Abstract
Overactive bladder syndrome (OAB) is a common condition affecting adults and children worldwide, resulting in a substantial economic and psychological burden. Percutaneous tibial nerve stimulation (PTNS) is derived from acupuncture used in Chinese traditional medicine and was first described in the early 1980s. It is a neuromodulation technique used to modulate bladder function and facilitate storage. Being a minimally invasive, easily applicable, but time-consuming treatment, future developments with implantable devices might be the solution for the logistical problems and economic burden associated with PTNS on the long term. This nonsystematic review provides a current overview on PTNS and its effectiveness in the treatment of OAB for both adults and children.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Overactive bladder syndrome (OAB) is a common condition defined as urgency to void, usually accompanying frequency and nocturia, with or without urge urinary incontinence (UUI), in the absence of urinary tract infection (UTI) or other obvious pathology.Citation1,Citation2 OAB with or without UUI affects millions worldwide, with a prevalence ranging from 1.5% to 36.4% resulting in a substantial economic and psychological burden.Citation3,Citation4 Health-related quality of life (QoL) is usually negatively affected, and patients with OAB experience more anxiety and depression compared to healthy controls.Citation4–Citation7 In these patients, social stigmatization frequently leads to less self-esteem and impaired interpersonal interactions.Citation8,Citation9 Although the etiology of OAB is multifactorial, some genetic predisposition might exist, as there is a reported 2.8 times increased risk for children with mothers suffering from OAB.Citation10
Neuromodulation, like sacral nerve stimulation (SNS) and percutaneous tibial nerve stimulation (PTNS) of the lower urinary tract, is a second-line treatment option for refractory OAB. In theory, neuromodulation should be minimally invasive, easily applicable, and not cause unnecessary embarrassment by stimulating specific areas of the body, for example, the genital area. Furthermore, sustainability and cost-effectiveness are important in view of the competitiveness with other treatments.
This nonsystematic review presents a summary of the history and theories on the pathophysiology behind neuromodulation, the clinical results, and latest developments in PTNS. The aim was to provide an up-to-date overview on PTNS and its effectiveness in the treatment of OAB.
Materials and methods
We searched the databases PubMed, MEDLINE, Cochrane, and Embase for relevant English-language articles. In addition, citations from the primary references were scrutinized for relevant articles that the databases could not locate. A combination of keywords used for the search included: percutaneous or posterior tibial nerve stimulation, neuromodulation, effectiveness, long-term outcome, patient perspectives, prognostic factors, cost-effectiveness, and implantable stimulator.
Results
General principle of neuromodulation
The innervation of the lower urinary tract comes from the lumbar, sacral, and coccygeal segmental nerves originating from L2-S4. A large network of both afferent and efferent fibers is formed after exiting the spinal cord, innervating all the pelvic organs. The sciatic nerve is composed of fibers from L4 to S3 and descends down toward the lower extremities.Citation11 One of its distal branches is the posterior tibial nerve (PTN). Neuromodulation is postulated to be the effect of cross-signaling between sympathetic and parasympathetic postganglionic nerve terminals and synapses, causing alteration of nerve signals involved in the voiding reflex. de Groat et alCitation12 described this neurophysiological process and the neural circuits involved in controlling the lower urinary tract. Stimulation of peripheral nerves and subsequent “cross-talk” at the level of the postganglionic neuroeffector junctions can modulate transmission. This implies that stimulating one area of the innervations system seems to alter the nerve behavior of other systems, leading to alteration in bladder function by stimulating peripheral nerves. The pudendal nerve, the dorsal genital nerve, and the PTN are examples of such peripheral nerves that can affect bladder behavior. Stimulating overlying skin or dermatomes, instead of actual nerves, is another option of peripheral neuromodulation.Citation13–Citation16
Effects on the peripheral nervous system
The mechanism behind neuromodulation is still not completely understood. Alteration of the afferent and efferent pathways between the brain, brain stem, and pelvic organs are thought to modulate the voiding reflex and facilitate storage. Symptoms of OAB (including UUI) may represent the clinical expression of an alteration of the pelvic neuromuscular environment via changes in the inhibitory and excitatory signals of the voiding reflex; this has been confirmed in animal studies. A normal spinal–brain stem–spinal reflex is seen in cats with an intact central nervous system (CNS), activated by non-nociceptive Aδ bladder afferent fibers which pass through the supraspinal relay stations in the periaqueductal gray (PAG) to the pontine micturition center (PMC). The PMC activates the efferent pelvic nerve which results in voiding.Citation12,Citation17
