Abstract
Control and elimination of human onchocerciasis using mass drug administration of ivermectin (Mectizan®) has proceeded with marked gains over the past 10 years, more so in the Americas than in Africa. In the Americas, the initial focus on elimination of ocular morbidity has shifted to interruption of transmission, and the program has refined both the process leading up to interruption of transmission as well as the critical period following cessation of mass drug administration to document that there is no recrudescence of transmission. This is called the post-treatment surveillance (PTS) period. This report describes the aims, phases, and methodology of PTS as operationalized by the endemic countries and the Onchocerciasis Elimination Program for the Americas. Successful completion of the PTS period without signs of recrudescence leads to a country request for certification of elimination by the World Health Organization. As elimination of onchocerciasis in the Americas proceeds and emphasis in Africa switches from control to elimination, the PTS guide should prove invaluable to those programs going forward.
Introduction
Onchocerciasis (river blindness) is caused by the vector-borne parasite Onchocerca volvulus and is endemic in 28 African countries, extending centrally across the continent from Senegal eastward to Ethiopia; a small focus also occurs in Yemen in the Arabian Peninsula. Onchocerca volvulus was also introduced several centuries ago into the Americas where it became endemic in 13 foci in six countries – Brazil, Colombia, Ecuador, Guatemala, Mexico, and Venezuela. Strategic approaches taken to combat the disease have varied from control emphasizing vector reduction to elimination of the parasite using drug monotherapy.Citation1 As a result of long-term mass drug administration (MDA) with Mectizan® (Merck and Co, Rahway, NJ) elimination of the parasite in certain foci in Africa and the Americas now appears to be possible.Citation2,Citation3 Consequently, there is a need for an operational guide to implement surveillance in those foci or transmission zones where it is believed that parasite elimination has been achieved.
The Onchocerciasis Elimination Program for the Americas (OEPA) is a regional partnership that includes program managers from the six endemic countries, the Pan American Health Organization (PAHO)/World Health Organization (WHO), The Carter Center, Lions Clubs International and local Lions clubs, the United States Centers for Disease Control and Prevention (CDC), the Bill and Melinda Gates Foundation, several universities, the Mectizan Donation Program, and Merck and Co.Citation4 The goal of the initiative has been to provide MDA at least twice per year with Mectizan® (donated by Merck and Co) to achieve regional elimination of O. volvulus. Treatment coverage was targeted to reach at least 85% of the eligible population. OEPA is now operating under a 2008 PAHO Directing Council Resolution CD48. R12 calling for the regional elimination of ocular morbidity caused by onchocerciasis and interruption of transmission of the parasite by 2012.Citation5 Technical and scientific guidance to achieve these goals is provided to OEPA by a program coordinating committee (PCC).
In 2001 WHO published the document “Certification of elimination of human onchocerciasis: criteria and procedures,”Citation6 which established the different phases to be followed by a country to achieve certification of elimination of onchocerciasis. Each phase is associated with an aspect of parasite transmission resulting in four categories (; see also ).
Figure 1 Four stages of evaluation of onchocerciasis transmission and subsequent action leading to application for certification of elimination.Citation6
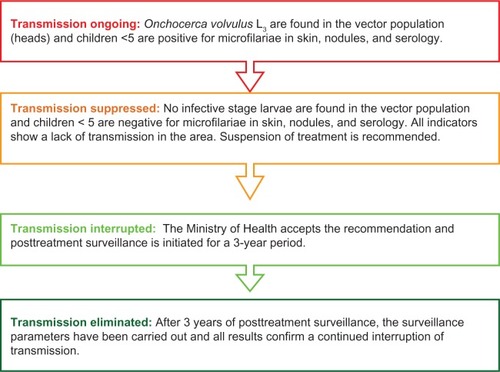
Table 1 Schedule of activities toward the elimination of onchocerciasis
We report here a guide developed by the PCC and OEPA as a field document that has as its focus the 3-year period defined by posttreatment surveillance (PTS) and describes the activities that distinguish and bridge category 3 (Transmission Interrupted) and category 4 (Transmission Eliminated). For this reason, the fundamental philosophy, structure, and many of the specific tenets developed for the Americas are widely applicable to onchocerciasis elimination programs in general.
Role of the PCC
Most major control and elimination programs benefit from an oversight committee that can provide impartial guidance to the country programs. The PCC is the technical steering committee of OEPA and makes recommendations to OEPA staff and to participating countries pertaining to the process of onchocerciasis elimination in each of the region’s 13 foci with respect to each of the stages of transmission described.
