancreas. In: Mulholland MW, Lillemoe KD, Doherty GM, Maier RV, Simeone DM, Upchurch GR Jr, editors. Greenfield’s Surgery Scientific Principles and Practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2011:857–871.
- Vig S, Lewis M, Foster KJ, Stacey-Clear A. Lessons to be learned: a case study approach insulinoma presenting as a change in personality. J R Soc Promot Health. 2001;121(1):56–61.
- Mathur A, Gorden P, Libutti SK. Insulinoma. Surg Clin North Am. 2009;89(5):1105–1121.
- Hirshberg B, Livi A, Bartlett DL, et al. Forty-eight-hour fast: the diagnostic test for insulinoma. J Clin Endocrinol Metab. 2000;85(9):3222–3226.
- Mehrabi A, Fischer L, Hafezi M, et al. A systematic review of localization, surgical treatment options, and outcome of insulinoma. Pancreas. 2014;43(5):675–686.
- Hashimoto LA, Walsh RM. Preoperative localization of insulinomas is not necessary. J Am Coll Surg. 1999;189(4):368–373.
- Guettier JM, Kam A, Chang R, et al. Localization of insulinomas to regions of the pancreas by intraarterial calcium stimulation: the NIH experience. J Clin Endocrinol Metab. 2009;94(4):1074–1080.
- Brown CK, Bartlett DL, Doppman JL, et al. Intraarterial calcium stimulation and intraoperative ultrasonography in the localization and resection of insulinomas. Surgery. 1997;122(6):1189–1193; discussion 1193–1194.
- Doppman JL, Chang R, Fraker DL, et al. Localization of insulinomas to regions of the pancreas by intra-arterial stimulation with calcium. Ann Intern Med. 1995;123(4):269–273.
- Finlayson E, Clark OH. Surgical treatment of insulinomas. Surg Clin North Am. 2004;84(3):775–785.
- Wayne M, Steele J, Iskandar M, Cooperman A. Robotic pancreatic surgery is no substitute for experience and clinical judgment: an initial experience and literature review. World J Surg Oncol. 2013;11:160.
- Park BJ, Alexander HR, Libutti SK, et al. Operative management of islet-cell tumors arising in the head of the pancreas. Surgery. 1998;124(6):1056–1061; discussion 1061–1062.
- Hirshberg B, Libutti SK, Alexander HR, et al. Blind distal pancreatectomy for occult insulinoma, an inadvisable procedure. J Am Coll Surg. 2002;194(6):761–764.
- Berends FJ, Cuesta MA, Kazemier G, et al. Laparoscopic detection and resection of insulinomas. Surgery. 2000;128(3):386–391.
- Zhao YP, Zhan HX, Zhang TP, et al. Surgical management of patients with insulinomas: Result of 292 cases in a single institution. J Surg Oncol. 2011;103(2):169–174.
- Grover AC, Skarulis M, Alexander HR, et al. A prospective evaluation of laparoscopic exploration with intraoperative ultrasound as a technique for localizing sporadic insulinomas. Surgery. 2005;138(6):1003–1008; discussion 1008.
- Simon D, Starke A, Goretzki PE, Roeher HD. Reoperative surgery for organic hyperinsulinism: indications and operative strategy. World J Surg. 1998;22(7):666–671; discussion 671–672.
- Goode PN, Farndon JR, Anderson J, Johnston IDA, Morte JA. Diazoxide in the management of patients with insulinoma. World J Surg. 1986;10(4):586–592.
- Fajans SS, Floyd JC, Thiffault CA, Knopf RF, Harrison TS, Conn JW. Further studies on diazoxide suppression of insulin release from abnormal and normal islet tissue in man. Ann N Y Acad Sci. 1968;150(2):261–280.
- Arnold R, Wied M, Behr TH. Somatostatin analogues in the treatment of endocrine tumors of the gastrointestinal tract. Expert Opin Pharmacother. 2002;3(6):643–656.
- Vezzosi D, Bennet A, Rochaix P, et al. Octreotide in insulinoma patients: efficacy on hypoglycemia, relationships with Octreoscan scintigraphy and immunostaining with anti-sst2A and anti-sst5 antibodies. Eur J Endocrinol. 2005;152(5):757–767.
- Caplin ME, Pavel M, CwikŁa JB, et al; CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–233.
- Ferrer-García JC, Iranzo González-Cruz V, Navas-DeSolís S, et al. Management of malignant insulinoma. Clin Transl Oncol. 2013;15(9):725–731.
- Hirshberg B, Cochran C, Skarulis MC, et al. Malignant insulinoma: spectrum of unusual clinical features. Cancer. 2005;104(2):264–272.
- Raymond E, Dahan L. Raoul JL. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–513.
- Yao JC, Shah MH, Ito T. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523.