This reflex is present even in decerebrated animals, but is blocked by the transection of the spinal cord because, then, the afferent signals from the bladder cannot go up and the efferent signals from the PMC to the bladder cannot go down. If the spinal transection is distal to the sacral segments, irrigation of the bladder by diluted acetic acid (AA) unmasks reflex contractions mediated by spinal reflex circuitry activated C fiber bladder afferents instead of non-nociceptive Aδ bladder afferent fibers.Citation18 It is postulated that detrusor overactivity (DO) in OAB is mediated through these C fibers.Citation19,Citation20
Animal studies have shown different effects of SNS, pudendal nerve stimulation (PNS) or tibial nerve stimulation (TNS) on voiding reflex pathways each including different neurotransmitter mechanism.Citation21,Citation22 In decerebrate cats under anesthesia, DO was evoked by direct electrical stimulation (ES) of the PMC, activating the excitatory efferent output from PMC to the bladder. Subsequently, PNS or TNS followed. PNS but not TNS was able to inhibit PMC-induced DO. Furthermore, propanolol (a non-selective B-adrenergic receptor antagonist) completely eliminated PNS-induced inhibition of DO. On the contrary, TNS facilitated PMC-induced DO and was blocked by propanolol. The authors concluded that B-adrenergic receptors are mainly involved in PNS-induced inhibition of DO acting on the efferent pathway of the voiding reflex while TNS might use the same receptors for the opposite effect, that is, facilitation of DO. Instead of influencing the efferent pathway of the voiding reflex like PNS, the inhibitory effect of DO after TNS is believed to be the effect of modulation of the afferent pathway. Strong inhibition of the ascending sensory pathway and weak excitation of the excitatory efferent pathway will result in the overall inhibition of DO seen after TNS.Citation22
While B-adrenergic receptors are thought to play a major role in PNS, opioid receptors and endogenous enkephalins seem to be important in the mechanism behind TNS. In cats with an intact CNS, intravenous naloxone (opioid receptor antagonist) blocked the TNS-induced inhibition of DO after AA bladder installation, the latter representing a model for OAB. Nalaxone was unable to block TNS-induced inhibition of normal reflex bladder activity provoked by saline bladder instillation.Citation18 Tai et al also studied the role of nalaxone administration in TNS-induced inhibition of DO in cats. Nalaxone was able to block TNS-induced inhibition of DO after AA installation but, furthermore, did not change cystometric bladder capacity (CMC). On the contrary, nalaxone was unable to alter TNS-induced normal bladder reflex activity after saline installation but reduced CMC instead.Citation23 Nalaxone could not alter PTNS-induced inhibition but reduced CMC, indicating that the voiding reflex was inhibited by the activation of opioid receptors and endogenous enkephalins. On the contrary, nalaxone suppressed PTNS-induced inhibition after AA installations but did not alter CMC, indicating that the enkephalinergic inhibition was inactive during AA-induced DO, but was activated by PTNS. This supports the idea that bladder activity in OAB is mediated through afferent C fibers being nociceptive, while normal reflex bladder activity is mediated through Aδ afferent fibers. Saline installations activate non-nociceptive Aδ afferent fibers which, in their turn, trigger a spinobulbospinal bladder reflex transmitted through the PAG and PMC. On the other hand, AA irritation of the bladder activates nociceptive C fibers that facilitate the supraspinal reflex. Enkephalinergic mechanisms are not involved in the control of the C-fiber-mediated spinal reflex, but are involved in the inhibitory modulation of this reflex induced by PTNS.Citation23
Li et alCitation24 implanted a sacral nerve stimulator in 7 pigs and evoked DO bladder by AA instillations. Consecutive cystograms at baseline, after infusion of AA and after SNS, were performed with or without intravenous naloxone and tramadol infusion. Remarkably, SNS combined with tramadol had a significantly better effect on CMC than SNS alone, suggesting the anti-nociceptive effect and subsequent inhibition of DO while naloxone blocked the effect of SNS leading to a decreased CMC.Citation24
Effects on the CNS
Blok et al studied the effects of acute and chronic SNS on the brain using positron emission tomography images.Citation25 Areas located in the right postcentral gyrus, left parietal cortex, right insula, and medial prefrontal cortex showed increased cerebral blood flow during acute SNS in newly implanted patients. Furthermore, increased activation in the ventromedial-orbitofrontal cortex and decreased activation in the left medial cerebellum occurred. This suggests modulation of areas involved in sensorimotor learning when starting SNS. During chronic SNS, there was decreased activity in the cerebellum, midbrain, and adjacent thalamus and limbic cortical areas, areas previously implicated in the control of bladder contractions, awareness of bladder filling, and initiation of voiding. This implies that different areas in the brain are involved in the learning process and start of SNS, which are taken over by other areas in the brain as time passes, resulting in a shift from dysfunctional to normal control of the voiding reflex.