The PCC should be engaged in this process up until the official request by the country to WHO for certification by an international certification team.Citation6 The PCC should formally review the results of the PTS activities with the National Ministry of Health team within 6 months of the data having been collected. If there is no evidence of recrudescence, then the PCC will issue a written opinion to the government that elimination has been achieved and, if the last focus in the country, suggesting that the government request that the final WHO certification process begin. However, as with the decision to halt interventions prior to PTS, it is the government’s decision to accept or not the interpretation of their PTS results by PCC and whether to accept the PCC recommendations related to the country request for WHO certification.
Origins of the PTS concept
The idea of posttreatment surveillance originated in the 2001 WHO guidelines in which a 3-year period was recommended that was called the “pre-certification period.” This was a “national period” (ie, related to the entire country rather than individual onchocerciasis foci) during which surveillance should be instituted to detect recrudescence of transmission of O. volvulus after all nation-wide interventions have been halted.
With the ceasing of interventions, a 3 year pre-certification period would start. At the end of this pre-certification period, it must be shown that, although intervention has ceased, no new incident onchocerciasis cases have been registered and no infected vectors identified.Citation6
Based on this original WHO statement, the PCC modified the original concept of a national pre-certification period to be applied to foci rather than entire countries. This concept (a posttreatment surveillance period rather than a pre-certification period) was necessary because of the focal nature of the infection in the Americas and the need to follow the progress of each focus through the four phases of elimination outlined above.
Definition of PTS
The PCC defines PTS in the following manner:
Post Treatment Surveillance (PTS) is a 3-year period that begins with the termination of ivermectin mass treatment for onchocerciasis. At the end of this period, it must be documented that, although intervention has ceased, no evidence of recurrent transmission has occurred based on polymerase chain reaction (PCR) testing for O. volvulus DNA (deoxyribonucleic acid) in a substantial sample of vectors. Should positive entomological results be found, then serologic antibody testing in children less than ten years of age in the endemic area, through the enzyme-linked immunosorbent assay (ELISA) Ov-16 antigen should be undertaken. Positive results may be confirmed if required using polymerase chain reaction (PCR) testing of skin snips, in accord with the accepted criteria. If the data indicate no recrudescence of Onchocerca volvulus transmission, then the infection can be declared eliminated. Post elimination (also termed “post endemic”) surveillance may continue in formerly endemic foci beyond the initial 3 year PTS if deemed necessary.
Principles of PTS
The evaluations comprising PTS should not be completely new to the programs but continuations of previous programmatic field impact evaluations.
Indices obtained during PTS would therefore be comparable with “stop treatment” surveys, the latter serving as the “PTS baseline.”
If a potential recrudescence event is detected, the PCC should be consulted immediately and before any other actions are taken.
The response to a potential recrudescence event (PRE) should involve flexibility based on the transmission dynamics along with other characteristics of the focus being considered.
In addition, further studies may be requested that are similarly tailored to the focus and the nature of the potential recrudescence “signals” emerging from the initial PTS studies.
The SIMON mathematical model developed for African onchocerciasisCitation7 has been adapted for the Americas and used by OEPA for the past decade (first as SIMONa and currently as EuSIMON), to test the validity of a potential recrudescence event under the epidemiological and entomological conditions of the focus. This model was originally developed to evaluate the impact of mass drug distribution or vector control, making it very suitable for present conditions where ivermectin serves as the principle intervention. Modeling activities should include interpretation of PTS results and subsequent studies relative to breakpoints, reproduction ratio (Ro) projections, statistical certainty of multiple simulations, and risk for slow versus explosive recrudescence.
In the event of a PRE, the PCC should take a measured analytical approach, ie, careful evaluation before re-initiating mass drug administration. If the PRE is judged to be a true recrudescence, then a prompt programmatic response will be devised in consultation with the national program.
Prompt programmatic response should include state-of-the-art methods specific to the affected focus, such as, vector control, increased frequency of treatments with ivermectin, nodulectomy, or use of doxycycline.
Care should be taken using new tools as yet untested in the Americas (research) as “evidence” for recrudescence until such tools have been fully evaluated and validated for the region.
In case of doubt or need of clarification regarding any of the guidelines presented in this document, the PCC and OEPA should be contacted.
Continuation of programmatic activities during PTS
After the suspension of treatment, programmatic activities, now in the form of PTS, must continue for a minimum of 3 years, in accordance with WHO guidelines. Ministries of Health, political leaders, and donors should recognize that the national onchocerciasis programs do not cease field programmatic operations when ivermectin treatments are halted.
Expected evolution of a recrudescence
The expected evolution of how a potential recrudescence would unfold is based on the natural history of onchocerciasis, ie, a pre-patent period which requires 9–18 months from the time of the inoculation of third stage larvae (L3 s) until the initial presence of microfilariae in the skin. Given this, the earliest signal of a possible recrudescence would be the presence of infective larvae in the fly; followed by the discovery of antibodies to the antigen Ov-16 in children; followed by the appearance of nodules and/or microfilariae in the skin; followed by increased incidence of microfilariae in the skin as infection spreads. Finally, as a late manifestation, microfilariae would appear in the eyes. This progression allows the process of PTS to be considered in the stages that are shown in .
