Abstract
Purpose
Combination chemotherapy of schistosomiasis mansoni has been studied previously, with praziquantel being the basis of combination. Artemether and myrrh are compounds of a natural origin that have been investigated experimentally and clinically against schistosomiasis. Artemether is used as an antimalarial drug, and has been used as a chemoprophylactic drug against Schistosoma japonicum in China whereas myrrh extract is manufactured and prescribed as an antischistosomal drug in Egypt. The present study investigated the experimental efficacy of combining artemether and myrrh using three different protocols in mice infected with the Egyptian strain of S. mansoni.
Methods
Experiments were performed on 40 eight-week-old female Swiss albino mice divided into three experimental groups and one control group. Assessment of efficacy was based on a suite of parasitologic and histopathologic parameters. Parasitologic parameters included reductions in total and female worm burdens, reductions in hepatic and intestinal wall tissue egg loads, and alterations in oogram patterns in the experimental groups compared to the infected untreated control. Histopathologic parameters comprised microscopic examination of liver sections stained with hematoxylin and eosin to study the reductions in the mean counts and diameters of hepatic granulomas as well as their healing ratios compared to the control.
Results
Reductions of 43.9%–58.2% in total worm burdens and 42.4%–63.7% in female worm burdens were induced. Meanwhile, significant reductions of 63.1%–77.8% in eggs per gram of small intestinal tissue and of 56.5%–66.3% in eggs per gram of liver tissue were also observed. The combination also caused alterations in the oogram pattern as well as amelioration of hepatic lesions as evidenced by increased ratios of healed granulomas in the treated groups compared to the control.
Conclusion
The experimental efficacy of the artemether–myrrh combination against the Egyptian strain of S. mansoni was evident, but not to an extent that would warrant clinical trials in humans.
Introduction
Combination chemotherapy is not a new concept, but was first developed for the treatment of bacterial infections.Citation1 The rationale was to delay the emergence of drug resistance or to minimize synergistic drug doses that might result in milder side effects.Citation1 Combination chemotherapy has also been attempted for schistosomiasis mansoni, both experimentally and clinically, with praziquantel being the main basis of the combination.Citation2 Combination chemotherapy studies published online regarding schistosomiasis are very few. The first experimental study on the chemotherapeutic potential of praziquantel–oxamniquine combination in mice infected with adult S. mansoni demonstrated the marked superiority of the combination over single drug use.Citation3 However, it was later found that low-dose (one-third of the curative dose) combinations of the two drugs against different stages of S. mansoni showed only slightly higher worm burden reductions compared to single-drug curative doses.Citation4
Pugh and Teesdale conducted a clinical study on the efficacy of praziquantel–oxamniquine in the treatment of school children infected with S. mansoni in Malawi.Citation5 It was concluded that the two drugs showed higher efficacy when administered simultaneously in single low doses of 15 mg/kg and 7.5 mg/kg, respectively. Additionally, the combined treatment was found to be well tolerated.Citation5 Another trial was carried out on 58 Zimbabwean schoolchildren concurrently infected with S. mansoni and Schistosoma haematobium.Citation6 A high cure rate of 89% for those with S. mansoni infections was achieved with the highest doses (praziquantel 20 mg/kg and oxamniquine 10 mg/kg). However, it was also concluded that the combination of the two drugs for the treatment of S. mansoni infection in Zimbabwean schoolchildren had no curative advantage over praziquantel alone.Citation6
The combination of praziquantel as a chemical drug with artemether as a natural, semisynthetic drug has also been suggested because they have efficacies on different stages of the life cycle of the major schistosomes. Utzinger et al compared S. mansoni worm burden reductions after administration of praziquantel and artemether, singly and in combination.Citation7 In this experiment, hamsters infected with schistosomula and adult S. mansoni worms were simultaneously treated with praziquantel and artemether.Citation7 The worm burden reduction after simultaneous drug administration was higher than that after praziquantel, but the reduction was not significantly different from that achieved by artemether.Citation7 In a study carried out on the Egyptian strain of S. mansoni in mice, it was found that the combined treatment with praziquantel and artemether did not enhance worm burden reduction compared to treatment using praziquantel alone.Citation8
Artemether is one of the artemisinin derivates obtained from the leaves of the Chinese wormwood shrub, Artemisia annua L., that are vital in the control of malaria, including chloroquine-resistant disease.Citation9 Myrrh is an oleogum resin obtained from the stem of Commiphora molmol (also called C. myrrha) and probably other species of the family Bursearacae, growing in northeast Africa and Arabia.Citation10 On searching well-known databases, we did not find any experimental study for the combination of these two drugs of natural origin in the treatment of S. mansoni infection. Therefore, the present study was carried out to evaluate the efficacy of combining artemether and myrrh.