Information processing in the brain after peripheral nerve stimulation can be visualized measuring somatosensory evoked potentials (SEPs). Especially long-latency somatosensory evoked potentials (LL-SEPs) seem to provide information on the function of somatosensory associative cortical structures. The presence of reproducible LL-SEPs are likely to be responsible for the neuroplastic changes induced by neuromodulation.Citation26 Finazzi-Agro et alCitation27 studied this more thoroughly. Both short-latency SEP (SL-SEP) and LL-SEPs were recorded after peripheral or sham stimulation.Citation27 Peak latency and peak-to-peak amplitude of so-called P80, P100, and P200 waves were measured at baseline and at the end of (sham) stimulation. Mean latency of the previously mentioned waves and the mean amplitude of P200 waves did not change significantly. However, the amplitude of LL-SEP changes of especially P80 and P100 waves in the active PTNS group was significantly higher as compared to the sham group. The recorded P80 and P100 amplitude increase suggests long-term modifications in the synaptic efficiency of the somatosensory pathway. Long-term potentiation and depression of excitatory synaptic transmission can contribute to experience-dependent modifications of the brain, including learning and memory.Citation27 This confirms the idea of the re-organization of the cortical network as a result of peripheral neuromodulation.
History of PTNS
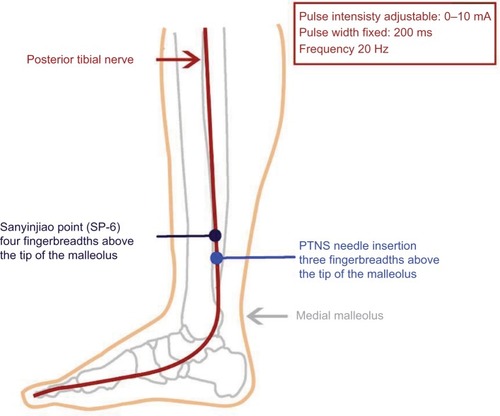
Peripheral neurostimulation is derived from techniques used in traditional Chinese medicine, better known as acupuncture. Acupuncture was already practised during the Stone Age. The earliest writings about “stone needles” (called Pien in Chinese) date from about 500 BC. Puncturing specific points was believed to restore “the energetic harmony” of the body.Citation28 In 1673, a Dutch physician (Wilhelmus ten Rhyne) discovered this Eastern traditional way of medicine which he published in a book entitled Dissertatio de Arthritide: Mantissa Schematica: De Acupunctura: Et Orationes tres. In this book, he was the first Western person to describe the technique he called “acupunctura,” in which needles were used to treat diseases. One of the most commonly used acupuncture points is the San-Yin-Jiao point or Spleen 6 (SP-6). This is located on the medial side of the lower leg, about 4 finger breadths cephalad to the medial malleolus. The location of the SP-6 point and the organs affected by its stimulation have remarkable similarities with PTNS (). When an electrical current is applied to the acupuncture needle, the technique is called electrical acupuncture. Especially when electroacupuncture is performed with similar stimulation parameters (2–15 Hz, 10–20 mA), it resembles PTNS.Citation29 The main difference lies in the actual anatomical substrate used in PTNS, instead of “energy pathways” stimulated in acupuncture.
Figure 1 Drawing showing the location of the Sanyinjiao point, or Spleen 6 (SP-6), the percutaneous tibial nerve stimulation (PTNS) point, and the technical details.