Figure 2 Sequential steps in the evolution of onchocerciasis recrudescence.

Given this sequence, the most cost-effective approach detecting the earliest signal of recrudescence with the least inconvenience to the population resident in the focus consists of screening first for positivity in the vectors, followed by screening for antibodies in children less than 10 years of age, and finally confirmation via PCR of skin biopsies taken from those children found to be serologically positive.
Basic PTS guidelines
The guidelines for PTS issued by the PCC comprise three components:
Educational intervention during the period of PTS
Each national program should guarantee that all communities and all individuals that have been involved in the onchocerciasis elimination process have a clear understanding of both the procedures undertaken to determine that an interruption of transmission has been achieved and the rationale behind the decision to suspend ivermectin treatment. In addition, each national program should work with the teams of health workers, leaders and volunteers, and the communities involved to define the specific activities that are to be undertaken during the 3-year PTS period, keeping in mind the recommendations put forth by the PCC. Of particular importance will be the need to maintain permanent ties of communication between the various health teams, leaders and volunteers, and the communities they serve through the strengthening of health promotional activities and keeping alive community interest and participation in achieving the goal of elimination.
Educational activities for program personnel
Each national program should guarantee that program workers and local health teams, along with local health agents, leaders, and volunteers that have been involved with the program, are thoroughly informed about the evaluations that have taken place to determine that transmission has been interrupted. The results of these evaluations should be reviewed jointly so as to provide clarity with regards to the rationale and decision making on the part of the Ministry of Health to suspend treatment with ivermectin in the communities.
The methods to be utilized to accomplish these educational goals should be defined by each national program in accordance with its particular characteristics and previous experience developed in the field of education.
Community educational activities
Program workers, local health teams and agents, and community leaders should thoroughly explain to the communities the activities that have taken place, the results of those activities, and the reasoning behind the suspension of treatment with ivermectin. Educational activities utilized within the community should be defined by each national program in relation to its previous experience.
Central level program accompaniment
It should be highlighted that central level program accompaniment is fundamental to the successful undertaking of educational activities at all levels of implementation and ensures that all persons and all communities involved in the program have the necessary information to understand changes in program activities. This is especially true with regards to the suspension of mass treatment with ivermectin. It is important that program workers, local health teams and agents, and community leaders and volunteers know that program coordinators at all levels are present at all activities to aid in the identification of needs and encourage the resolution of problems that are encountered along the way.
Evaluations during PTS
If recrudescence of onchocerciasis were to occur during the period of PTS, it would not occur in an abrupt manner (see ). For this reason, the PCC recommends that the participant countries conduct an entomological evaluation during the second and/or third year of the PTS. This evaluation should be conducted during the period of peak transmission and would confirm whether or not transmission remains interrupted and, in effect, whether or not it has been eliminated. The principle element of the PTS period will be entomological evaluations to determine the presence of parasite DNA in the Simulium vectors.
Entomological evaluationCitation8–Citation10
| – | Entomological evaluation by PCR technique (see Appendix 1). | ||||||||||||||||
| – | A minimum of 10,000 flies by focus (if available), collected in sentinel and/or extra-sentinel communities, and processed in pools of up to 50 flies by PCR and PoolScreen (version 3.0; University of Alabama, Birmingham, AL) analysis so as to obtain the infectivity rate. Extra-sentinel villages may be needed to increase focal representation and statistical certainty that transmission is interrupted. | ||||||||||||||||
| – | Timing: Fly collections will be conducted during the peak transmission season for each focus and initiated no later than Year 3 of PTS. Where the peak of transmission seasons span 2 years (October–February, for example), collections would need to be launched in Year 2. | ||||||||||||||||
| – | Body pools are analyzed by PCR first and upon finding the first positive body pool (containing stages L1 and L2), the analysis is switched to head pools (possibly containing infective stage larva [L3]). | ||||||||||||||||
| – | To confirm that transmission continues interrupted, or in effect, eliminated, the following results should be obtained:
| ||||||||||||||||
Serological surveyCitation11–Citation13
| – | A survey using the ELISA technique to determine the presence of antibodies to the antigen Ov-16 should be conducted in Year 3, only if the entomological evaluation indicates that a recrudescence in transmission has occurred (see Appendix 2). | ||||
| – | 3000 children <10 years of age will be tested via ELISA to detect immunoglobulin (Ig) G4-specific antibodies to the recombinant antigen Ov-16. | ||||
| – | WHO Certification GuidelinesCitation6 ask for a 5-year cumulative incidence rate of <1/1000 (<0.1%); here, the prevalence of Ov-16 antibodies will be taken as the equivalent to this cumulative incidence rate. Consequently, to calculate a prevalence rate of <0.1% with a 95% CI, assuming no positives, a sample size of at least 3000 children <10 years of age is required. | ||||
| – | If this number does not exist within the focus, then as many children in this age group as can be found should be surveyed. | ||||
| – | Sampling should be representative of the entire focus. | ||||
| – | Analysis should allow for stratification by age. | ||||
Ov-16 is a circulating pre-patent antigen, and antibodies to this antigen indicate exposure and possible pre-patent infection, ie, an infection that is incubating and not a full-blown patent infection. Therefore, if children are found positive by Ov-16 serology and the value of the indicator is above 0.1%, re-testing by PCR skin biopsy (to determine infection) should be considered. If these serologically positive persons were found negative by PCR, they will then be considered negative for a patent infection with O. volvulus but could still be considered as O. volvulus “exposed.”
