Abstract
Improved diagnosis and treatment regimens have resulted in greater longevity for men with prostate cancer. This has led to an increase in both androgen deprivation therapy (ADT) use and duration of exposure, and therefore to its associated adverse effects, such as sexual dysfunction, osteoporosis, reduced muscle mass, increased fat mass, and increased incidence of cardiovascular disease and type 2 diabetes. Given that the adverse effects of ADT are systemic, often debilitating, and difficult to treat, efforts continue in the development of new strategies for long-term management of prostate cancer. The PubMed database was searched to select trials, reviews, and meta-analyses in English using such search terms as “prostate cancer” and “androgen deprivation therapy”, “cardiovascular risk”, “lean body mass”, “exercise”, and “diet”. The initial searches produced 379 articles with dates 2005 or more recent. Articles published after 2004 were favored. This review utilizes the latest data to provide a status update on the effects of exercise and diet on patients with prostate cancer, focusing on ADT-associated side effects, and it discusses the evidence for such interventions. Since the evidence of large-scale trials in patients with prostate cancer is missing, and an extrapolation of supporting data to all patient subgroups cannot be provided, individualized risk assessments remain necessary before the initiation of exercise and diet programs. Exercise, diet, and nutritional supplementation interventions have the potential to provide effective, accessible, and relatively inexpensive strategies for mitigating ADT-associated toxicities without introducing additional adverse effects.
Introduction
Prostate cancer is the most frequently diagnosed cancer among men in developed countries.Citation1 Widespread adoption of programs to screen for prostate-specific antigen has revolutionized the diagnosis and treatment of prostate cancer. Early detection and development of new treatment options have improved outcomes, increasing 5-year survival from 67.8% to 99.7% over the past 25 years, with 10- and 15-year survival presently at 99% and 94%, respectively.Citation2 Thus, prostate cancer has moved from an aggressive, often fatal, disease to a chronic condition requiring comprehensive management strategies to maintain patients’ quality of life (QoL).
Androgen deprivation therapy (ADT) has long been the frontline treatment for advanced prostate cancer.Citation3–Citation8 Indeed, over a third of the roughly 3 million men in the USA presently diagnosed with prostate cancer have received or are receiving ADT.Citation2 Although ADT has been found to improve the overall survival, it is also associated with adverse side effects, including sexual dysfunction, gynecomastia, hot flashes, osteoporosis, cognitive defects, reduced muscle mass, increased fat mass, and increased incidence of both cardiovascular disease and type 2 diabetes.Citation9–Citation12
Although earlier diagnosis and treatment have greatly extended patient longevity, they have also led to a considerably longer duration of ADT. An alternative, intermittent ADT has been proposed to mitigate the adverse effects of continuous ADT with the premise that it will not impact efficacy.Citation13,Citation14 Although intermittent ADT offers some relief from the side effects of continuous ADT, in particular during treatment pauses, comprehensive management plans aimed at helping patients cope with the long-term effects of ADT throughout all phases of therapy are essential, but are often missing.
Ninety percent of the patients with prostate cancer in the USA are aged 60 years or older,Citation2 a population known to be at high risk for cardiovascular and diabetic comorbidities.Citation15 Exercise and diet programs are important factors in reducing the risks of these conditions.Citation16 Exercise and improved diet, therefore, have the potential to improve the QoL and also possibly the long-term survival of patients with prostate cancer (who may or may not be on ADT).Citation17,Citation18 Some clinical trial data, including that from multiple randomized controlled trials, have demonstrated the ability of exercise and dietary modifications to ameliorate ADT-associated adverse effects, and patients and physicians need to be informed about these nonpharmacologic approaches. Here, we review recent studies of exercise and nutritional interventions to assess their potential impact on the outcomes in patients with prostate cancer receiving ADT.
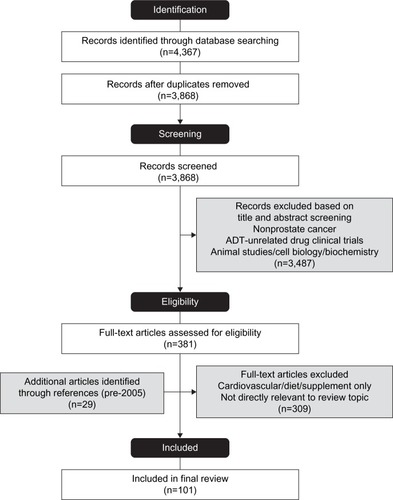
We searched the PubMed database to select trials, reviews, and meta-analyses in English in the last 10 years (2005–2015) using search terms “prostate cancer and cardiovascular diseases/diet/exercise/sexual dysfunction”, “androgen deprivation therapy and diet/exercise/bone”, and “cancer and body composition”. The initial searches produced 3,863 records after the removal of duplicates. shows the flowchart of manuscript selection.