Before the introduction of PTNS, SNS was the main neuromodulation technique used. SNS was developed by Tanagho and Schmidt in the late 1980s, and the first device was implanted in Europe in 1989. At first, it was believed that stimulation would lead to actual contraction of the pelvic floor and sphincter complex to prevent urinary leakage. Instead, during urodynamic studies, it actually led to the inhibition of DO. Nowadays, SNS is an evidence-based clinical tool for patients with OAB or non-obstructive retention.Citation30,Citation31 After SNS was introduced, the next aim was to develop more easily accessible and less invasive techniques, such as PTNS.
McGuire et al were the first to describe PTNS in 1983. In 22 patients with neurogenic OAB, TNS was applied and 87% showed complete or partial improvement of their symptoms.Citation32 Subsequently, Stoller et al further developed PTNS, also known as Stoller afferent nerve stimulation (SANS), as a treatment for OAB in pig-tailed monkeys and, later, in humans, with promising results.Citation33 This new initiative was the start of the worldwide development and exploration of PTNS.
Technique
PTNS is given in supine position with the medial malleolus pointing upwards. In children, previous administration of a topical anesthetic agent (eg, lidocaine) can help to reduce pain and fear associated with needle insertion. A 34-gauge stainless steel needle is inserted ~3 finger breadths cephalad to the medial malleolus, between the posterior margin of the tibia and soleus muscle (). The goal is to place the tip of the needle close to the PTN without actually touching it. Given the varying size of ankles, the optimal depth and angulation may be varied. On average, insertion depth is about 2–4 cm with an angulation of 60°–90°. A stick electrode is placed on the same leg near the arch of the foot. The needle and the electrode are connected to a low voltage (9 V) stimulator (Urgent PC®; Cogentix Medical Inc., Minnetonka, MN, USA) with an adjustable pulse intensity of 0–10 mA, a fixed pulse width of 200 microseconds, and a frequency of 20 Hz ().
Stimulation of the PTN leads to effects in both efferent and afferent nerve fibers. Flexion of the great toe, or fanning, is a result of an efferent effect. The sensory afferent effect is a radiation tickling sensation of the foot sole. During the initial test stimulation, the amplitude is slowly increased until the large toe starts to curl, or toes start to fan. If the patient complains about discomfort or “buzzing” immediately around the needle site, the needle may not be deep enough. In contrast, the needle may be too close to the PTN if the stimulation is extremely uncomfortable. Needle repositioning or reinsertion at the ipsilateral or contralateral ankle is advised. Once optimal position is assured, stimulation is applied at an intensity level well tolerated by the patient and can be increased or decreased during the treatment.Citation34
Most treatment schedules consist of 12 outpatient consecutive treatment sessions lasting 30 minutes each, given 1–3 times per week.Citation35,Citation36
Clinical results
Since its introduction, many clinical trials have used PTNS to treat either OAB or non-obstructive urinary retention. Outcomes in these studies are mainly based on frequency voiding charts (FVCs) and QoL questionnaires.Citation34,Citation37–Citation39 Overall subjective success, defined as improved QoL or willingness to continue treatment, was found in 56%–63%. Overall objective success with ≥50% decrease in urge or UUI and 25% reduction in daytime and/or nighttime frequency was found in 33%–71%.Citation34,Citation37–Citation39
Urodynamics done to provide more objective data show conflicting results. PTNS performed in eight neurological patients as soon as DO was observed during cystometry failed to suppress detrusor contractions.Citation40 However, another study showed a significant increase in both the volume of the first involuntary DO and the mean CMC in 29 patients with multiple sclerosis stimulated with PTNS.Citation41 Studies examining pre- and posttreatment urodynamic data in non-neurogenic OAB also show contradictory results. Vandonick et al found complete suppression of DO in only a few cases, while others report elimination of DO in 76.9%.Citation42,Citation43 Nevertheless, in both these studies, CMC increased significantly. Furthermore, patients without DO at baseline were 1.7 times more prone to respond to PTNS than those with urodynamic proven DO.Citation42
Peters et al performed the pivotal study on PTNS. In a multicenter, double-blind randomized controlled trial (RCT) (SUmiT trial), 12 weeks of PTNS was compared to sham stimulation. This was the first study including a validated sham arm providing more information on the placebo effect. In total, 220 patients were included. Outcome parameters were improvement in global response assessment (GRA), frequency voiding charts (FVC) data, and QoL. The GRA is a self-reported 7-point scale measuring the individual perception of treatment changes. Success was defined as moderate or marked improvement in the GRA. Patients receiving PTNS showed 55% moderate or marked improvement compared to 21% in the sham group. After 12 weeks, FVC parameters showed significant improvements in frequency, nocturia, voids with moderate/severe urgency, and UUI in the PTNS group compared to the sham group.Citation36 Finazzi-Agro et al performed another sham-controlled RCT with similar results.Citation35
Interestingly, there is no standardized treatment regimen. Different protocols are described (3, 6, 8, 12 weeks) with the most objective data to be in favor of the 12-week regimen based on previously mentioned RCTs.Citation35,Citation36 However, in the study of Peters et al weekly sessions were given compared to sessions 3 times a week in the study of Finazzi-Agro et al. Given the fact that both the studies show positive results, stimulation once a week seems to be effective and less time-consuming. Shorter schedules are reported by Yoong et al who found an overall 67.5% positive response in 43 women who received a shortened 6-week PTNS treatment protocol with a 50% symptom reduction and a 25% improvement in QoL, which is broadly comparable to the conventional 12-week results.Citation44 However, with the 6-week regimen, the median time to relapse was 3 weeks so the authors concluded that it was more cost-effective to provide 12 weekly sessions in newly diagnosed patients.