Skin biopsy PCR in serologically positive children
Ov-16 serology (using the ELISA technique) detects an exposure to the parasite without being able to determine when that exposure may have occurred, while the technique of PCR establishes whether or not there is an O. volvulus infection. To this end, biopsies should be conserved in ethanol or absolute isopropanol for PCR processing.
Age of children to be evaluated by serology
An issue of the age of children in this evaluation is worthy of mention here as it was a source of considerable debate within the PCC. In the criteria for the certification of onchocerciasis elimination, WHO establishes as one of the indicators for the interruption of transmission
the absence of detectable infection (evidenced by microfilariae [mf], nodules, immunological tests and other analysis) in children up to 5 years of age that have not received treatment (eg, those that are becoming eligible for their first dose of ivermectin). A 5 year cumulative incidence rate with less than 1 new case per 1000 susceptible children is acceptable (provided that the appropriate population size is available).
WHO guidelinesCitation6 sought to calculate a 5-year cumulative incidence of <1/1000 so that only 5-year-old children can provide the “5 year cumulative incidence” data sought because each 4-year-old has only a 4-year incidence density experience, each 3-year-old a 3-year experience, etc. However, from an operational perspective, in most endemic countries in the Americas it is difficult to find grouped preschool children under the age of 5 years accessible for sampling. In addition, parents are reluctant to let very young children submit to bloodletting, even if a finger prick.
The PCC recommendation for testing children less than 10 years of age would still fulfill the WHO criteria, as using older children would provide even stronger support for transmission interruption (ie, each negative 8-year-old contributes 8 years’ incidence density to the formula, etc). Secondly, Ov-16 serology is not affected by ivermectin treatment.
Finally, acquisition of infection rises fastest between 5 and 20 years of age. However, children >10 years of age may misrepresent transmission status since these children could be seropositive from exposure or infection in the distance past, yet transmission could have been interrupted in the area for many years. For the same reason, adults would not be a good indicator age group for serology studies because of the possibility of persistent antibodies due to exposure to pre-control parasite transmission levels.
Complementary methods in studying recrudescence skin biopsy in sentinel communities
Some foci have established sentinel villages that have had serial longitudinal evaluations for microfilariae in skin, eyes, and nodules during the treatment phase of the elimination program. For this reason, the PCC recommends that these sentinel villages be re-examined by skin-snip survey (using microscopy) in the last year of the PTS period, if results of the vector PCR at that time are ≥1/2000 and the ATP calculation is >20. Ophthalmological evaluations are not deemed necessary by the PCC.
Nodule surveillance
Due to the nonspecificity of subcutaneous nodules in areas where onchocerciasis prevalence is low, the PCC does not recommend nodule surveillance during PTS. If countries elect to implement nodule surveillance, the contents of suspicious masses should be determined by histology (eg, by resection of the mass, sectioning, and staining) and initial finding of O. volvulus worms on microscopic examination confirmed by a recognized expert, in consultation with OEPA (see Appendix 3). Alternatively, a more rapid and equally specific methodology could be needle aspiration of a suspected nodule to obtain fluid for PCR testing in a suitable laboratory, again in consultation with OEPA.