Materials and methods
Eight-week-old female Swiss albino mice of the CD-1 strain, weighing 20 ± 2 g, were obtained from the Schistosome Biologic Supply Center, Theodore Bilharz Research Institute, Cairo, Egypt. The mice were bred under environmentally controlled conditions, and fed with a standard pellet diet and water ad libitum. The animals were handled and the experiments were conducted according to the institutional guidelines of our affiliations. An Egyptian S. mansoni (CD) strain of cercariae was obtained from the Schistosome Biologic Supply Center of the same institute. Artemether (Mether®; Kunming Pharmaceutical Corp, Kunming, China) was used as a freshly prepared suspension in 7% Tween-80 and 3% ethanol before intragastric administration. Myrrh (Mirazid®; Pharco Pharmaceuticals, Alexandria, Egypt) was used as a freshly prepared suspension in 2% Cremophor EL before intragastric administration.
Three dosing protocols of combined drugs were applied intragastrically using a stomach tube in the treatment groups from day 46 post-infection onwards, ie, when adult S. mansoni stages are harbored in the mice. Forty mice infected with S. mansoni were divided into three experimental groups (groups I, II, and III) and one untreated control group, (n = 10 in each group). The dosing protocols are shown in . The artemether and myrrh dosing protocols were in accordance with those used in previous studies.Citation11,Citation12
Table 1 Dosing protocols used in this study
Parasitologic procedures
Mice were infected by subcutaneous injectionCitation13 with (60 ± 10) cercariae of S. mansoni suspended in 0.1 mL solution.Two weeks later, worms were recovered from the hepatic and portomesenteric veins using the Smithers and Terry perfusion technique for mice.Citation14 The recovered worms were then counted and sexed. Weighed fragments of liver and small intestinal tissues were removed for the estimation of tissue egg loads. These fragments were processed separately by the potassium hydroxide digestion technique for counting S. mansoni eggs in tissues.Citation15 Alterations in oogram patterns in the mucosa of the small intestines of treated mice groups were studied and compared to the infected untreated control.Citation16
Histopathologic investigations
Liver sections were prepared and stained with hematoxylin and eosin to determine reductions in the mean counts and diameters of hepatic granulomas as well as their healing in the treated groups compared to the infected untreated control. Histopathologic examination was performed in the Pathology Department of Medical Research Institute, Alexandria University, Egypt.
Statistical analysis
Data were expressed as mean ± standard error of the mean, and analyzed using SPSS software (version 11.5; SPSS Inc, Chicago, IL). One-way analysis of variance followed by Tukey’s honestly significant difference (HSD) post hoc test was used to test the difference in means between each experimental group and the control.
Results
Worm burden reductions
Artemether–myrrh combination caused significant (P < 0.01) total worm reductions of 58.2% and 57.7% in groups I and II, respectively, followed by 43.9% in group III compared to the control. There were also significant (P < 0.01) female worm reductions of 63.7% and 57.5% in groups I and II, respectively. Group III showed a lower female worm burden reduction of 42.4% (P < 0.05) compared to the control (). shows male and female schistosomes recovered from the mice.
Figure 1 Male (pinkish-colored) and female (dark-colored) S. mansoni worms recovered by perfusion of mice (Actual size).
