 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
This study tested whether natural cocoa powder ingestion could mitigate hepatic injury coincident with murine malaria. Plasmodium berghei infection causes liver damage including hepatic sinusoidal distension, and elevated serum alanine transaminase (ALT) and aspartate transaminase (AST) levels. According to literature, these pathologies largely result from activity of reactive oxygen species (ROS) and may be extenuated by antioxidants.
Animals and methods
Thirty Balb/c mice were randomly assigned to three equal groups. One of two groups of mice inoculated with 0.2 mL of P. berghei-parasitized red blood cells (RBCs) was given unrestricted 24-hour access to a natural cocoa powder beverage (2% by weight) in place of water. The third group of mice were neither infected nor given cocoa. All mice were fed the same standard chow. After 6 days, mice were sacrificed and their livers processed for histomorphometric assessment of mean hepatic sinusoidal diameter as a quantitative measure of altered morphology. Serum ALT and AST were measured as a gauge of functional impairment.
Results
Compared with uninfected mice, hepatic sinusoidal diameter in P. berghei-infected mice not given cocoa increased by 150%, whereas a smaller increase of 83% occurred in infected mice that ingested cocoa. Mean serum ALT increased by 127% in infected mice not given cocoa and 80% in infected mice that consumed cocoa, compared with the value for uninfected mice. Similarly, mean serum AST was raised by 141% in infected mice not given cocoa and 93% in infected mice that drank cocoa.
Conclusion
Distension of hepatic sinusoidal diameter in P. berghei-infected mice was reduced by 67%, whereas respective elevations of serum ALT and AST concentrations were reduced by 47% and 48% via ingestion of cocoa. Anti-inflammatory and antioxidant components of cocoa probably mediated the demonstrated hepatoprotective benefit by blunting pernicious ROS activity in P. berghei-infected mice.
Introduction
Extensive efforts made over the last century to understand and control malaria have yet to minimize its significance as a major cause of morbidity and mortality in humans, and a number of factors have led to its presenting with unusual features.Citation1 In mice, significant liver ultrastructural pathology has been demonstrated in advanced stages of Plasmodium berghei malarial infection.Citation2 The liver is of great interest in malaria parasitemia for at least three reasons. Firstly, it is a target organ that plays a key role in the parasite’s developmental cycle.Citation3 Secondly, parasite activity combine with the host’s immune response to give rise to chronic inflammatory insults,Citation4,Citation5 which predisposes the organ to deleterious conditions including dysfunction and fulminant hepatic failure,Citation1 as well as hepatocellular cancer, and non-alcoholic fatty liver disease.Citation6 Thirdly, understanding of the liver stage of malaria parasites offers a promising target for antimalarial strategies that aim to establish immunity against the malaria parasite.Citation7
After subcutaneous deposition by a biting female anopheles mosquito, malaria sporozoites are transported to the liver via the bloodstream where they invade hepatocytes and undergo many rounds of schizogony.Citation8 The parasites migrate through several hepatocytes causing cell death before eventually settling down in a final hepatocyte for multiplication and differentiation into merozoites.Citation9,Citation10 Hepatocellular damage results from the generation of free radicals produced during malaria infection.Citation11 A link between free radicals, reactive oxygen species (ROS), and oxidative stress in tissue damage is now well established. It has been shown that increased oxidative stress during malaria infections,Citation12 arises from both the parasite’s metabolism,Citation4 and the host’s immune response.Citation5 With respect to liver pathology, oxidative stress is one of the causes of DNA damage associated with hepatocellular carcinoma in chronic viral hepatitis;Citation13 whilst ROS and lipid peroxidation products contribute to both onset and progression of hepatic fibrosis.Citation14
The mouse has a liver with four major lobes, just as in humans,Citation15 and has a gall bladder (which rats lack),Citation16 making it a good model for the study being reported. Moreover, P. berghei (murine malaria) is one of the most widely used experimental models to study malaria transmission.Citation17 One striking histological feature of the acute stage of malarial parasitemia is gross congestion in the sinusoids and hypertrophy of hepatic macrophages (Kupffer cells) that arises as they engulf parasitized and unparasitized red blood cells, remnants of parasites, granules and masses of hemozoin containing hemosiderin.Citation18 Hepatic damage is also characterized by markedly elevated levels of alanine transaminase (ALT), aspartate transaminase (AST) and bilirubin, coupled with a marked hepatic oxidative stress.Citation19
Cocoa, a product derived from the beans of the Theobroma cacao plant, has been consumed since 600 BC by ancient civilizations, such as the Mayans and Aztecs.Citation20 A rich source of flavonoid and theobromine, cocoa has been used for centuries as a medicine to combat inflammation, pain, and numerous other ailments.Citation21 Cocoa flavanols are particularly notable for their powerful antioxidative properties, which is mainly related to their inherent capacity to scavenge free radicals, thereby counteracting conditions of oxidative stress and coincident tissue damage.Citation22,Citation23 This antioxidant activity has been proven with isolated cocoa flavonoids, including the main compounds, catechin, epicatechin, and procyanidins;Citation24,Citation25 as well as the cocoa metabolites.Citation26 For instance, the flavonol quercetin (a cocoa metabolite) has been shown to prevent hepatotoxicity and nephrotoxicity caused by oxidative damage in rats.Citation27,Citation28 Moreover, consumption of cocoa powder enhances the antioxidant capacity of plasma, and decreases the content of lipid oxidation products in humanCitation29 and rat plasma.Citation30 This study used natural cocoa because Gu et alCitation31 showed that, being the least processed of consumed cocoa products, it contains the highest levels of total antioxidant capacity and procyanidins.