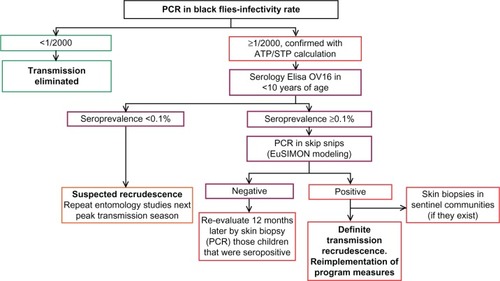
Decision tree illustrating the PTS process
| – | If in the PCR entomological evaluation there are no positive head pools or the infectivity rate is <1/2000 and the ATP/STP is <20, then it is assumed that transmission has been eliminated. It is at this point in the process that testing multiple runs through the mathematical model are encouraged to determine if the model predictions are compatible with the existing biological data. Nevertheless, the PCC/OEPA could recommend extending PTS in some areas in relation to particular epidemiological and vector considerations. | ||||
| – | Where there are cases of PCR head pools found to be positive where the infectivity rate is ≥1/2000 (95% CI) and the ATP/STP is >20, then a serological survey should be undertaken using a sample of 3000 resident children <10 years of age (if that number of children with those characteristics were available) with the goal of determining their seroposivity (ie, the presence of IgG4-specific antibodies against the recombinant antigen Ov-16 in capillary blood drawn from the children). | ||||
| – | If the serological survey yields an antibody prevalence of <1/1000 or <0.1% (95% CI), the results indicate a suspected recrudescence and it is recommended that entomological evaluations be repeated during the next period of peak transmission. | ||||
| – | If the serological survey yields an antibody prevalence of ≥1/1000 or ≥0.1% (95% CI), then there is the possibility of a recrudescence event. For confirmation, skin biopsies should be obtained from seropositive children and the biopsies processed via PCR. | ||||
| – | If PCR results confirm the positive serological results, then it is considered that the children are infected and that there is a definite recrudescence in transmission. The families of the children found to be positive should be interviewed to exclude the possibility of travel to other endemic areas or exposure to the vectors. This type of situation will need to be managed on a case by case basis in consultation with OEPA. | ||||
| – | The PCC additionally recommends a re-evaluation via skin biopsy of the sentinel villages, if they were to exist (see Appendix 4). | ||||
If the seropositive children have negative PCR analyzed skin biopsies, they should be considered negative for O. volvulus infection. In this situation, recrudescence of transmission is not confirmed but could be occurring. Consequently, these children should be re-examined in 12 months’ time via skin biopsy, again processed by PCR, so as to confirm their status. This second result should be considered definitive.
shows the decision tree/flow chart that illustrates the possible choices.
What to do in case of detecting a potential recrudescence
Should entomological and serological evidence indicate that recrudescence has occurred in a particular focus, then appropriate measures should be initiated to prevent further infections and, where possible, to eliminate the parasite or reduce transmission levels below the maintenance threshold. The PCC requests that countries contact it immediately so that a flexible solution can be determined using state-of-the-art methods specific to the affected focus. This may include vector control, increased treatments with ivermectin, nodulectomy, or use of doxycycline.
Preparation of a country report in support of the request for PAHO/WHO certification of elimination
During the PTS period, the national programs will not only have to join in the two components already mentioned (Education and Evaluations) but will also need to dedicate efforts in the organization of data and the preparation of the report that will accompany the request for certification. This report should meet the requisites established by PAHO/WHO in Appendices II, “Directives for the Preparation of a Country Report,” of the guide to criteria for certification.Citation6 WHO as well as OEPA are ready to assist in the development of this report.
Certification of elimination request to PAHO/WHO, by country
Requests to WHO for certification of elimination are not made by focus, but by country. The PCC and OEPA will assist country programs in the process leading up to a request for certification. When the final focus in a given country enters its PTS period, then by definition the country has entered the “precertification period” after which a national request for formal certification procedures can be made to PAHO/WHO.
Elimination of onchocerciasis has now become a realistic goal in both Africa and the Americas as result of many years of MDA using Mectizan®. In addition to 11 of the 13 foci in the Americas where transmission has been interrupted (representing four of the six countries in the region where the disease was endemic), foci in Mali, Senegal, and northern Sudan have also reported interruption of transmission.Citation14,Citation15 Thus, the development of this document serves a central epidemiological and, ultimately, a policy purpose that will be far-reaching in its utilitarian value. As for most guides of this type, it is also anticipated that changes will be made to adapt parts to more specific regional or country epidemiological situations. For example, historical entomological data recorded by the Onchocerciasis Control Programme in some hyperendemic locations in Africa demonstrated parous rates as high as 70%–80%, fly densities of the order of 20–50 per day or less, and infection rates as high as 10%.Citation16 However, these are unusual and vector populations characteristically fall in the range # 50% parity. For this reason, epidemiological models used in both Africa and the Americas typically assume a 50% parous rate. We therefore believe that the core information presented will serve as a central source of information to be used wherever onchocerciasis elimination is a goal.
Acknowledgments
Members of the Program Coordinating Committee/OEPA staff contributing to the writing of this document were: Ed Cupp (chair), Steve Ault, Alfredo Dominguez, Mark Eberhard, Maria-Eugenia Grillet, Alba Lucia Morales, Santiago Nicholls, Frank Richards, and Mauricio Sauerbrey. The Committee thanks D Rios and G Zea-Flores for their keen interest and important comments offered during the development of this document and N Cruz for analysis and discussion of ELISA and PCR results. We also thank C González for assistance in translation of the Spanish version of the earlier working document and the six OEPA country programs for their continued efforts in elimination of Robles disease in the Americas. We also acknowledge the expert assistance of S Sagastume in preparation of figures and earlier versions of this article.