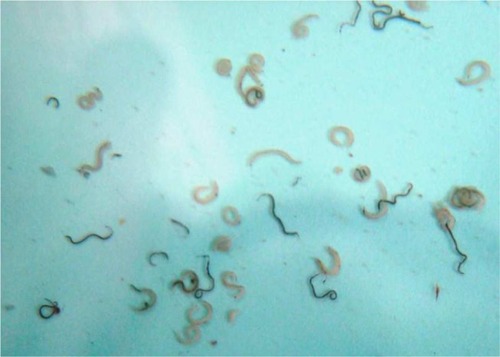
Table 2 Effects of different artemether–myrrh protocols on worm burdens, tissue egg loads, and oogram patterns in experimentally infected mice harbouring adult S. mansoni (Egyptian CD strain)
Tissue egg loads
Artemether–myrrh combination induced significant reductions in eggs per gram liver tissue compared to the control. The highest reduction was 66.3% (P < 0.001) in group II, followed by comparable, significant (P < 0.001) reductions of 58.8% and 56.5% in groups I and III, respectively. In addition, statistically significant (P < 0.001) reductions of 77.8% and 74.2% in eggs per gram of the small intestinal tissue were observed in groups II and I, respectively, followed by a reduction of 63.1% (P < 0.001) in group III ().
Oogram patterns
There was a significant decrease in the percentage of total immature stages to 6.14% (P < 0.001) in group I compared to 66.14% in the control. In addition, a statistically significant increase of 57.57% (P < 0.001) was observed in the percentage of dead eggs in group I compared to 5.15% in the control. The oogram pattern showed eggs of all developmental stages, with a significant (P < 0.001) decrease in the percentages of total immature stages to 30.50% and 23.77% in groups II and III, respectively, compared to 66.14% in the control. In addition, statistically significant (P < 0.001) increases in the percentages of dead eggs to 36.50% and 46.79% were observed in groups II and III, respectively, compared to 5.15% in the control ().
Hepatic granuloma morphometries
Microscopic examination of liver sections stained with hematoxylin and eosin revealed intact liver architecture in all groups. The liver parenchyma was studded with schistosomal granulomas surrounding newly laid eggs which were very numerous in the controls (). These were either active consisting of a central ovum surrounded by epithelioid cells, lymphocytes, eosinophils, and a few giant cells, or healed whereby the inflammatory cellular infiltrate was replaced by fibrosis (). Only group II showed a statistically significant (P < 0.01) reduction of 52.2% in the mean count of hepatic granulomas. However, groups I and III showed statistically nonsignificant (P > 0.05) reductions of 27.8% and 24.3%, respectively, compared to the control. On the other hand, only group III showed a statistically significant reduction of 26.4% (P < 0.05) in the mean diameter of hepatic granulomas compared to the control, with nonsignificant (P > 0.05) reductions of 23.5% and 19.6% observed in groups II and I, respectively. While healed granulomas represented only about a half of the active granulomas (a healing ratio of 1:2) in the control, group III showed the highest healing ratio of 5:1 followed by a ratio of 2:1 in groups I and II ().
Figure 2 A liver tissue section of a control group showing intact architecture studded with numerous schistosomal granulomas (H&E, ×40).
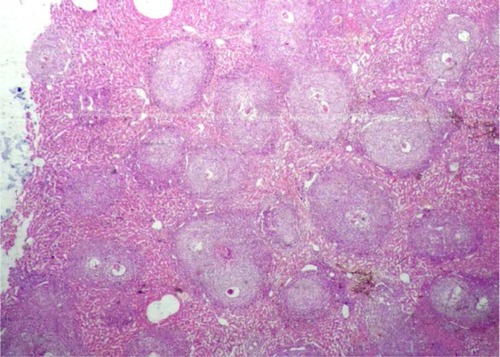
Figure 3 A liver section from a treated group showing one healed and two active hepatic schistosomal granulomas (H&E, ×40).
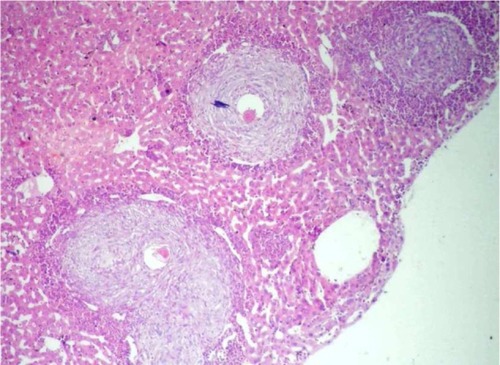
Table 3 Effects of different artemether–myrrh combination protocols on granuloma characteristics in experimentally infected mice harbouring adult S. mansoni (Egyptian CD strain)
Discussion
The present study investigated the in vivo therapeutic efficacy of combining artemether and myrrh in female Swiss albino mice harboring adult S. mansoni (Egyptian CD strain). This was performed using three combination dosing protocols according to defined parasitologic and histopathologic parameters.