Material and methods
Animals
Thirty male Balb/c mice aged 6–8 weeks and of body weight 12–25 g were used. All mice were kept under the same laboratory conditions of temperature (22°C ± 2°C), relative humidity (70% ± 4%), and were exposed to a 12-hour light and dark cycle, and adequate ventilation. Mice were transferred from the breeding unit to the infectious unit of the animal experimentation unit of the Noguchi Memorial Institute for Medical Research for 7 days acclimatization before commencement of the experiments. During this period their body weights were recorded and they were fed with commercially obtained standard feed from Ghana Agro Food Company (GAFCO, Tema, Ghana), and given freshly filtered tap water every morning. The study protocol was approved by the Ethical and Protocol Review Committee of the University of Ghana Medical School. Procedures involving the care and use of mice conformed to the institutional guidelines in compliance with national and international laws and guidelines for the use of animals in biomedical research.
P. berghei (NK65) was procured from the Immunology Department of the Noguchi Memorial Institute for Medical Research.
Experimental protocol
Mice were randomly assigned to three experimental groups of ten animals per group, and were separated in three different cages of dimension 30 cm × 22 cm × 16 cm. Mice in group 1 (G1), were inoculated with 0.2 mL of parasitized blood containing 105 parasites per μL of blood and given unrestricted 24 hours access to 2% (weight/volume) aqueous natural cocoa powder in place of drinking water. Mice in group 2 (G2), were inoculated with 0.2 mL of parasitized blood containing 105 parasites per μL of blood and were not given cocoa, but had an unrestricted 24 hours access to drinking water. Mice in group 3 (G3) represented the control group which were neither infected nor fed cocoa, but had an unrestricted 24 hours access to drinking water. Animals in all three groups were fed with commercially obtained standard feed from Ghana Agro Food Company (GAFCO) during the experiment.
Cryopreserved parasites were taken through routine procedures to prepare an inoculum in complete parasite medium. After inoculation of stock (donor) mice with parasites, a series of passages was run in order to establish infection. Blood films were prepared from donor mice to estimate parasite density and parasitemia required to achieve infection of experimental mice. Blood from the tail vein of infected mice was placed on a clean glass slide (All Pro Processed Microscope Slide, Cat #7105, Surgifriend Medicals, Middlesex, England) and taken through the routine procedure for preparation of thick and thin films and examined under a light microscope (Leica, Galen III, Cat# 317505; Leica Microsystems, Wetzlar, Germany) with immersion oil and ×100 objective lens. When the required parasite density (105 parasitized red blood cells [RBC]) was achieved in donor mice, blood was drawn by cardiac puncture for inoculation of the experimental mice.
A hypodermic needle containing 0.2 mL of trisodium citrate (to prevent blood from clotting) was inserted into the thorax using the xiphoid process as a guide, and blood was drawn by cardiac puncture. The blood was then put into Ependorff tubes (Reagiergefab, Sarstedt AG and Co, Numbrecht-Rommelsdorf, Germany) containing 0.5 mL normal saline. The diluted blood was then transferred into a 15 mL falcon tube (Rohrchen; Greiner Bio-One International AG, Kremsmuenster, Austria) containing 2 mL of trisodium citrate. Mice in G1 and G2 were inoculated intraperitoneally with 0.2 mL of diluted parasitized blood containing 105 P. berghei (NK65) parasites per μL of blood.