Impact of exercise on patients with prostate cancer
Exercise, prostate cancer, and mortality risk
The beneficial impact of regular physical activity on many chronic ailments, including cardiovascular disease, osteoporosis, diabetes, obesity, fatigue, and depression, has been reported in many studies, not least in the 1996 US Department of Health and Human Services Report of the Surgeon General.Citation16 There is a paucity of good data with respect to the effect of exercise on the mortality of men with prostate cancer. Studies of cancer (including prostate cancer) have shown that there is a link between increased physical activity and improved physical functioning in cancer survivors. For example, the Reach out to Enhance Wellness study enrolled 641 elderly (mean age, 73 years), sedentary, overweight, or obese (mean body mass index [BMI], 29 kg/m2) cancer survivors (including 94 prostate cancer survivors) and utilized a home-based diet and exercise intervention. The Reach out to Enhance Wellness intervention consisted of workbooks, equipment, and quarterly newsletters, endorsing 15 minutes of strength training every other day and 30 minutes of endurance exercise daily to evaluate the effects on physical function. The intervention produced a decrease in the rate of physical function decline, according to various self-reported measures, including the Medical Outcomes Study Short Form-36 (SF-36); the intervention also produced a decrease in BMI compared with the control (delayed intervention) population.Citation19,Citation20
Kenfield et alCitation17 studied a population of 2,705 male health care professionals (mean age at prostate cancer diagnosis about 70 years) with nonmetastatic prostate cancer and found that those participating in vigorous physical activity (metabolic equivalent task [MET] valueCitation21 ≥6) for a duration ≥3 hours/week demonstrated a 49% lower risk of all-cause mortality and a 61% lower risk of death specifically from prostate cancer, compared with men who did <1 hour/week of vigorous activity. MET value is a way of defining the energy cost of physical activity in adults, and examples range from meditation (1 MET), stationary bike or jogging (7 METs), playing squash (12 METs), running up stairs (15 METs) to running at 14 mph (25 METs).Citation17 The men engaging in nonvigorous exercise (MET value <6, eg, walking at a normal to very brisk pace) ≥90 minutes/week had an all-cause mortality risk 46% less than those who walked <90 minutes/week. There was, however, no statistically significant difference between nonvigorous exercise and walking with respect to prostate cancer-specific mortality.Citation17 Other studies in large populations of men who were cancer-free at enrollment, however, have shown no significant association between physical activity, body weight, and waist girth and prostate cancer risk,Citation22,Citation23 although Patel et alCitation23 suggest that physical activity may be associated with reduced risk of aggressive prostate cancer.Citation23
Strength, lean body mass, and functional ability
A systematic review of ten studies (five randomized and five uncontrolled clinical trials) examined the effects of exercise on patients receiving ADT.Citation24 Study populations included both patients with metastatic and nonmetastatic prostate cancer, with mean ages ranging from 63 to 72 years, and with ADT duration of 4 to >44 months. The number of patients on ADT undergoing exercise interventions ranged from five in a pilot studyCitation25 to 74.Citation26 Although most interventions included two to six exercise-based sessions per week for 24 weeks, the interventions were heterogeneous, varying in duration, frequency, intensity, and degree of supervision. Despite this heterogeneity, the evidence demonstrated that physical performance was improved by exercise. Randomized controlled trials found exercise to be consistently beneficial for muscular performance: reported as increases in muscular strength and increases in upper and lower limb strength, compared with the control population (see, eg, Bourke et alCitation27 and Galvão et alCitation28). Although one study found improvement in cardiovascular fitnessCitation27 and others showed improvements in 400-mCitation29 or 6-minute walk times,Citation25 still other studies showed no improvement in 6-minute walk times or in cardiorespiratory fitness.Citation30,Citation31
Body composition is a component of many studies investigating exercise effects on prostate cancer patients on ADT,Citation24 and resistance training has been shown to either increase lean body massCitation28 or reduce its declineCitation26 in randomized controlled trials. Other uncontrolled studies have shown increased lean body mass,Citation29 decreased BMI, or decreased weight with resistance exercise.Citation29,Citation32 The data on adiposity are not clear, as some studies report an adiposity reduction,Citation30,Citation33 whereas others report no differences between exercising and control groups.Citation26–Citation28
Fatigue
Fatigue measurements, assessed by patient-reported questionnaires, such as the Functional Assessment of Cancer Therapy-Fatigue (FACT-F)Citation34 or the Functional Assessment of Chronic Illness Therapy-Fatigue,Citation35 are frequently assessed together with exercise outcomes in these studies.Citation24,Citation36,Citation37 In general, the evidence is equivocal as to whether exercise helps diminish patient-reported fatigue. Some studies show clinically meaningful improvement versus controls,Citation26,Citation27,Citation33,Citation38 whereas another failed to show benefit.Citation30 Some uncontrolled studies show benefit; for example, Hanson et alCitation29 showed that in 17 black men on ADT, strength training reduced fatigue perception by 38%, which exceeds changes reported in other cancer survivor studies. On the other hand, for example, Hansen et alCitation25 found that exercise training in ten men with prostate cancer produced no significant difference in fatigue between those on ADT and those who were not. Neither of these studies was large enough to allow statistically meaningfully comparisons to be made between the groups.
QoL
Many controlled and uncontrolled studies report that physical activity improves aspects of patient-reported QoL. The most widely used measures for self-reported general and psychosocial QoL (mental health/emotional well-being) are the Medical Outcomes Study 12-item Short Form (SF-12) and the SF-36.Citation24,Citation39 A 3-month exercise program consisting of aerobic and resistance exercises produced clinically significant improvement in the SF-36 scores of 32 men with prostate cancer starting ADT, compared with 31 men starting ADT on usual care but not exercising.Citation38 Likewise, in a pilot study, resistance exercise training sessions three times a week for 12 weeks produced clinically significant prostate cancer-specific improvement in the QoL for five men on ADT, compared with those on usual care, as measured by the FACT-Prostate scale.Citation25 In contrast, however, Cormie et alCitation36 studied 20 men with bone metastases secondary to prostate cancer and found no significant between-group differences in QoL or psychological distress comparing those who underwent a 12-week resistance exercise program and those on usual care.
Interestingly, a study of 66 prostate cancer survivors compared home-based aerobic training with home-based resistance training and found that after 6 months, the aerobic group had undertaken significantly more physical activity than the resistance training group, although fatigue and QoL were not significantly different between the two groups.Citation31 This suggests that aerobic exercise may be more attractive to the prostate cancer population than resistance exercises. This has implications as aerobic exercise is anticipated to have a greater impact on cardiovascular health, whereas resistance exercise is more associated with increases in muscular strength and lean body mass.