Disclosure
The authors report no conflicts of interest in this work.
References
- CuppEWSauerbreyMRichardsFElimination of human onchocerciasis: history of progress and current feasibility using ivermectin (Mectizan®) monotherapyActa Trop2010120Suppl 1S100S10820801094
- MackenzieCDHomeidaMMHopkinsADLawrenceJCElimination of onchocerciasis from Africa: possible?Trends Parasitol2012281162210.1016/j.pt.2011.10.00322079526
- GustavesenKHopkinsASauerbreyMOnchocerciasis in the Americas: from arrival to (near) eliminationParasit Vectors2011420510.1186/1756-3305-4-20522024050
- SauerbreyMThe Onchocerciasis Elimination Program for the Americas (OEPA)Ann Trop Med Parasitol2008102Suppl 1252918718151
- Pan American Health Organization Resolution CD48/R12Toward the elimination of onchocerciasis (River Blindness) in the AmericasXLVIII Directing Council of the Pan American Health Organization2008 http://www.paho.org/english/gov/cd/cd48.r12-e.pdf
- World Health OrganizationGuidelines: Certification of elimination of human onchocerciasis: criteria and procedures2001GenevaWHO/CDS/CPE/CEE/200118b http://whqlibdoc.who.int/hq/2001/WHO_CDS_CPE_CEE_2001.18b
- DaviesJBPrediction of feasibility of onchocerciasis eradicationFilaria J2003226468 http://www.filariajournal.com/content/pdf/1475-2882-2-2.pdf
- KatholiCRToeLMerriweatherAUnnaschTRDetermining the prevalence of Onchocerca volvulus infection in vector populations by polymerase chain reaction screening of pools of black fliesJ Infect Dis19951725141414177594692
- UnnaschTRMeredithSEOThe use of degenerate primers in conjunction with strain and species oligonucleotides to classify Onchocerca volvulusClappJPMethods in Molecular Biology vol. 50 Species Diagnostics Protocols: PCR and Other Nucleic Acid Methods Totowa, NJHumana Press1996293303
- KatholiCRBarkerJPoolScreenBirmingham, ALUniversity of Alabama at Birmingham2002
- LindbladeKAAranaBZea-FloresGElimination of Onchocerca volvulus transmission in the Santa Rosa Focus of GuatemalaAm J Trop Med Hyg200777233434117690408
- LobosEWeissNKaramMAn immunogenic Onchocerca volvulus antigen: a specific and early marker of infectionScience19912515001160316052011741
- LipnerEMDembeleNSouleymaneSField applicability of a rapid-format anti-Ov-16 antibody test for the assessment of onchocerciasis control measures in regions of endemicityJ Infect Dis2006194221622116779728
- DiawaraLTraoréMOBadjiABissanYDoumbiaKFeasibility of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: First evidence from studies in Mali and SenegalPLoS Negl Trop Dis200937e49719621091
- HigaziTBZarrougIMAMohamedHAPolymerase chain reaction pool screening used to compare prevalence of infective black flies in two onchocerciasis foci in Northern SudanAm J Trop Med Hyg201184575375621540385
- WalshJFDaviesJBCliffBWHO Onchocerciasis Control Programme in the Volta River BasinLairdMBlackflies: The Future for Biological Methods in Integrated ControlLondon, UKAcademic Press198185103
Appendix 1
Entomologic evaluation
There are diverse considerations in preparing for the entomological evaluation, including the composition of the capture team (a collector and an attractant), the elaboration of a calendar in accordance to the period of peak transmission, and the acquisition of materials necessary for the packaging and conservation of the collected flies.
Selection of communities for evaluation
The communities to be evaluated are those that have served as sentinel and extra-sentinel communities, which is where in-depth epidemiological evaluations (EEPs) have taken place. The EEPs in these communities have permitted the evaluation of the impact of mass treatment with ivermectin on transmission and will, in the end, demonstrate that transmission has been interrupted.
Selection of capture sites within the community
In each of the communities to be evaluated, the capture sites should be identified and should be the same sites used during the EEPs.
Selection of season and hours of collection
Transmission seasons can vary by focus. For example, transmission of O. volvulus in Guatemala occurs annually between November and April, thus making these the best months for captures. In addition, the greatest numbers of flies are found between 12 noon and 5 pm, so that daily captures should be made during these hours.
The number of collection days depends on the known biting density for the community. If biting rates are low then it would be necessary to collect flies over a greater number of days to assure a sufficient total number of flies collected (about 10,000 flies), and in that way making for a more precise annual transmission potential (ATP).