Parasite density (of inoculum) per μL of blood was determined by counting the number of parasites against the total white blood cells (∼200 white blood cells) in Giemsa-stained thick blood films and the result multiplied by 8000 (the standard white blood cell count per μL of blood). The calculation was done as follows:
(1)
Parasitemia was monitored every 2 days post inoculation through Giemsa-stained thin blood films and expressed as percentages of at least 500 RBCs. Between 50–1000 RBCs were counted per slide with a mechanical hand tally counter (WJT-002A, Wenzhou Hualong Instrument & Apparatus, China) and percentage parasitemia was calculated as follows:
(2)
Biochemical analysis
On day 6 post inoculation, about 0.8 mL blood was collected by cardiac puncture from each experimental animal, into a clean dry centrifuge tube. The blood sample was allowed to stand for 2 hours to clot, and then centrifuged at 10,000 rpm for 10 min using a Wisperfuge centrifuge (model 1384; GEA Westfalia Separator, Oelde, Germany). The blood clot was gently loosened from the sides of the tube with an applicator stick to facilitate serum separation. Serum was then collected from the clot by micropipette, into sterile Ependorff tubes (Reagiergefab) for the measurement of biochemical indices. Activity of serum ALT and AST (both expressed as μ/L) were assayed with an automated biochemical analyzer (Vitalab Flexor E; 115W × 49H × 56D cm; Vital Scientific, Dieren, Netherlands). Serum from individual blood samples was measured separately and average values calculated for each group of mice.
Preparation of unsweetened natural cocoa drink
Two grams of GoodFood® Natural Cocoa Powder (batch number KK1004Al; Kakawa Enterprise Ltd, Accra, Ghana) was weighed using a chemical weighing balance (P1200; Mettler-Toledo International Inc, Greifensee, Switzerland) and dissolved in 100 mL of hot water. The dissolution of cocoa powder was ensured by adequate stirring to a uniform suspension. The hot cocoa drink was cooled under running tap water to 35°C before being made available to the mice. Fresh cocoa drink was prepared every morning and G1 mice had unrestricted access to it in place of drinking water. The drink was administered in a bottle with a tube from which the animals could suck. The bottles were washed each morning and filled with a freshly prepared drink. Mice in G2 and G3 were given tap water without cocoa powder. The cocoa drink in the feeding bottles was periodically shaken to prevent sedimentation of the particles of cocoa powder, which otherwise could clog the teats of the bottles and reduce the free flow of the drink when sucked by the mice. The volume of cocoa drink ingested by the mice was estimated by subtracting the final volume of the drink from the initial volume served. The composition of the cocoa powder used in this study is given in .
Table 1 Composition of Goodfood® Natural Cocoa Powder according to manufacturer’s specification
Measurement of liver weight
All mice were sacrificed on day 6 post inoculation after first anesthetizing them with chloroform in a tight fitted jar. Freshly dissected liver was put in ice-cold physiological saline to wash off blood, and blotted dry with filter paper. The liver was then weighed (in grams) using the Mettler balance. Two weight readings were taken for each liver and the average was determined. The hepatosomatic index (HSI) was determined by dividing the liver weight of each mouse by its respective body weight at termination of experiment.
Sampling and processing of mouse liver for stereological assessment
Mice livers were fixed in 10% (by volume) buffered formalin solution for 7 days, and sampled using a stepwise systematic random sampling technique. The liver of each mouse was separated into the right, left, median, and caudate lobes and each lobe cut with a disposable microtome blade, into 2 mm thick slices. Four slices each were obtained from the right, left and median lobes, whilst two slices were obtained from the caudate lobe because it was the smallest of all the lobes. A slice was selected at random to represent each of the four lobes of the liver of each mouse, for routine histological processing. In all, a total of 120 liver slices were processed for microscopic examination. Paraffin wax blocks of liver tissues were prepared using a fully automated tissue processor (Leica Tp 1020; Leica Microsystems; Ernst-Leitz-Strasse, Wetzlar, Germany) and tissue blocks were cast using a molten wax dispenser, plastic cassettes and stainless steel mould boxes. Sectioning of blocked tissues was done using a rotary microtome (Leica 2235; Leica Microsystems) at 5 μm thickness. The sections were stained with hematoxylin and eosin using an automatic stainer (Leica Autostainer XL; Leica Microsystem; Ernst-Leitz-Strasse, Wetzlar, Germany). To identify the slides, the code of each mouse was written on the frosted sides of the slides.
Measurement of sinusoidal diameters
A design-based stereological assessment was conducted to determine the diameter of the liver sinusoids, as a quantitative measure of altered liver morphology. Five slides of liver tissues out of ten per mouse were randomly sampled for the morphometry. Twenty microscopic fields were randomly captured from each of the selected slides onto a computer equipped with Microsoft Publisher software (Publisher software version 2007, Microsoft, Redmond, Wash), using a digital eye piece (Catalogue # MA 88;C & A Scientific Co Inc, Manassas, Virginia) attached to a light microscope (Leica Galen III). Microscope field sampling was randomized manually using the X and Y axes of the microscope stage micrometer. This was done by alternately moving three graduations on the X-axis and subsequently three graduations on the Y-axis. When at any point, a tissue field was in focus, the image was captured and saved on the computer. This was done until 20 micrographs were obtained from each slide. Two hundred micrographs were obtained from each experimental group. A microscope stage graticule (Graticules Ltd, Kent, UK) was photographed at the same microscopic magnification as the micrographs of liver and used to calibrate a ruler, which was in turn used to measure the short diameter of sinusoids. Ten sinusoids were randomly measured per micrograph. In all, a total of three thousand sinusoidal diameters were measured.