All combination protocols showed significant reductions in total and female worm burdens. The two-day protocol of artemether 400 mg/kg + myrrh 500 mg/kg daily as well as the four-day protocol of artemether 200 mg/kg + myrrh 250 mg/kg daily induced the greatest reductions of >50% in total and female worm burdens. However, the six-day protocol of artemether 100 mg/kg + myrrh 125 mg/kg daily caused reductions of only 43.9% and 42.4% in the total and female worm burdens, respectively. In addition, the artemether–myrrh combination caused a significant reduction in tissue egg loads. Eggs in the wall of the small intestine appeared to be more affected by all three combination dosing protocols than eggs retained in liver tissue. The combination reduced intestinal tissue egg loads in the range of 63.1%–77.8% and liver egg loads in the range of 56.5%–66.3%. A moderate alteration in the oogram pattern was also induced with a significant reduction in the total immature stages and an increase in the percentages of dead eggs. The two-day protocol of artemether 400 mg/kg + myrrh 500 mg/kg daily was the most efficacious in changing the oogram pattern, causing a reduction to 6.14% in total immature eggs and inducing the greatest increase in the percentage of dead eggs to 57.57%. The other two protocols showed lower effects on oogram pattern with the presence of all developmental egg stages, but there was still a significant ovicidal effect with the percentage of dead eggs increased to 36.50% and 46.78%, compared to 5.10% in the control. With respect to the effect of the combination of low-dose praziquantel and oxamniquine on different stages of S. mansoni (Egyptian strain), it has been found that the combination had a potentiating effect.Citation4 It was reported that the combination regimen was most effective four hours after infection, producing 96% worm reduction with absence of eggs in the liver and intestine. Five weeks post-infection, the same regimen was reported to reduce the tissue egg load by 98%.Citation4
To the best of our knowledge, no studies on the combination of artemether and myrrh have been published, and this is the first study combining these two drugs. The combination of artemether with praziquantel has been suggested due to the activity of these agents on different stages of the life cycle of schistosomes.Citation1 In accordance with this suggestion, it was found that the combination of artemether and praziquantel was more efficacious than either drug alone against S. mansoni (Liberian strain).Citation7 A single combined treatment with low doses of praziquantel 75 mg/kg and artemether 150 mg/kg was more efficacious in hamsters infected with 14- and 21-day-old schistosomula and 49-day-old adult S. mansoni (Liberian strain).Citation7 The investigators reported that the combination of the two drugs simultaneously caused a total worm reduction of 77% compared to only 2% and 66% with single administration of praziquantel and artemether, respectively.Citation7 In addition, a female worm reduction of 85% was attained with the combination compared to 12% and 81% with praziquantel and artemether, respectively.Citation7 In fact, the low-dose praziquantel–artemether combination in that study was more efficacious than the efficacy of the higher-dose artemether–myrrh combination in the present study. However, it should be considered that the praziquantel–artemether combination was tried on hamsters harboring schistosomula and adult worms togetherCitation7 in comparison to the present study that was conducted in mice harboring the adult stage only. In contrast, artemether was not found to be a good adjuvant to praziquantel in mice experimentally infected with S. mansoni (Egyptian CD strain) as regards worm burden reduction.Citation8 It was found that administration of praziquantel in addition to artemether in double dose (four and six weeks post-infection) led to a reduction of 90% in comparison to 95% in those treated with praziquantel alone, a difference that was not statistically significant.Citation8 However, co-administration of grapefruit juice 0.5 mL with a reduced dose (150 mg/kg) of artemether for two consecutive days four weeks post-infection enhanced the killing of female worms, but not males, in mice experimentally infected with S. mansoni.Citation17 This combination caused a female worm reduction of 79.8% compared to only 54.4% when artemether was used alone in the same reduced dose.Citation17 Moreover, the combined treatment induced a complete absence of eggs in hepatic and intestinal tissues.Citation17 The combination of artemether and myrrh in the present study appears to be less efficacious in targeting the female worm population than that induced by the artemether–grapefruit combination. This may be attributed to the fact that grapefruit juice significantly increases the oral bioavailability of artemether without having an effect on the elimination half-life.Citation18