Cardiovascular disease
In 2006, the Surveillance, Epidemiology, and End Results Medicare study reported an observed 11% increase in myocardial infarction risk and a 16% increased risk of coronary heart disease and death from cardiac arrest in an observational study of prostate cancer patients receiving ADT, versus those not on hormone therapy (n=73,196).Citation3 In a later study of men in the Veterans Healthcare Administration database, ADT was also found to be associated with stroke.Citation40 These reports, along with observational evidence from several additional studies linking ADT to an increased risk of cardiovascular events, prompted the US Food and Drug Administration to issue a communication in 2010 requiring that manufacturers of drugs for ADT include a warning label citing an increased risk of diabetes and certain cardiovascular diseases (eg, heart attack, sudden cardiac death, and stroke) with their use.Citation41 The link between ADT and cardiovascular events remains somewhat controversial; however, as subsequent studies indicate that although ADT appears to be linked to an increased risk for cardiovascular events, it is not associated with higher rates of related mortality.Citation11,Citation42
Although there are no studies to date that are specifically powered to evaluate the effect of exercise on ADT-mediated cardiovascular events, there is a large body of evidence supporting the role of physical activity in the prevention and management of cardiovascular disease in broader populations.Citation43–Citation46 Many plausible mechanisms could be involved in the protective effects of physical activity, including protection against atherosclerosis, improvement of plasma lipid and lipoprotein profile, dilation of peripheral blood vessels/attenuation of sympathetic nervous activity leading to reduction in blood pressure, adaptations to coronary circulation, reduction in thromboses due to enhanced fibrinolysis, and decrease in the aggregation and adhesion of platelets.Citation16,Citation47,Citation48 By extension, it seems likely that implementation of an exercise program would help mitigate the cardiovascular risk factors associated with ADT, although further research is necessary.
Bone health
Bone density reduction is a serious consequence of ADT, with the frequency of osteoporosis and osteopenia being directly proportional to treatment duration.Citation49–Citation51 This is particularly significant in men, as a high mortality rate (up to 37.5%) is associated with minimal trauma fractures.Citation52 The greatest loss of bone mineral density occurs in the first year of ADT, with noticeable changes seen within months as androgen levels decline.Citation53 Many epidemiologic studies have reported that low body weight and low BMI are risk factors for diminished bone mineral density and fragility fracture. An explanation for the correlation of higher body weight and bone resilience is that this may be due to increased mechanical load on the bones in heavier persons stimulating an increase in bone mineral density.Citation54,Citation55 There is considerable evidence that physical activities involving mechanical loading, such as resistance training and high-impact load-bearing exercises, are beneficial for bone health in the general population.Citation16,Citation52 In contrast to this view, however, a recent study of a heterogeneous cohort of 8,833 men aged 18–64.9 years used computed tomography to show an inverse relationship between adiposity (BMI and visceral and subcutaneous adiposity) and bone quality.Citation56
Despite the known effects of ADT on bone health, there is a paucity of data available regarding the impact of exercise on the bone health of patients receiving ADT. Recently, a randomized controlled study in 63 men with prostate cancer examined the effect of moderate- to high-intensity aerobic and resistance exercises in preventing ADT-associated toxicity in the first 3 months of treatment and found no significant difference in bone mineral density loss and in blood biomarkers of bone turnover between the exercise and usual care groups.Citation38 The authors speculated that the efficacy of exercise-based interventions will likely require incorporation of targeted, high-impact activities that provide skeletal loading, such as jumping and hopping. Indeed, similar results have been observed in resistance training studies in older men and women, where only high-intensity, and not moderate-intensity, strength training resulted in increased bone mineral density.Citation57–Citation59
A clinically important issue is whether high-impact or moderate- to high-intensity exercise might put patients with prostate cancer at risk of fracture, particularly in 70%–80% of advanced prostate cancer patients who have bone metastases.Citation60 The risk increases further given the effect that ADT has on bone health. For example, a study of 19,079 Canadian men with prostate cancer demonstrated a 65% increased fragility risk for those on at least 6 months of ADT, compared with those not on ADT.Citation61 Exercise, therefore, is commonly not recommended for this population, due to fears over fragility and potential fracture. However, a recent randomized, preliminary, 12-week study of targeted, moderate- to high-intensity exercise in 20 prostate cancer patients with bone metastases, found that the exercise was well tolerated, with 93% patient compliance and no reported adverse events.Citation36 This provides initial evidence that supervised resistance exercise may be safe for prostate cancer patients with metastatic bone disease, although the data from this trial showed that the intervention made no significant changes to the patients’ bone mineral density. The supervised exercise did, however, improve other measures, such as physical activity, muscular strength, and lean body weight. More studies involving larger numbers of subjects are required to build on these results.
Sexual dysfunction
About 30%–90% of prostate cancer patients have been reported to experience adverse sexual side effects from their care treatments.Citation62–Citation64 Sexual dysfunction can profoundly affect patients’ QoL and lead to depression, loss of connection with their partner, and decline in their identity as a man.Citation62,Citation64 Addressing ADT complications will likely play an important role in improving the overall outcomes.
Kratzik et alCitation65 observed that in healthy men aged 45–60 years (n=674), risk of severe erectile dysfunction was 83% less among men who exercised ≥3,000 kcal/week, compared with those who exercised ≤3,000 kcal/week. In two studies, Cormie et alCitation38,Citation66 examined the effect of moderate- to high-intensity exercise (resistance and aerobic) over a 12-week intervention. Using the sexual function section of the European Organization for Research and Treatment of Cancer prostate cancer-specific module questionnaire, the researchers found significant differences between the usual care (nonexercise) group and the exercise group, with the latter reporting maintenance of sexual activity and a higher percentage of participants with a major interest in sex (17.2% vs 0%, P=0.024).Citation38,Citation66 In-depth interviews with 18 men (mean age, 63.1 years) receiving ADT for prostate cancer suggest that exercise may be helpful in alleviating sexual dysfunction in a broader, less specific manner. The role of exercise in mitigating the reduction in lean body mass and muscular strength seen with ADT may be important in improving the subjects’ perceptions of their masculinity, which in turn leads to a better psychological frame of mind with respect to their sexuality.Citation67 Further research is needed, but this preliminary data suggest that exercise may be able to play a role in reducing ADT-associated sexual dysfunction.