Statistical analysis
All results were expressed as arithmetic mean and standard deviation (SD). Statistical significance of the difference between group means was performed by Student’s t test and one-way analysis of variance (ANOVA). Differences with P < 0.05 were considered to be significant. SPSS software (version 16.0; SPSS Inc, Chicago, IL) was used for these analyses. Further analysis of percentage RBC parasitemia was performed using GraphPad Prism software (version 3.0; GraphPad, La Jolla, CA).
Results
Each mouse in the control (G3) and in infected mice not given cocoa (G2) groups took on a daily basis, an average of 13.86 mL (SD 1.07) and 10.71 mL (SD 4.50) of water, respectively. Each infected mouse in the group given cocoa (G1) consumed on daily basis an average of 10.48 (SD 3.24) mL of natural cocoa drink. ANOVA showed that these volumes of fluid consumed by the three groups of mice were not significantly different.
Significantly increasing RBC parasitemia occurred in infected mice () over the six days of the study, both in animals that drank cocoa and those that did not. Parasitized RBCs and liver macrophages (Kupffer cells) that had phagocytosed infected cells were observable in the central veins of liver sections of infected mice (). Bonferroni post-hoc tests affirmed increasing parasitemia over the three days of blood sampling in P. berghei-infected mice regardless of whether they drank cocoa or not, except between days two and four in animals that did not drink any cocoa () Whether or not mice drank cocoa failed to exert statistical main effect or interaction on percentage parasitized RBCs () in two-way ANOVA comparisons with cocoa intake and days of parasitemia as the variables. In agreement with data in , the two-way ANOVA produced significant main effect of days of parasitemia (). However, post-hoc tests showed nonsignificant differences between percentage RBC parasitemia in mice that drank cocoa and those that did not, when data were compared according to corresponding number of days after parasite inoculation (). Indeed, the only fatality recorded in this study was a rodent that drank cocoa, albeit it died before day four after inoculation with parasitized RBCs at the time when parasitemia was relatively low (); absence of veterinary post mortem service did not permit establishment of the cause of death.
Figure 1 H&E stained liver sections showing profiles of central veins of (A) infected mouse given cocoa and (B) infected mouse not given cocoa containing parasitized RBCs (yellow arrows) and phagocytosed parasitized RBCs (red arrows). Central vein of control mouse (C) contain unparasitized RBCs (yellow arrows).
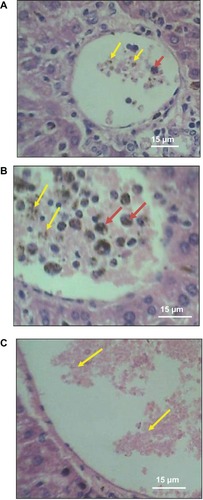
Table 2 Means, and comparative statistics on parasitized RBCs coincident with or without cocoa ingestion in Plasmodium berghei-infected mice
Table 3 Bonferroni’s post hoc statistical analyses of percentage parasitized RBCs coincident with or without cocoa ingestion in Plasmodium berghei infected mice within the groups
Table 4 Two-way ANOVA comparisons of percentage of parasitized RBCs coincident with or without cocoa ingestion in mice infected with Plasmodium berghei
Table 5 Bonferroni post hoc comparisons of percentage of parasitized RBCs coincident with or without cocoa ingestion in mice infected with Plasmodium berghei
Before they were inoculated with parasitized RBCs, mean body weight of infected mice given cocoa was 24.11 g (SD 1.85) and this decreased to 20.77 g (SD 2.23) after 6 days of infection. Similarly mean body weight of infected mice not given cocoa was 22.90 g (SD 1.79) before their inoculation and 20.34 g (SD 1.83) after 6 days infection. Control mice had an average body weight of 22.35 g (SD 1.62) at commencement and 22.24 g (SD 1.41) after the 6 days (of experimentation with infected mice). ANOVA yielded a nonsignificant F-statistic (2.48, P = 0.10, DF = 2) for mean baseline body weight and body weight after 6 days of infection (F = 2.93, P = 0.07, df = 2). The respective mean liver weights on day 6 after infection were 1.29 g (SD 0.15) for infected mice given cocoa, 1.32 g (SD 0.16) for infected mice not given cocoa, and 1.27 g (SD 0.16) for control mice. One-way ANOVA indicated no statistical differences among the groups (P = 0.77). The mean HSI obtained in the three groups of mice on day 6 after infection was 6.21 (SD 0.35) for infected mice given cocoa, 6.49 (SD 0.53) for infected mice not given cocoa, and 5.76 (SD 1.03) for control mice. Again, one-way ANOVA showed nonsignificant difference (P = 0.08) among the groups.