In the present study, the four-day protocol of artemether 200 mg/kg and myrrh 250 mg/kg daily was the most efficacious protocol for reducing hepatic granulomas. Nevertheless, the highest healing ratio was achieved when the combination was used with the six-day protocol of artemether 100 mg/kg and myrrh 125 mg/kg daily. Using the latter protocol, the amount of healed granulomas was five times higher than the active ones (5:1) compared to a ratio of 1:2 in the control. In contrast, the two-day protocol of artemether 400 mg/kg and myrrh 500 mg/kg daily as well as the four-day protocol of artemether 200 mg/kg and myrrh 250 mg/kg daily caused the healed granulomas to increase to two times the amount of the active ones (2:1) compared to a ratio of 1:2 in the control. This may be explained by the longer period of treatment, because artemether has a rapid elimination time. The artemether–myrrh protocols induced nonsignificant reductions of only 19.6%–26.4% in the mean diameter of hepatic granulomas. It was only the six-day protocol of artemether 100 mg/kg and myrrh 125 mg/kg daily that induced a significant reduction of 26.4% compared to the control.
Another study showed that artemether 400 mg/kg/day given during weeks four and six post-infection caused a complete absence of classic granulomas in mice experimentally infected with S. mansoni (Egyptian CD strain) either alone or after addition of praziquantel 500 mg/kg/day for two consecutive days six weeks post-infection.Citation8 Combination of grapefruit with a reduced dose (150 mg/kg) of artemether four weeks post-infection resulted in a complete absence of granulomatous reaction, with only occasional degenerated eggs seen trapped in the hepatic venules.Citation17 This can also be explained by the increased bioavail-ability of artemether when combined with grapefruit.Citation18
Abdul-Ghani et al reported that using myrrh with the same protocols used in the present study had no significant efficacy against S. mansoni (Egyptian CD strain) in experimentally infected mice.Citation12 However, using artemether with the same dosing protocols used in the present study showed a greater efficacy regarding all parasitologic and histopathologic parameters,Citation19 with total worm reductions ranging from 40.7% to 59.7% and female worm reductions ranging from 69.3% to more than 90%.Citation19 In addition, significant reductions, ranging from 75.2% to 82.6%, in the liver tissue egg loads as well as significant reductions, ranging from 68.8% to 78.9%, in the intestinal wall egg loads were reported.Citation19 Significant alterations in the oogram pattern in the intestinal mucosa of infected mice with cessation of oviposition and increased rates of dead eggs were also observed.Citation19 Antipathologic activities were also evident in the amelioration of granulomas in the liver with increased ratios of healed to active ones.Citation19 An important fact that should be addressed is that the possible mode of action of artemether is believed to involve an interaction with haemin that cleaves the endoperoxide bridge of the drug and produces carbon-centered free radicals exerting oxidative stress on the parasites.Citation20 However, myrrh is known to possess strong antioxidant propertiesCitation21 that may counteract the action of the oxidant free radicals released by artemether.
In conclusion, although the combination of artemether and myrrh showed some degree of efficacy against experimental schistosomiasis mansoni, it does not encourage further clinical trials. This is based on the fact that previous experimental studies with praziquantel and/or artemether gave much better results. For the sake of not being dependent on a single drug against schistosomiasis, systematic research and development of other novel antischistosomal drugs, preferably herbal, as well as new combinations should be actively pursued.
Acknowledgments
We thank the staff of Schistosome Biologic Supply Center of Theodore Bilharz Research Institute, Cairo, Egypt, for their cooperation. We also extend our utmost gratitude to Prof. Dr. Amel el Sahn, High Institute of Public Health, Alexandria University, and Prof. Dr. Nibal Hammouda, Faculty of Medicine, Alexandria University, for their invaluable comments and suggestions. We also thank Mr. Hany Hussein, Biostatistics Department, High Institute of Public Health, Alexandria University, for assistance in the statistical analysis.