Metabolic syndrome
ADT is associated with significant adverse metabolic effects, including elevated serum triglycerides (≥150 mg/dL); fasting serum glucose ≥100 mg/dL; weight gain, especially of the abdomen; peripheral insulin resistance; and increased diabetes risk.Citation3,Citation9,Citation40,Citation68 These effects are characteristics of metabolic syndrome and increase the risk of heart disease and stroke, in addition to diabetes.Citation69
We reviewed the data on cardiovascular fitness earlier, and there is good evidence that exercise improves or mitigates the decline in lean body mass and decreases the BMI of patients on ADT. There is also the additional evidence that exercise may impact the overall all-cause mortality, of which cardiovascular events are a large proportion. Exercise that is considered desirable in a noncancer population is also likely to show benefit in men with prostate cancer. Segal et alCitation26 reported that 12 weeks of resistance training in 155 men with prostate cancer and receiving ADT resulted in significantly greater upper and lower body muscular fitness, compared with those on usual (nonexercise) care; however, waist circumference, BMI, or subcutaneous adiposity differences between groups were not significant.
Recently, two studiesCitation38,Citation70 have shown that exercise-based interventions are promising methods for reducing few ADT-specific metabolic effects. Results from a randomized pilot study assessed the impact of over 6 months of combined metformin, a low-glycemic-index diet, and exercise in 20 prostate cancer patients at ADT initiation and compared this with 20 men who were on ADT alone. The metformin and exercise group had decreased abdominal girth, weight, BMI, and systolic blood pressure, compared with the group on ADT treatment alone, although insulin-resistant biochemical markers were not significantly different. In this small study, however, it was not possible to separate the metformin and dietary effects from the exercise components.Citation70 In the study by Cormie et alCitation38, 63 prostate cancer patients were randomized to receive either a 3-month aerobic and resistance exercise program or usual care, concomitant to initiation of ADT. Patients receiving the exercise-based intervention demonstrated significant reductions in ADT-associated metabolic effects, including decreased whole body fat mass, trunk fat mass, and percentage fat, compared with the usual care control group. The ratio of total cholesterol to high-density lipoprotein (HDL) cholesterol improved in the exercise group, which is clinically significant, as this ratio is a common cardiovascular disease risk marker. There were no significant changes, however, in other cardiovascular and metabolic biomarkers, such as triglycerides, insulin and glucose levels, or glycated hemoglobin.Citation38
Evidence from trials has also shown that ADT can cause significant increases in HDL.Citation38,Citation71–Citation73 A feature of ADT-associated metabolic changes is that HDL is usually elevated, which is not typical with metabolic syndrome (where HDL levels are low).Citation72,Citation73 Potentially, HDL increases could be further amplified by the addition of prescriptive exercise. Although these are preliminary studies, they suggest that exercise may provide some benefit in lessening the adverse metabolic effects of ADT. Two randomized trials are presently being conducted to investigate this further.Citation74,Citation75
C-reactive protein, an inflammation marker commonly elevated in metabolic syndrome, showed a clinically meaningful reduction in a randomized controlled trial of exercise in 57 men on ADT.Citation28 Going forward, further study is required to investigate the correlations between C-reactive protein level and exercise.
Although the final verdict from large-scale trials is still outstanding, exercise programs should be considered an integral part of treatment for men with prostate cancer, as they can provide low-cost, scalable, and widely accessible strategies to help counter the systemic effects associated with prostate cancer therapies, including ADT. Exercise impacts patient well-being, both physical and psychological, potentially leading to improved physical functioning and survival.Citation24,Citation39,Citation75–Citation77 However, before a general recommendation can be issued, treating physicians should exercise caution when considering to institute exercise and diet programs, and patients should be carefully assessed prior to any intervention.