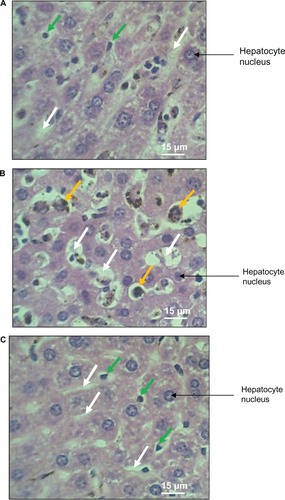
Liver histoarchitecture from a P. berghei-infected mouse given and another not given natural cocoa to drink, as well as an uninfected control mouse, are all illustrated in . The mean hepatic sinusoidal diameter (MHSD) for infected mice given cocoa was 15.22 μm (SD 5.42), for infected mice not given cocoa was 20.80 μm (SD 6.08), and for control mice was 8.31 μm (SD 2.64). One-way ANOVA gave a significant F-statistic of 844.22 with P = 0.001 and DF = 2. Bonferroni post-hoc multiple comparison tests yielded significant differences (P < 0.001) among all three groups. Thus, infected mice that were not given cocoa had the highest and control mice the lowest MHSD. Infected mice given cocoa had an intermediate MHSD that was significantly higher than the value for control mice and significantly lower than the value for infected mice not given cocoa.
Figure 2 H and E stained sections showing liver architecture and sinusoids of (A) infected mouse given cocoa, (B) infected mouse not given cocoa, and (C) control mouse. White arrows indicate hepatic sinusoids. Kuppfer cells (orange arrows) in (B) have undergone hypertrophy compared with those in (A) and (C) green arrows.
The average serum ALT (in μ/L) in P. berghei-infected mice given cocoa, infected mice not given cocoa, and control mice were 67.18 (SD 16.50), 84.38 (SD 28.74), and 37.23 (SD 7.08), respectively. One-way ANOVA yielded significant F-statistic of 5.95 (P = 0.023, DF = 2). Bonferroni post-hoc multiple comparison test was nonsignificant (P = 0.18) between infected mice given cocoa and control mice; significant (P = 0.02) between infected mice not given cocoa and control mice, and nonsignificant (P = 0.74) between infected mice given cocoa and infected mice not given cocoa.
Average AST (in μ/L) was 257.30 (SD 74.99) for infected mice given cocoa, 320.60 (SD 68.30) for infected mice not given cocoa, and 133.08 (SD 65.75) for control mice. One-way ANOVA gave significant F-statistic of 7.47 (P = 0.012; DF = 2). Post-hoc multiple comparison test was nonsignificant (P = 0.10) between infected mice given cocoa and control mice, significant (P = 0.01) between infected mice not given cocoa and control mice, and nonsignificant (P = 0.70) between infected mice given cocoa and infected mice not given cocoa.
Discussion
This study was undertaken to ascertain the hepatoprotective activity of cocoa in reducing damage caused by plasmodial malaria. HSI was measured as a proxy for injurious activity of malaria infection in this study. Although the trend indicated that infected mice given cocoa had intermediate value between higher HSI for infected mice not given cocoa and lower HSI for control mice, these differences were not significant.
This study has confirmed the power of MHSD to distinguish the extent of damage caused to the liver by P. berghei infection. Probably for the first time, this study has shown that voluntary consumption of natural cocoa by mice reduced sinusoidal distension coincident with parasitemia by 67% compared with controls and infected mice that did not drink cocoa. Voluntary ingestion of cocoa was preferred to other routes of administration because it better reflected the typical route of administration that would be used by humans who were encouraged to drink natural cocoa as antimalarial prophylaxis.Citation32 The finding in this study that MHSD was higher in the infected mice (G1 and G2) than in control mice, is consistent with previous work that also showed inoculation of mice with P. berghei resulted in liver damage evidenced by distension of hepatic sinusoids.Citation33,Citation34 Evidence of hepatic damage in the present study included disruption of the trabecular structure of hepatocytes due to dilatation of hepatic sinusoids, as well as hypertrophy of Kupffer cells, also previously reported.Citation18
A direct in vitro inhibitory activity of extract and fractions of natural cocoa powder against P. falciparum has already been demonstrated,Citation35 so the hepatoprotective effect of cocoa in the present study suggests that prophylactic antimalarial benefit of regular ingestion of natural cocoa would take multiple forms. With respect to possible mechanism(s) of action of natural cocoa in mitigating the damage to liver that accompanies plasmodial infection, it is attractive to speculate that the notable antioxidativeCitation24–Citation26 and antiinflammatoryCitation36 properties of dietary flavanols play a key role. For instance it has been noted that enhanced glutathione release by liver parenchymal cells traps ROS generated by Kupffer cells and neutrophils in the vasculature, thereby attenuating liver injury.Citation37 It has also been observed that apoptosis of endothelial cells caused by severe malaria could be reduced by antioxidants.Citation38,Citation39 Furthermore, the flavonoid quercetin is reported to be capable of preventing hepatotoxicity caused by oxidative damage.Citation27,Citation28 Following from these, it is postulated that in the current study, components in ingested natural cocoa exerted anti-inflammatory and antioxidative properties that blunted the damage caused to hepatic sinusoids by ROS, oxidative stress, and immune-responsive proinflammatory factors engendered by P. berghei infection.