While most objective data published favor a 12-week protocol, shortened regimes might also be effective, but perhaps less sustainable. Until further data are published, treatment protocols remain mainly dependent on individual patient–physician preferences.
Relapse of symptoms after successful treatment is likely to occur after PTNS. In a study on 11 patients with a successful outcome of 12-week PTNS, an interval of 6 weeks without stimulation was introduced. After this interval, 7 of 11 (63%) experienced a ≥50% worsening of their complaints, which returned to baseline after re-starting PTNS.Citation45
Yoong et alCitation44 published the 2-year follow-up of 23 patients without deterioration of initially achieved results if a maintenance schedule of PTNS was given. Their study consisted of an open-door policy whereby patients could receive PTNS whenever they felt it necessary. A median of 8.42 treatments per year with a median length between the treatments of 64.3 days was given. Nocturnal frequency decreased with 57%. Daytime frequency and UUI episodes at 2 years were significantly lower than at pretreatment (6.6 vs 11.8 and 2.0 vs 3.5, respectively; p<0.05) and comparable to those at 6 weeks. The median satisfaction score after 2 years of maintenance PTNS therapy was comparable to that recorded at 6 weeks (7.25/10 vs 9.6/10; p=0.25).Citation44
Peters et alCitation46 followed 50 participants from the SUmiT Trial who met the primary effectiveness endpoint after 12 weekly PTNS. These patients were prescribed a fixed-schedule 14-week tapering protocol followed by a personal treatment. Of this group, 29 patients completed the 36-month protocol receiving a median of 1.1 treatments per month. At 3 years, 77% remained relapse free with 8.7 median voids per day (baseline 12.0) and 0.3 UUI episodes per day (baseline 3.3). QoL remained markedly improved from baseline through 3 years.Citation46 Other studies confirm sustainability with a maintenance schedule.Citation47,Citation48 Maintaining PTNS once every 2 or 3 weeks seems to be sufficient to sustain therapeutic effect in those patients who benefit from PTNS.