Effect of nutrition and dietary supplements on the complications of ADT
Nutrition and dietary supplements
There is a lack of data on how nutritional adjustments might affect ADT-associated adverse effects and the few studies that have investigated the integration of either nutritional counseling or modified diet have done so in conjunction with another intervention, making it difficult to specifically assess the impact of dietary changes.Citation70,Citation78 Given the similarities to what is observed in postmenopausal women, however, some researchers have made specific recommendations for lifestyle modifications, including smoking cessation, moderation of alcohol and caffeine intake, and supplementation with vitamin D and calcium.Citation79,Citation80 As mentioned in the previous section, a low-glycemic-index diet, regular exercise, and metformin treatment have been shown to significantly improve abdominal girth, weight, BMI, and systolic blood pressure in patients receiving ADT.Citation70 Caffeine is also garnering research as a method to potentially improve workout energy levels and duration and postworkout discomfort.Citation81 Studies with prostate cancer patients are lacking, although caffeine consumed (0.16 mg/kg anhydrous caffeine) 1 hour before completing a number of functional performance and exercise capacity tests has been reported to increase the exercise capacity by 3.0% versus placebo in a randomized, double-blind crossover study in 30 prostate cancer survivors. There was no significant difference, however, in postexercise fatigue and perception of exertion compared with placebo, nor in other functional performance measures.Citation82
Bone health
Despite the debilitating effects of osteoporosis observed in men receiving ADT, few studies have examined two key modulators of bone health in this population: vitamin D and calcium. Bone density deterioration is known to occur shortly after initiation of ADT.Citation49,Citation50 A meta-analysis of calcium and vitamin D studies in women and men aged ≥50 years (92% of study participants were women and those with secondary osteoporosis were excluded) provided evidence that calcium and vitamin D supplementation is associated with a reduced rate of bone mineral density loss in the hip and the spine, and also with decreased risk of fracture.Citation83 On the basis of this evidence, the European Association of Urology has recommended that calcium and vitamin D supplements be monitored both prior to and during ADT if serum concentrations fall below the lower limit of normal (normal range: calcium, 2.2–2.6 nmol/L; vitamin D, 100–160 nmol/L).Citation84 In its most recent guidelines, the European Association of Urology recommends that a daily intake of ≥1,200 mg/day of calcium and 1,000 IU of vitamin D may be useful for improving bone mineralization in prostate cancer patients.Citation84 The National Osteoporosis Foundation, on the other hand, supports the Institute of Medicine guidelines recommending that men aged 50–70 years consume 1,000 mg/day of calcium, and those ≥71 years, 1,200 mg/day of calcium.Citation85,Citation86
A systematic review of twelve clinical trials, however, failed to find conclusive support for these recommendations in prostate cancer patients receiving ADT, noting that the frequently recommended daily doses of calcium (500–1,000 mg) and vitamin D (200–500 IU) were insufficient to prevent ADT-associated bone density loss.Citation87 More recently, in a prospective longitudinal study, Alibhai et alCitation88 examined the long-term effects of calcium and vitamin D supplementation in 160 prostate cancer patients (mean age, 69 years), with and without ADT. Vitamin D, but not calcium, was shown to provide some protection from bone density loss, especially in the first year of ADT. It seems likely that the efficacy of vitamin D and calcium supplementation may require implementation of concomitant exercise programs to stimulate bone accrual, as discussed earlier.Citation36,Citation38,Citation75
Fat and muscle mass
Although there are a number of randomized controlled trials that have demonstrated exercise to be effective for reducing fat mass in overweight older men,Citation89 the absence of testosterone in men on ADT has proven to be a more difficult challenge. It has been reported that during the early phase of ADT, fat loss or maintenance of BMI can be achieved through exercise alone.Citation38,Citation90 This concept remains controversial, however, as other studies have suggested that, in addition to exercise, caloric restriction is required for consistent fat loss in men.Citation89,Citation91
Loss of muscle mass, which has major consequences for physical function and risk of metabolic disease, is a frequently reported adverse effect of ADT.Citation11 Resistance training has consistently been associated with muscle hypertrophy in men on ADT.Citation28,Citation31 This effect is likely to be amplified by appropriate nutritional supplementation, including protein, carbohydrates, and creatine monohydrate, as has been demonstrated in studies of both younger and older men not on ADT.Citation92 Recently, a meta-analysis of 14 randomized controlled trials in a total of 626 adults showed an increase in weight loss, lean body mass, and a decrease in visceral fat among men receiving supplemental whey protein in combination with resistance training.Citation93 Although more studies are needed, the potential for whey protein or another similar protein isolate to mitigate the adverse effects of prostate cancer therapies is considerable.
Fatigue
Encouraging data from a randomized, double-blind, phase III trial of American ginseng (2,000 mg/day) found a statistically significant and clinically meaningful decrease in cancer-related fatigue in 171 patients (4% of whom had prostate cancer), compared with 170 patients on placebo.Citation94 The primary endpoint was the Multidimensional Fatigue Symptom Inventory-Short Form. American ginseng is the first treatment option, besides exercise, to show efficacy in addressing cancer-related fatigue, as no other drugs, including stimulants, have been proven to be effective. Interestingly, similar to what was observed with exercise,Citation38 greater benefits were found in patients who started ginseng at the same time as their cancer treatment, compared with patients who started ginseng after treatment initiation. This suggests that treatment with American ginseng should be started at the same time or prior to ADT initiation; however, this needs to be confirmed with a larger study population of patients with prostate cancer.
Cardiovascular disease
The impact of ADT on cardiovascular disease development and progression remains contested in the literature. Regardless, heart disease is a leading cause of mortality in all men, including those on ADT.Citation42 Lifestyle changes, such as modifications to diet and exercise, may significantly affect cardiovascular disease risk in prostate cancer patients on ADT.Citation18
Although it has not been studied in patients on ADT, fish oil has been approved by the Food and Drug Administration for use in lowering triglycerides.Citation95 Correspondingly, the American Heart Association recommends that individuals with heart disease take 1,000 mg of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), the primary omega-3 fatty acid components of fish oil, daily.Citation96 Indeed, a large, randomized controlled trial (Japan EPA Lipid Intervention Study) in 18,645 Japanese subjects has shown synergistic effects between omega-3 acids and statins in reducing serum triglycerides in patients with high cholesterol.Citation97,Citation98 Interestingly, further subgroup analysis found that fish oil significantly reduced the incidence of coronary artery disease in subjects with impaired glucose metabolism (−22%), even greater than that observed for subjects with normal glucose metabolism (−18%).Citation97
Conclusions
The advent of prostate-specific antigen screening programs, greater public awareness of prostate cancer, and improved treatment regimens have fundamentally altered the disease landscape. A consequence of greater patient longevity is a more extended exposure to ADT and its associated adverse effects. Given that the adverse effects of ADT are systemic, often debilitating, and difficult to treat, efforts are being made in the development of new strategies for long-term management of prostate cancer.
As summarized in , exercise, diet, and nutritional supplementation interventions have the potential to provide relatively inexpensive and accessible strategies for mitigating many ADT-associated toxicities and are unlikely to introduce additional adverse effects. However, since the evidence of large-scale trials in patients with prostate cancer is missing, and an extrapolation of supporting data to all patient subgroups cannot be provided, individualized risk assessments remain necessary before the initiation of exercise and diet programs. The coordinated efforts of health care professionals, including exercise physiologists and dieticians, coupled with increased patient awareness, will be critical for effective implementation of such intervention programs, and future research would help to further our understanding of the effectiveness of diet and exercise in this complex patient population.