In the present investigation, challenging mice with P. berghei-parasitized RBCs resulted in a significant increase of serum ALT and AST levels, as compared with the control mice sera. The increased activities of the enzymes AST and ALT in serum indicate hepatocellular damage since the levels of these enzymes are elevated in acute hepatotoxicity.Citation40 Our findings that ALT and AST levels were lower in infected mice given cocoa compared with the infected mice not given cocoa, affirms implied hepatoprotective activity of components in cocoa. Other researchers such as Ko et alCitation41 have shown that antioxidants reduce AST and ALT levels in experimental rats and mice with liver disorders. Furthermore, extract of Antrodia camphorata (an antioxidant) has been reported to ameliorate the increase in ALT and AST levels caused by chronic repeated carbon-tetrachloride intoxication in mice, by mediating antioxidative and free-radical scavenging activities.Citation42 Our finding that serum AST and ALT could not distinguish between infected mice given cocoa and control mice, provokes an inference that these functional markers are less acute indicators of hepatic damage than MHSD, and/or may lag behind structural damage to the liver.
There is a possibility that apart from polyphenols, other components in cocoa such as methylxanthines, peptides and minerals,Citation43 may have accounted individually or synergistically for the observed hepatoprotective activity of ingested cocoa in the present study. However, support for antioxidative mediation of the observed liver protection in the mice that drank cocoa in the work being reported, resides in the well-established role of oxidative stress in the pathophysiological processes of liver injury.Citation44,Citation45 Despite recent skepticism, literature abounds with the in vivo antioxidative benefits of the polyphenols in cocoa.Citation43,Citation46,Citation47 Moreover, our own laboratory has a previous study which provides supportive evidence for in vivo antioxidative benefit of ingested cocoa.Citation48 In that study streptozotocin-induced diabetic rats were given 2% Goodfood natural cocoa powder for 13 weeks. The concentration of plasma isoprostane, an oxidative marker, rose by 43.82% in streptozotocin-induced diabetic rats and by only 9.14% in streptozotocin-induced diabetic rats that voluntarily ingested cocoa as a drink, signifying a 34.68% decrease in the plasma oxidative marker. Furthermore, EtseyCitation48 showed that total antioxidative power of testicular tissue, measured as a uric acid equivalent, decreased by 80.5% in animals not given cocoa versus 43.4% in animals that drank cocoa over 13 weeks of the study. This indicated that ingestion of 2% natural cocoa powder coincided with 37.1% retention of total antioxidative power in the testicular tissue of streptozotocin-induced diabetic rats.
It is noteworthy that cocoa intake failed to ameliorate progression of parasitemia in the present study. On the one hand, it indicates that the hepatoprotective benefit observed did not result from less severe parasitemia in the mice that drank cocoa. On the other hand, it provokes the inference that cocoa intake did not treat the malaria infection in the mice. Questions arise as to whether this finding resulted from failure of the mice to ingest an adequate amount of cocoa, or whether the unnatural concentration of parasites in the inoculum exceeded the antiplasmodial activity of ingested cocoa. Future studies would seek to address these issues. Although postprandial plasma concentrations of cocoa metabolites were not determined in our study, it has been reported that recovery of total polyphenol was 100% in the prepared hot cocoa drink.Citation49 It is therefore unlikely that the mode of preparation of cocoa in our study reduced the quantity of available polyphenols in the drink ingested by the mice. Regardless of the absence of antimalarial activity in the present report, it unequivocally demonstrated that natural cocoa ingestion has potential for controlling liver damage coincident with malaria parasitemia. Pertinently, our laboratory has reported a comparable finding that voluntary ingestion of natural cocoa extenuated hepatic injury in rats with experimentally induced chronic alcoholic toxicity.Citation50
Conclusion
Voluntary intake of 2% aqueous suspension of natural cocoa powder significantly reduced structural and functional damage of the liver in mice infected with P. berghei for six days. Given the known role of oxidative stress and inflammation in liver injury in general and referable to plasmodium parasitemia in particular, it is postulated that the hepatoprotective activity of natural cocoa is attributable to its antioxidative/anti-inflammatory properties. Available literature suggests that theobromine and polyphenols in cocoa may have exerted antioxidative/anti-inflammatory activity that blunted pernicious activity of ROS and proinflammatory immune-response macromolecules generated in infected mice. This study does not rule out the possibility that other components in natural cocoa may have produced, or contributed to, the hepatoprotective benefit reported via nonantioxidative properties.