Side effects
From the studies examined, it was found that PTNS has no serious adverse events. Side effects described in the literature are mild and mainly related to needle insertion, bruising (0.9%), discomfort (1.8%), and slight bleeding.Citation34–Citation36
PTNS compared to other treatments
Several studies aimed to establish the effectiveness of PTNS compared to other treatments (). Two RCTs compared PTNS versus tolterodine in patients with non-neurogenic OAB.Citation49,Citation50 UUI decreased significantly after 3 months in both the groups and QoL increased. However, in both the RCTs, no significant difference was seen between both the treatments regarding QoL, 24-hour voiding episodes and UUI. Fewer side effects were seen in the PTNS group.Citation49,Citation50 Other antimuscarinics compared to PTNS yielded similar outcomes.Citation14,Citation51 A large Cochrane review compared seven trials with various types of peripheral neuromodulation (intravaginal ES, PTNS/SANS, and transcutaneous nerve stimulation) to antimuscarinics. Subjective improvement rates were observed in favor of ES. In 54%, no improvement was seen with antimuscarinics versus 33% with ES (risk ratio 0.64, 95% CI 1.15–2.34). However, this was significant only for PTNS (risk ratio 2.21, 95% CI 1.13–4.33) and was not supported by significant changes in voiding parameter or QoL. The authors concluded that antimuscarinics were a well-established therapy for OAB, and limited evidence from small trials might suggest ES to be a better option in patients refractory to antimuscarinics.Citation52
Table 1 Overview of trials comparing PTNS to other treatments
A subsequent Cochrane review compared ES with non-implantable electrodes for OAB to no treatment or other available treatments. They included 63 studies and found moderate-quality evidence indicating that ES was better for the perception of improvement of OAB symptoms than pelvic muscle floor training (PMFT) (risk ratio [RR] 1.60, 95% CI 1.19–2.14), drug treatment (RR 1.20, 95% CI 1.04–1.38) and placebo or sham treatment (RR 2.26, 95% CI 1.85–2.77). The authors concluded that ES has better results than PMFT. Low evidence suggested participants receiving ES plus PMFT, compared to PMFT training alone were more likely to report improvement in UUI (RR 2.82, 95% CI 1.44–5.52). They concluded that ES shows promising results compared to no treatment, placebo/sham treatment, PMFT, and drug treatment and that adding ES to other treatments might be beneficial.Citation53
Sancaktar et alCitation54 also studied PTNS as part of a multimodal treatment. They compared antimuscarinics with or without PTNS in 40 women with OAB. Frequency decreased from 12.8±1.3 at baseline to 6.4±0.6 in the antimuscarinics group and 12.2±1.2 to 4.5±0 in the antimuscarinics/PTNS group (p<0.05). Urgency, UUI, and QoL improved in both the groups but was significantly better if multimodal treatment was given.Citation54 In a study by Karademir et al, the combination of PTNS and oxybutinine led to an overall response of 83.2% compared to 61.6% response rate with PTNS alone; however, this difference was not significant (p=0.24).Citation55 Other studies were also unable to confirm the additional effects of antimuscarinics with PTNS.Citation56
Patients with OAB, especially when refractory to first-line treatments, pose a therapeutic challenge. Efficacy of anti-muscarinics may be limited by their intolerable side effects and/or inadequate response. Furthermore, a high number of patients discontinue antimuscarinics on the long term. One study showed that the adherence rates for tolterodine and oxybutinine after 12 months were 9% and 6%, respectively.Citation57 PTNS might be a good alternative treatment. Other options include SNS or Botox. However, to our knowledge, no RCT has compared these second-line treatments.
Prognostic factors for PTNS
Few data are available on the prognostic factors for PTNS. Urodynamic studies in patients with OAB receiving PTNS suggest better treatment outcome in those patients without actual DO.Citation42 In a study of 132 patients, numerous clinical parameters were evaluated and most of them could not reveal any prognostic value.Citation58 Even a history of sexual and/or physical abuse did not alter PTNS treatment outcome. The only factor that seemed to show any influence was a low total score at baseline in the SF-36 general QoL questionnaire. This proved to be predictive for not obtaining objective or subjective success. Patients with a low SF-36 Mental Component Summary were especially prone to fail. These patients also scored worse on disease-specific QoL questionnaires, despite that they had no difference in disease severity compared to patients with good mental health.Citation58
Therefore, mental health questionnaires might be helpful as an additional tool to select optimal candidates for PTNS.
Patient perspectives
Some studies evaluated actual patient preferences concerning treatment options for refractory OAB.Citation59,Citation60 A best-worst scaling, together with surveys with different individual attributes, was used to assess the preferences of 245 patients from the USA and the UK. Most patients (98.8%) were willing to try at least one of the different treatments. On a scale from 0% to 100%, the mean percentage likelihoods of trying SNS, Botox, and PTNS were 45%, 43%, and 62%, respectively. The main attributes in general considered important in decision-making were “lasting improvement,” “minimal side effects,” and “send signals to the brain.” Worst-rated attributes were “be willing to catheterize” and “complications of implant.” However, preferences for the attributes differed mainly based on which treatment patients preferred; for example, patients preferring PTNS favored the attributes “needle insertion in the ankle” and “multiple visits required” more than patients favoring SNS.Citation59,Citation60
Thus, incorporation of a decision tool addressing these attributes might help patients to increase compliance, treatment effect, and satisfaction.