Table 1 Adverse effects reported with ADT and potential lifestyle, supplemental, and prescription medication solutions
Acknowledgments
Medical writing and editorial support was provided by Audrey Vandervelde, PhD, and Robin Smith, PhD, of The Curry Rockefeller Group, LLC, Tarrytown, NY. Funding for this support was provided by AbbVie.
Disclosure
Mark Moyad has served as a consultant and on the speaker’s bureau for AbbVie; has served as a consultant for Farr Labs, and is an author of the Promoting Wellness Series of Books. Robert Newton and Ulf Tunn report no conflicts of interest in this work. Damian Gruca is an employee of and owns stock in AbbVie.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- DeSantisCELinCCMariottoABCancer treatment and survivorship statistics, 2014CA Cancer J Clin201464425227124890451
- KeatingNLO’MalleyAJSmithMRDiabetes and cardiovascular disease during androgen deprivation therapy for prostate cancerJ Clin Oncol200624274448445616983113
- Lu-YaoGLAlbertsenPCMooreDFSurvival following primary androgen deprivation therapy among men with localized prostate cancerJAMA2008300217318118612114
- MottetNBellmuntJBollaMEAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancerEur Urol201159457258321315502
- PerlmutterMALeporHAndrogen deprivation therapy in the treatment of advanced prostate cancerRev Urol20079Suppl 1S3S817387371
- SharifiNGulleyJLDahutWLAndrogen deprivation therapy for prostate cancerJAMA2005294223824416014598
- SharifiNGulleyJLDahutWLAn update on androgen deprivation therapy for prostate cancerEndocr Relat Cancer2010174R305R31520861285
- AhmadiHDaneshmandSAndrogen deprivation therapy: evidence-based management of side effectsBJU Int2013111454354823351025
- CheungASZajacJDGrossmannMMuscle and bone effects of androgen deprivation therapy: current and emerging therapiesEndocr Relat Cancer2014215R371R39425056176
- NguyenPLAlibhaiSMBasariaSAdverse effects of androgen deprivation therapy and strategies to mitigate themEur Urol201567582583625097095
- Van PoppelHTombalBCardiovascular risk during hormonal treatment in patients with prostate cancerCancer Manag Res20113495521448299
- TunnUThe current status of intermittent androgen deprivation (IAD) therapy for prostate cancer: putting IAD under the spotlightBJU Int200799Suppl 11922 discussion 23–1417229164
- GrucaDBacherPTunnUSafety and tolerability of intermittent androgen deprivation therapy: a literature reviewInt J Urol201219761462522435512
- GrundySMBenjaminIJBurkeGLDiabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart AssociationCirculation1999100101134114610477542
- Centers for Disease Control and PreventionA Report of the Surgeon General: Physical Activity and Health1996Atlanta (GA)US Department of Health and Human Services Available from: www.cdc.gov/nccdphp/sgr/pdf/sgrfull.pdfAccessed March 3, 2016
- KenfieldSAStampferMJGiovannucciEChanJMPhysical activity and survival after prostate cancer diagnosis in the health professionals follow-up studyJ Clin Oncol201129672673221205749
- MoyadMARoachM3rdPromoting wellness for patients on androgen deprivation therapy: why using numerous drugs for drug side effects should not be first-line treatmentUrol Clin North Am201138330331221798392
- Demark-WahnefriedWMoreyMCSloaneRReach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivorsJ Clin Oncol201230192354236122614994
- MoreyMCSnyderDCSloaneREffects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trialJAMA2009301181883189119436015
- AinsworthBEHaskellWLHerrmannSD2011 Compendium of Physical Activities: a second update of codes and MET valuesMed Sci Sports Exerc20114381575158121681120
- LeeIMSessoHDPaffenbargerRSJrA prospective cohort study of physical activity and body size in relation to prostate cancer risk (United States)Cancer Courses Control2001122187193
- PatelAVRodriguezCJacobsEJSolomonLThunMJCalleEERecreational physical activity and risk of prostate cancer in a large cohort of U.S. menCancer Epidemiol Biomark Prev2005141275279
- GardnerJRLivingstonPMFraserSFEffects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic reviewJ Clin Oncol201432433534624344218
- HansenPADechetCBPorucznikCALaStayoPCComparing eccentric resistance exercise in prostate cancer survivors on and off hormone therapy: a pilot studyPM R20091111019102419942188
- SegalRJReidRDCourneyaKSResistance exercise in men receiving androgen deprivation therapy for prostate cancerJ Clin Oncol20032191653165912721238
- BourkeLDollHCrankHDaleyARosarioDSaxtonJMLifestyle intervention in men with advanced prostate cancer receiving androgen suppression therapy: a feasibility studyCancer Epidemiol Biomark Prev2011204647657
- GalvãoDATaaffeDRSpryNJosephDNewtonRUCombined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trialJ Clin Oncol201028234034719949016
- HansonEDSheaffAKSoodSStrength training induces muscle hypertrophy and functional gains in black prostate cancer patients despite androgen deprivation therapyJ Gerontol A Biol Sci Med Sci201368449049823089339
- Culos-ReedSNRobinsonJWLauHPhysical activity for men receiving androgen deprivation therapy for prostate cancer: benefits from a 16-week interventionSupport Care Cancer201018559159919609570
- Santa MinaDAlibhaiSMMatthewAGA randomized trial of aerobic versus resistance exercise in prostate cancer survivorsJ Aging Phys Act201321445547823238110
- Culos-ReedSNRobinsonJLLauHO’ConnorKKeatsMRBenefits of a physical activity intervention for men with prostate cancerJ Sport Exerc Psychol200729111812717556779
- SegalRJReidRDCourneyaKSRandomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancerJ Clin Oncol200927334435119064985