Acknowledgments
FK Addai is nationally (Ghana) and regionally (West Africa) known as a researcher and promoter of natural cocoa consumption for better health. He is the Founder and Director of Kakawa Enterprise Limited, an ultramicroscale company that produces GoodFood Unsweetened Natural Cocoa Powder. Kakawa Enterprise Limited made no financial contribution towards the study or publication of this paper.
Funding for the study was provided by the Department of Anatomy, University of Ghana, from its Departmental Development Fund. The College of Health Sciences and University of Ghana Medical School are gratefully acknowledged for their indispensable administrative and technical support. The authors are grateful to the Departments of Pathology (University of Ghana Medical School), and Departments of Animal Experimentation and Epidemiology (Noguchi Memorial Institute for Medical Research), for financial, material, and technical support. Dr Nii Ayite-Aryee, and Rev Prof Andrew S Ayettey are acknowledged for serving as scientific advisors.
References
- ZakiSAShanbagPAtypical manifestations of malariaResearch and Reports in Tropical Medicine20112922
- Rodríguez-AcostaAFinolHJPulido-MéndezMLiver ultrastructural pathology in mice infected with Plasmodium bergheiJ Submicrosc Cytol Pathol19983022993079648294
- VanderbergJPFrevertUIntravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporo-zoites injected into skin by mosquitoesInt J Parasitol200434999199615313126
- MüllerSRedox and antioxidant systems of the malaria parasite Plasmodium falciparumMol Microbiol20045351291130515387810
- UrbanBCIngRStevensonNMEarly interactions between blood-stage plasmodium parasites and the immune systemCurr Top Microbiol Immunol2005297257016265902
- PanMHLaiCSHoCTAnti-inflammatory activity of natural dietary flavonoidsFood Funct201011153121776454
- HoffmanSLGohLMLukeTCProtection of humans against malaria by immunization with radiation-attenuated Plasmodium falci-parum sporozoitesJ Infect Dis200218581155116411930326
- KappeSHKaiserKMatuschewskiKThe Plasmodium sporozoite journey: a rite of passageTrends Parasitol200319313514312643997
- MotaMMPradelGVanderbergJPMigration of Plasmodium sporozoites through cells before infectionScience2001291550114114411141568
- FrevertUEngelmannSZougbédéSIntravital observation of Plasmodium berghei sporozoite infection of the liverPLoS Biol200536e19215901208
- ClarkIAHuntNHCowdenWBOxygen-derived free radicals in the pathogenesis of parasitic diseaseAdv Parasitol1986251443022568
- GriffithsMJNdunguFBairdKLMullerDPRMarshKOxidative stress and erythrocyte damage in Kenyan children with severe Plasmo-dium falciparum malariaBr J Haematol2001113248649111380421
- HagenTMHuangSCurnutteJExtensive oxidative DNA damage in hepatocytes of transgenic mice with chronic active hepatitis destined to develop hepatocellular carcinomaProc Natl Acad Sci U S A1994912612808128127809125
- PoliGPathogenesis of liver fibrosis: role of oxidative stressMol Aspects Med2000213499810978499
- HummelKPRichardsonFLFeketeEAnatomyGreenELBiology of the Laboratory MouseNew YorkMcGraw-Hill1968247307
- MacSweenRNMDesmetVJRoskamsTScothorneRJDevelopmental anatomy and normal structureMacSweenRNMBurtADPortmannBCIshakKGScheuerPJAntonyPPPathology of the LiverNew YorkChurchill Livingstone2002166
- Jaramillo-GutierrezGRodriguesJNdikuyezeGPovelonesMMolina-CruzABarillas-MuryCMosquito immune responses and compatibility between Plasmodium parasites and anopheline mosquitoesBMC Microbiology2009915419643026
- EdingtonGMGillesHMPathology in the Tropics2nd edLondonEdward Arnold1976
- TirkeyNPilkhwalSKuhadAChopraKHesperidin, a citrus biofla-vonoid, decreases the oxidative stress produced by carbon tetrachloride in rat liver and kidneyBMC Pharmacol20055215683547
- HurstWJTarkaSMJrPowisTGValdezFJrHesterTRCacao usage by the earliest Maya civilizationNature2002418689528929012124611
- DillingerTLBarrigaPEscarcegaSJimenezMLoweDSGrivettiLEFood of the gods: cure for humanity? A cultural history of the medicinal and ritual use of chocolateJ Nutr20001308 SupplS2057S2072
- Rice-EvansCFlavonoid antioxidantsCurr Med Chem2001879780711375750
- LeeKWKimYJLeeHJLeeCYCocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wineJ Agric Food Chem200351257292729514640573