Collection procedures
| – | Standard methods to assess the biting rate and collect vector specimens should be followed. Each team consists of a collector and an attractant. | ||||
| – | Each national program has standardized their hours for fly collecting in relation to the highest number of parous flies (eg, from 8 am to 5 pm, from 11 am to 5 pm, etc). | ||||
| – | Flies are collected for 50 minutes each hour, by aspiration, and before they have a chance to take blood. | ||||
| – | Collected flies are stored in absolute ethanol in tubes labeled with the hour, date, site, and community. | ||||
| – | At the end of each day, flies are separated according to species, using a stereoscope. | ||||
| – | The numbers of the vector in the area are recorded for each hour. | ||||
Laboratory analysis
For the PCR analysis of the collected flies, they should be placed in a tube or a jar with no more than 50 flies each. The heads and bodies of the flies are separated using standard procedures. A representative sample is tested by PCR to detect O. volvulus DNA. Body pools are analyzed first; if any of the body pools are positive, testing of putative positives is repeated. If the positive body pool is confirmed then body pool testing is suspended and all of the head pools are then analyzed. As part of a process to standardize this procedure, positive controls obtained from the University of South Florida are used. Positives are confirmed by a second PCR.
Data analysis
The geometric mean number of vectors caught per hour is calculated as [exp(∑log(x+1)/n)-1]/0.833, where x + 1 is the number of flies caught in a 50-minute collection period plus 1 (to avoid log[0]), n is the number of collection periods, and 0.833 is the conversion factor to convert a 50-minute collection period into 1 hour. This geometric mean hourly landing rate (which approximates the biting rate, as it will be called hereafter) is calculated for the vector over the capture period. The total biting density for this period (called the seasonal biting density [SBD]) is calculated as the geometric mean hourly biting rate multiplied by 10 potential hours of biting per day and the number of days in the season.
The PoolScreen® software program (version 3.0; University of Alabama, Birmingham, AL) employs a statistical model to calculate the probability of infection of an individual black fly from the number of positive pools and the size of the pools and will be used to calculate the proportion of infective flies with 95% CI computed using the Bayesian method.
The seasonal transmission potential (STP) is calculated as the product of the SBD, the proportion of flies with infective-stage O. volvulus larvae, and the mean number of infective larvae per infective fly (assumed to be one in an area of low transmission). The STP may be equal to, or slightly less than, the ATP.
The criteria used by the former Onchocerciasis Control Program of West Africa (OCP)Citation1 and in recent APOC/Gates-supported evaluations of transmission interruption in West Africa (Mali and Senegal)Citation14 are a prevalence of infective flies below 0.1% in parous flies or a prevalence less than 0.05% in all flies (assuming a parity rate of 50%). The sample size required to exclude a prevalence of infective flies of 0.05% in all flies at a 95% CI, given that no infective fly is found, is roughly 6000. This metric differs from the original World Health Organization criterion that called for sampling 10,000 flies. In cases where collections are unable to reach minimum sample size despite collections over the entire transmission season, the ATP or STP is critical to assessing the status of onchocerciasis transmission.
An OEPA-convened meeting of entomologists in September 2006 recommended the use of the ATP or STP, although there was controversy surrounding the levels at which transmission breakpoints occur. All entomologists at that meeting agreed that an ATP > 20 represented ongoing transmission and <5 represented interrupted transmission. However, the ATP or STP below which the reproduction ratio (Ro) of the parasite is <1, ie, the threshold transmission potential that indicates the parasite population is moving towards eradication, has yet to be identified and is likely to vary according to characteristics of the vector species. In actuality, estimates of this threshold transmission potential have ranged from 5 to 54 L3 s/person/year using mathematical models, from 7.6 to 18 using field observations, and in general a range of 5–20 is considered acceptable by most entomologists.
Appendix 2
Serologic evaluation
The objective is to measure the prevalence of IgG4 antibodies to Ov-16, a recombinant pre-patent antigen of O. volvulus, in children under 10 years of age.
To determine the population to be included in the serological evaluations, two methods have been employed in the region, each drawing their sample from a differing frame of reference:
| – | includes only endemic communities that had received treatment, and | ||||
| – | includes communities that are potentially endemic (that had or had not received treatment). | ||||
Method that includes only endemic communities under ivermectin treatment
| – | A census is made of all children <10 years of age in each community. | ||||
| – | A list of these communities is made with the number of children <10 years of age for each one. | ||||
| – | If the total number of children in the focus communities is more than 3000, then a sample is taken. | ||||
| – | For this, all communities in the focus are considered and a random sample of these communities made for inclusion in the evaluation. | ||||
Method that includes potentially endemic communities
This method of sampling in schools was used by Lindblade et alCitation11 in Guatemala in relation to foci with a large number of communities initially identified as potentially endemic but which eventually were excluded from treatment. This fact made it necessary to include these communities in the evaluations to determine an interruption of transmission.