- CellaDThe Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigueSemin Hematol1997343 Suppl 213199253779
- YellenSBCellaDFWebsterKBlendowskiCKaplanEMeasuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement systemJ Pain Symptom Manage199713263749095563
- CormiePNewtonRUSpryNJosephDTaaffeDRGalvaoDASafety and efficacy of resistance exercise in prostate cancer patients with bone metastasesProstate Cancer Prostatic Dis201316432833523917308
- O’NeillRFHaseenFMurrayLJO’SullivanJMCantwellMMA randomised controlled trial to evaluate the efficacy of a 6-month dietary and physical activity intervention for patients receiving androgen deprivation therapy for prostate cancerJ Cancer Surviv20159343144025916660
- CormiePGalvaoDASpryNCan supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: a randomised controlled trialBJU Int2015115225626624467669
- HasenoehrlTKeilaniMSedghi KomanadjTThe effects of resistance exercise on physical performance and health-related quality of life in prostate cancer patients: a systematic reviewSupport Care Cancer20152382479249726003426
- KeatingNLO’MalleyAJFreedlandSJSmithMRDiabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancerJ Natl Cancer Inst20101021394619996060
- FDA drug safety communication: update to ongoing safety review of GnRH agonists and notification to manufacturers of GnRH agonists to add new safety information to labeling regarding increased risk of diabetes and certain cardiovascular diseases2010Silver Spring (MD)US Food and Drug Administration Available from: http://www.fda.gov/Drugs/DrugSafety/ucm229986.htmAccessed April 29, 2016
- EfstathiouJABaeKShipleyWUCardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85-31J Clin Oncol2009271929919047297
- BlairSNJacksonASPhysical fitness and activity as separate heart disease risk factors: a meta-analysisMed Sci Sports Exerc200133576276411323545
- FagardRHExercise characteristics and the blood pressure response to dynamic physical trainingMed Sci Sports Exerc2001336 SupplS484S492 discussion S493–S49411427774
- LeonASRiceTMandelSBlood lipid response to 20 weeks of supervised exercise in a large biracial population: the HERITAGE Family StudyMetabolism200049451352010778878
- ThompsonPDBuchnerDPinaILExercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity)Circulation2003107243109311612821592
- Rafieian-KopaeiMSetorkiMDoudiMBaradaranANasriHAtherosclerosis: process, indicators, risk factors and new hopesInt J Prev Med20145892794625489440
- RidkerPMLDL cholesterol: controversies and future therapeutic directionsLancet2014384994360761725131980
- GalvãoDASpryNATaaffeDRChanges in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancerBJU Int20081021444718336606
- GreenspanSLCoatesPSereikaSMNelsonJBTrumpDLResnickNMBone loss after initiation of androgen deprivation therapy in patients with prostate cancerJ Clin Endocrinol Metab200590126410641716189261
- MoroteJMorinJPOrsolaAPrevalence of osteoporosis during long-term androgen deprivation therapy in patients with prostate cancerUrology200769350050417382153
- EbelingPRClinical practice. Osteoporosis in menN Engl J Med2008358141474148218385499
- HamiltonEJGhasem-ZadehAGianattiEStructural decay of bone microarchitecture in men with prostate cancer treated with androgen deprivation therapyJ Clin Endocrinol Metab20109512E456E46320881261
- De LaetCKanisJAOdenABody mass index as a predictor of fracture risk: a meta-analysisOsteoporos Int200516111330133815928804
- GonnelliSCaffarelliCNutiRObesity and fracture riskClin Cases Miner Bone Metab201411191425002873
- ZhangPPetersonMSuGLWangSCVisceral adiposity is negatively associated with bone density and muscle attenuationAm J Clin Nutr2015101233734325646331
- KerrDMortonADickIPrinceRExercise effects on bone mass in postmenopausal women are site-specific and load-dependentJ Bone Miner Res19961122182258822346
- MaddalozzoGFSnowCMHigh intensity resistance training: effects on bone in older men and womenCalcif Tissue Int200066639940410821873
- VincentKRBraithRWResistance exercise and bone turnover in elderly men and womenMed Sci Sports Exerc2002341172311782642
- CarlinBIAndrioleGLThe natural history, skeletal complications, and management of bone metastases in patients with prostate carcinomaCancer20008812 Suppl2989299410898342
- AlibhaiSMDuong-HuaMCheungAMFracture types and risk factors in men with prostate cancer on androgen deprivation therapy: a matched cohort study of 19,079 menJ Urol2010184391892320643458
- BoberSLVarelaVSSexuality in adult cancer survivors: challenges and interventionJ Clin Oncol201230303712371923008322
- NgEWooHHTurnerSLeongEJacksonMSpryNThe influence of testosterone suppression and recovery on sexual function in men with prostate cancer: observations from a prospective study in men undergoing intermittent androgen suppressionJ Urol201218762162216622503022
- ResnickMJKoyamaTFanKHLong-term functional outcomes after treatment for localized prostate cancerN Engl J Med2013368543644523363497
- KratzikCWLacknerJEMarkIHow much physical activity is needed to maintain erectile function? Results of the Androx Vienna Municipality StudyEur Urol200955250951618359146
- CormiePNewtonRUTaaffeDRExercise maintains sexual activity in men undergoing androgen suppression for prostate cancer: a randomized controlled trialProstate Cancer Prostatic Dis201316217017523318529
- HamiltonKChambersSKLeggMOliffeJLCormiePSexuality and exercise in men undergoing androgen deprivation therapy for prostate cancerSupport Care Cancer201523113314225005233
- Braga-BasariaMDobsASMullerDCMetabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapyJ Clin Oncol200624243979398316921050
- AlbertiKGEckelRHGrundySMHarmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of ObesityCirculation2009120161640164519805654
- NobesJPLangleySEKlopperTRussell-JonesDLaingRWA prospective, randomized pilot study evaluating the effects of metformin and lifestyle intervention on patients with prostate cancer receiving androgen deprivation therapyBJU Int2012109101495150221933330
- MoroteJGómez-CaamañoAAlvarez-OssorioJLThe metabolic syndrome and its components in patients with prostate cancer on androgen deprivation therapyJ Urol201519361963196925541340
- SmithMRFinkelsteinJSMcGovernFJChanges in body composition during androgen deprivation therapy for prostate cancerJ Clin Endocrinol Metab200287259960311836291
- SmithMRLeeHMcGovernFMetabolic changes during gonadotropin-releasing hormone agonist therapy for prostate cancer: differences from the classic metabolic syndromeCancer2008112102188219418348297
- GalvãoDASpryNDenhamJA multicentre year-long randomised controlled trial of exercise training targeting physical functioning in men with prostate cancer previously treated with androgen suppression and radiation from TROG 03.04 RADAREur Urol201465585686424113319
- NewtonRUTaaffeDRSpryNCan exercise ameliorate treatment toxicity during the initial phase of testosterone deprivation in prostate cancer patients? Is this more effective than delayed rehabilitation?BMC Cancer20121243223013489
- BrownJKByersTDoyleCNutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choicesCA Cancer J Clin200353526829114570227
- SchmitzKHCourneyaKSMatthewsCAmerican College of Sports Medicine roundtable on exercise guidelines for cancer survivorsMed Sci Sports Exerc20104271409142620559064
- BourkeLSohanpalRNantonVCrankHRosarioDJSaxtonJMA qualitative study evaluating experiences of a lifestyle intervention in men with prostate cancer undergoing androgen suppression therapyTrials20121320823151126
- HolzbeierleinJMCastleEThrasherJBComplications of androgen deprivation therapy: prevention and treatmentOncology (Williston Park)2004183303309 discussion 310, 315, 319–32115065701
- MoyadMAPromoting general health during androgen deprivation therapy (ADT): a rapid 10-step review for your patientsUrol Oncol2005231566415885584
- SprietLLExercise and sport performance with low doses of caffeineSports Med201444Suppl 2S175S18425355191
- CornishRSBolamKASkinnerTLEffect of caffeine on exercise capacity and function in prostate cancer survivorsMed Sci Sports Exerc201547346847524977700
- TangBMEslickGDNowsonCSmithCBensoussanAUse of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysisLancet2007370958865766617720017
- MottetNBastianPJBellmuntJGuidelines on Prostate Cancer2014European Association of Urology Available from: https://uroweb.org/.../1607-Prostate-Cancer_LRV3.pdfAccessed June 28, 2016
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and CalciumRossACTaylorCLYaktineALDel ValleHBDietary Reference Intakes for Calcium and Vitamin DWashington (DC)The National Academies Press2011
- CosmanFde BeurSJLeBoffMSClinician’s Guide to Prevention and Treatment of OsteoporosisOsteoporos Int201425102359238125182228
- DattaMSchwartzGGCalcium and vitamin D supplementation during androgen deprivation therapy for prostate cancer: a critical reviewOncologist20121791171117922836449
- AlibhaiSMMohamedaliHZGulamhuseinHChanges in bone mineral density in men starting androgen deprivation therapy and the protective role of vitamin DOsteoporos Int201324102571257923563932
- KleinSBurkeLEBrayGAClinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology FoundationCirculation2004110182952296715509809
- GalvãoDATaaffeDRSpryNJosephDNewtonRUAcute versus chronic exposure to androgen suppression for prostate cancer: impact on the exercise responseJ Urol201118641291129721849187
- WingRRPhysical activity in the treatment of the adulthood overweight and obesity: current evidence and research issuesMed Sci Sports Exerc19993111 SupplS547S55210593526
- CermakNMResPTde GrootLCSarisWHvan LoonLJProtein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysisAm J Clin Nutr20129661454146423134885
- MillerPEAlexanderDDPerezVEffects of whey protein and resistance exercise on body composition: a meta-analysis of randomized controlled trialsJ Am Coll Nutr201433216317524724774
- BartonDLLiuHDakhilSRWisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2J Natl Cancer Inst2013105161230123823853057
- US FDA News Release P04-89FDA Announces Qualified Health Claims for Omega-3 Fatty Acids2004Silver Spring (MD)US Food and Drug Administration Available from: http://www.fda.gov/SiteIndex/ucm108351.htmAccessed March 3, 2016
- Kris-EthertonPMHarrisWSAppelLJAHA Nutrition CommitteeOmega-3 fatty acids and cardiovascular disease: new recommendations from the American Heart AssociationArterioscler Thromb Vasc Biol200323215115212588750
- OikawaSYokoyamaMOrigasaHSuppressive effect of EPA on the incidence of coronary events in hypercholesterolemia with impaired glucose metabolism: sub-analysis of the Japan EPA Lipid Intervention Study (JELIS)Atherosclerosis2009206253553919447387
- YokoyamaMOrigasaHMatsuzakiMEffects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysisLancet200736995671090109817398308
- GrossmannMZajacJDHematological changes during androgen deprivation therapyAsian J Androl201214218719222231300
- BaileyCABrooke-WavellKOptimum frequency of exercise for bone health: randomised controlled trial of a high-impact unilateral interventionBone20104641043104920004758
- FriskJManaging hot flushes in men after prostate cancer – a systematic reviewMaturitas2010651152219962840