- AdamsonGELazarusSAMitchellAEHPLC method for the quantification of procyanidins in cocoa and chocolate samples and correlation to total antioxidant capacityJ Agric Food Chem199947104184418810552788
- ZhuQYHoltRRLazarusSAOrozcoTJKeenCLInhibitory effects of cocoa flavanols and procyanidin oligomers on free radical-induced erythrocyte hemolysisExp Biol Med (Maywood)2002227532132911976402
- SpencerJPSchroeterHShenoyBSraiSKDebnamESRice-EvansCEpicatechin is the primary bioavailable form of the procyanidin dimers B2 and B5 after transfer across the small intestineBiochem Biophys Res Commun2001285358859311453632
- PeresWTuñónMJColladoPSHerrmannSMarroniNGonzález-GallegoJThe flavonoid quercetin ameliorates liver damage in rats with biliary obstructionJ Hepatol200033574275011097482
- SatyanarayanaPSSinghDChopraKQuercetin, a bioflavonoid, protects against oxidative stress-related renal dysfunction by cyclosporine in ratsMethods Find Exp Clin Pharmacol200123417518111676225
- WangJFSchrammDDHoltRRA dose-response effect from chocolate consumption on plasma epicatechin and oxidative damageJ Nutr20001308 SupplS2115S2119
- BabaSOsakabeNNatsumeMCocoa powder enhances the level of antioxidative activity in rat plasmaBr J Nutr200084567368011177180
- GuLHouseSEWuXOuBPriorRLProcyanidin and catechin contents and antioxidant capacity of cocoa and chocolate productsJ Agric Food Chem200654114057406116719534
- AddaiFKNatural cocoa as diet-mediated antimalarial prophylaxisMedical Hypothesis2010745825830
- PariLAmaliDRProtective role of tetrahydrocurcumin (THC) an active principle of tumeric on chloroquine induced hepatoxicity in ratsJ Pharm Pharm Sci20058111512315946605
- KakkilayaBS20062008Pathology of malaria Available from: http://www.malariasite.comAccessed: March 7, 2009
- AmponsahSKBugyeiKAOsei-SarfoDIn vitro activity of extract and fractions of natural cocoa powder on Plasmodium falciparumJ Med Food201215547648222248179
- PanMHLaiCSHoCTAnti-inflammatory activity of natural dietary flavonoidsFood Funct201011153121776454
- JaeschkeHEnhanced sinusoidal glutathione efflux during endotoxin-induced oxidant stress in vivoAm J Physiol19922631 Pt 1G60G681636717
- UemuraMManabeHYoshidaNAlpha-tocopherol prevents apoptosis of vascular endothelial cells via a mechanism exceeding that of mere antioxidationEur J Pharmacol20024561–3293712450566
- HemmerCJLehrHAWestphalKUnverrichtMKratziusMReisingerECPlasmodium falciparum Malaria: reduction of endothelial cell apoptosis in vitroInfect Immun20057331764177015731077
- ObiEOrisakweOEAsomughaLAUdemezueOOThe Hepatotoxic effect of halofantrine in guinea pigsIndian J Pharmacy200436303305
- KoKMIpSPPoonMKEffect of a lignan-enriched fructus schisandrae extract on hepatic glutathione status in rats: protection against carbon tetrachloride toxicityPlanta Med19956121341377753920
- HsiaoGShenMYLinKHAntioxidative and hepatoprotective effects of Antrodia camphorata extractJ Agric Food Chem200351113302330812744658
- JalilAMIsmailAPolyphenols in cocoa and cocoa products: is there a link between antioxidant properties and health?Molecules20081392190221918830150
- LieberCSAlcohol and the liver: metabolism of alcohol and its role in hepatic and extrahepatic diseasesMt Sinai J Med2000671849410677787
- AlbanoEOxidative mechanisms in the pathogenesis of alcoholic liver diseaseMol Aspects Med2008291–291618045675
- TzounisXRodriguez-MateosAVulevicJGibsonGRKwik-UribeCSpencerJPPrebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention studyAm J Clin Nutr2011931627221068351
- ZomerEOwenAMaglianoDJLiewDReidCMThe effectiveness and cost effectiveness of dark chocolate consumption as prevention therapy in people at high risk of cardiovascular disease: best case scenario analysis using a Markov modelBMJ2012344e365722653982
- EtseyANEffects of Regular Ingestion of Natural Cocoa on Testicular Histology in Streptozotocin-Induced Diabetic Rats [thesis]AccraUniversity of Ghana2009
- StahlLMillerKBApgarJPreservation of cocoa antioxidant activity, total polyphenols, flavan-3-ols, and procyanidin content in foods prepared with cocoa powderJ Food Sci2009746C456C46119723182
- SokporGAddaiFKGyasiRKBugyeiKAAhenkorahJHottorBVoluntary ingestion of natural cocoa extenuated hepatic damage in rats with experimentally induced chronic alcoholic toxicityFFHD201225166187