Identification of potentially endemic communities
Using historical onchocerciasis transmission maps, a list of communities having at least one of the following characteristics should be compiled:
| – | past evidence of onchocerciasis transmission (defined as the documented presence of microfilariae in skin or eye, or confirmed onchocercomas in at least one community resident); | ||||
| – | suspicion of past transmission (a survey having taken place but no positive residents were found); or | ||||
| – | history of having been under mass drug administration with ivermectin. | ||||
The communities that satisfy these criteria are termed “potentially endemic communities” and form the basis for sample selection for serological assessments. If the estimated number of participants was below the targeted sample size of 3000, then all the children of eligible age in the area should be included. Efforts should be made to find absent children to ensure maximum participation in the evaluation.
Procedures
Sterile procedures are used to prick the fingers of all participants and four to six drops of blood (80–120 µL) are absorbed onto Whatman No 2 filter paper. The filter paper blood samples are dried, separated by sheets of paper, and then bundled and stored in sealed plastic bags in a cooler until they are returned to the laboratory where they are stored at 4°C, if immediately processed. If processing is not to take place within a short period of time, then the samples should be stored at −20°C.
Laboratory analysis
Two 6 mm punches of blood-saturated filter paper are placed in a phosphate-buffered saline–Tween 0.05% and bovine serum albumin 5% buffer and eluted overnight at 4°C. The elution is then run in duplicate in a standard ELISA to detect IgG4 antibodies against the Ov-16 recombinant antigen. A standard curve is used on each plate to identify positive samples and permit comparisons between plates and over days. Any positive results are repeated before being reported as positive.
Appendix 3
Nodule surveillance
Some programs have elected to undertake nodule assessments during PTS. After a series of discussions about the merit of such surveillance, PCC decided not to endorse nodule surveillance activities since a number of conditions could give rise to subcutaneous masses that are clinically suspicious for onchocercomas. Thus, false positives can easily result, and care must be given to distinguish between the following categories:
| – | “Masses” as reported by untrained personnel or by patients themselves that often are not clinically suspicious for onchocercomas; | ||||
| – | “Suspicious masses” clinically resembling onchocercomas; and | ||||
| – | “Onchocercomas” confirmed histologically or by PCR using fluid drawn from the nodule. | ||||
The process that has generally been followed includes:
| – | educational activities in coordination with health teams and communities to allow them to report the appearance of any suspicious subcutaneous mass that could be an onchocercal nodule; | ||||
| – | each “subcutaneous mass” should be documented as to its clinical details (size, pain, consistency, anatomical location, mobility) and if the mass appeared after treatment was suspended; | ||||
| – | such masses must be identified first by trained staff from the program to distinguish them from “suspicious masses;” | ||||
| – | likely onchocercomas are then surgically removed and submitted for proper histopathologic testing, in consultation with the Onchocerciasis Elimination Program for the Americas, for confirmation. | ||||
Active nodule surveillance has been a routine activity in some programs (especially Mexico), and so this mode of field work will undoubtedly continue during the PTS period. However, outside of Mexico it is assumed that the search of nodules will be mostly passive, with self-reporting of masses or examination by inexperienced health personnel.
Appendix 4
Sentinel villages
In its initial phase, the Onchocerciasis Elimination Program for the Americas (OEPA) established a methodology to determine the impact of mass treatment with ivermectin in endemic communities, which included the selection of a group of sentinel villages or communities that have been subjected to special follow-up activities and to the periodical holding of EEPs. It is important to point out that not all 13 foci in the region established sentinel communities given the fact that not all foci contained hyper-endemic communities. However, as OEPA advanced in the process of elimination, additional communities were selected in areas previously not evaluated. These communities are known as extra-sentinel communities.
| – | Sentinel communities were chosen by each national program at the beginning of their operations, and the majority of these were hyper-endemic (with a baseline prevalence of >60%). The extra-sentinel communities were chosen using the same criteria. EEPs took place at regular intervals (programmatic impact evaluations); first before treatment started, then after 2 years and finally at 4-year intervals thereafter. In the extra-sentinel communities, EEPs have also been carried out but not at previously established intervals. | ||||
| – | The evaluations include parasitological (microfilariae and nodules), ophthalmological, serological, and entomological indicators. | ||||
| – | Entomological evaluation will be carried out in sentinel and extra-sentinel communities during the PTS period to determine the interruption of transmission. | ||||
| – | The PCC recommends that only skin snip surveys be done in sentinel communities under the following circumstances: | ||||
| – | The entomological evaluations conducted during the PTS period showed positive results (infectivity rate of ≥1/2000 and ATP >20); | ||||
| – | The serological evaluations demonstrate an IgG4 antibody prevalence against O. volvulus of >0.1%; | ||||
| – | PCR results from biopsies taken from serologically positive children are positive. | ||||