Disclosure
The authors report no conflicts of interest in this work.
References
- UtzingerJKeiserJShuhuaXTannerMSingerBHCombination chemotherapy of schistosomiasis in laboratory studies and clinical trialsAntimicrob Agents Chemother20034751487149512709312
- ColleyDGSecorWEImmunoregulation and World Health Assembly resolution 54.19: Why does treatment control morbidity?Parasitol Int200453214315015081946
- ShawJRBrammerKWThe treatment of experimental schistosomiasis with a combination of oxamniquine and praziquantelTrans R Soc Trop Med Hyg198377139406679362
- BotrosSSolimanAel-GawharyNSelimMGuirguisNEffect of combined low dose praziquantel and oxamniquine on different stages of schistosome maturityTrans R Soc Trop Med Hyg198983186892513673
- PughRNTeesdaleCHSynergy of concurrent low dose oxamniquine and praziquantel in schistosomiasisBr Med J (Clin Res Ed)19832876396877878
- CreaseyAMTaylorPThomasJEDosage trial of a combination of oxamniquine and praziquantel in the treatment of schistosomiasis in Zimbabwean schoolchildrenCent Afr J Med19863271651673107836
- UtzingerJCholletJYouJMeiJTannerMXiaoSEffect of combined treatment with praziquantel and artemether on Schistosoma japonicum and Schistosoma mansoni in experimentally infected animalsActa Trop200180191811495639
- MahmoudMRBotrosSSArtemether as adjuvant therapy to praziquantel in murine Egyptian schistosomiasis mansoniJ Parasitol200591117517815856895
- AshleyEAWhiteNJArtemisinin-based combinationsCurr Opin Infect Dis200518653153616258328
- GreeneDAGold, frankincense, myrrh, and medicineN C Med J199354126206228302372
- UtzingerJCholletJTuZXiaoSTannerMComparative study of the effects of artemether and artesunate on juvenile and adult Schistosoma mansoni in experimentally infected miceTrans R Soc Trop Med Hyg200296331832312174787
- Abdul-GhaniRLoutfyNShetaMHassanAEfficacy of low-dose myrrh protocols in the treatment of experimental schistosomiasis mansoni: hepatic improvement without parasitologic cureRes Reports Trop Med201016571
- PetersAPWarrenKSA rapid method of infecting mice and other laboratory animals with Schistosoma mansoni by subcutaneous injectionJ Parasitol1969553558563
- SmithersSRTerryRJThe infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of adult wormsParasitology19655546957004957633
- CheeverAWConditions affecting the accuracy of potassium hydroxide digestion techniques for counting Schistosoma mansoni eggs in tissuesBull World Health Organ19683923283314881073
- PellegrinoJOliveiraCAFariaJCunahASNew approach to the screening of drugs in experimental schistosomiasis mansoni in miceAm J Trop Med Hyg196211220121514484966
- El-LakkanyNMSeif el-DinSHBadawyAAEbeidFAEffect of artemether alone and in combination with grapefruit juice on hepatic drug-metabolising enzymes and biochemical aspects in experimental Schistosoma mansoniInt J Parasitol200434121405141215542101
- van AgtmaelMAGuptaVvan der WöstenTHRuttenJPvan BoxtelCJGrapefruit juice increases the bioavailability of artemetherEur J Clin Pharmacol199955540541010456492
- Abdul-GhaniRLoutfyNShetaMHassanAArtemether shows promising female schistosomicidal and ovicidal effects on the Egyptian strain of Schistosoma mansoni after maturity of infectionParasitol Res2010in press
- XiaoSCholletJUtzingerJMatileHMeiJTannerMArtemether administered together with haemin damages schistosomes in vitroTrans R Soc Trop Med Hyg2001951677111280070
- AssimopoulouANZlatanosSNPapageorgiouVPAntioxidant activity of natural resins and bioactive triterpenes in oil substratesFood Chem2005924721727