Cost-effectiveness
Patient with refractory OAB remain a therapeutic challenge, and the effectiveness of second-line treatment options should be weighed against their costs. An economic model comparing SNS with Botox was developed using a probabilistic Markov analytic model in Dutch patients with refractory OAB. Different modeling scenarios were used. The 5-year costs were € 25,780 for SNS and € 19,353 for Botox, leading to € 6,428 additional costs per patient for SNS. SNS became cost-effective (incremental cost-effectiveness ratio [ICER] <40,000) from the third yearly treatment of Botox onward if given under general anesthesia.Citation61 A comparable study conducted in Italy calculated the 10-year costs for SNS at € 32,975 versus € 33,309 for Botox, with cumulative quality-adjusted life years (QALYs) of 7.52 and 6.93, respectively. It was concluded that the relatively higher initial costs of early SNS can be offset by the favorable long-term outcomes.Citation62 Staskin et alCitation63 compared the costs of different treatment modalities for OAB in the USA. PTNS was the least costly ($4,999 for a 3-year treatment), followed by Botox ($7,651) and SNS ($26,269) for the same treatment period.Citation63 Martinson et al also concluded that PTNS had substantially lower costs compared to SNS in the USA. Furthermore, an additional 1% of patients would remain on therapy at 2 years if SNS was used rather than PTNS, but average additional costs per patient would be >$500,000.Citation64 Despite variation between countries PTNS appears not to be cost-effective as a primary treatment option compared to antimuscarinics but might be a good alternative in therapy refractory patients. However, on the long term, SNS might be more cost-effective considering the necessity of repeated clinical visits for PTNS.
Implantable PTNS
While maintenance therapy seems necessary to sustain therapeutic effect, soon after its clinical introduction, it was realized that repeated visits would finally lead to a logistic problem. Besides overfull clinics, the travel burden for patients would be high and PTNS treatment-on-demand would be impossible. Transcutaneous stimulation was tried with surface electrodes, but results indicate that it might be less effective because of the impendence of the skin.Citation65 A new promising development came with the introduction of an implantable stimulator near the ankle. Van der Pal et alCitation66 were the first to study the subcutaneous implant Urgent-SQ (Uroplasty, Inc, Minnetonka, MN, USA) in 8 patients with refractory OAB. The Urgent-SQ was surgically implanted ~5 cm above the medial malleolus near the PTN, without actually exposing it. During the procedure, the implant was activated to confirm the correct position. Motor and sensory responses were evaluated postoperatively at day 10 and after 3–6–12 months. The primary objective was ≥50% reduction in the number of UUI and/or voids on bladder diary. At 3, 6, and 12 months, 5, 6, and 4 patients, respectively, met the primary objective. At 3- and 6-month follow-up, voiding and QoL parameters had significantly improved in these patients; at 12 months, it remained stable compared to 6 months. UTI temporary walking difficulties and spontaneous radiating sensations were reported as adverse events, and there was no local infection, erosion, or dislocation. As in studies concerning sacral stimulation, not all patients who respond well to PTNS have similar results with implantable devices. For example, in one patient, the implant was removed after 12 months because of technical failure. During the procedure, the implant was activated which did not result in a motor response. The device was examined but results not mentioned.Citation66
Janssen et alCitation67 published the long-term efficacy and safety of these patients in an open-label study. The 7 patients with the implant still in situ were contacted after 9 years and evaluated with an interview, physical examination, ankle X-ray, FVC, questionnaires addressing adverse events, performance, efficacy, safety, and QoL. Results showed that 6 of the 7 patients still had sensory and locomotor responses on stimulation at 9-year follow-up. Also, 3 patients, who had a successful treatment response at 1 year, still used the device. The implants were intact without migration and/or displacement; 2 patients experienced minor discomfort. The conclusion was that, after 9 years, the Urgent-SQ implant was a safe device and well tolerated.Citation67
Results of a new tibial implanted device (BlueWind Medical, Herzliya, Israel) have recently been published.Citation68 The installation procedure resembled that of the Urgent-SQ. For the 15 patients in whom the device was implanted, a significant improvement was seen in both frequency and UUI. At 3-months follow-up, a significant change was seen in 24-hour voiding frequency from a mean 11.8 (SD 3.5) to 8.1 (SD 2.0) per day (p=0.002). The number of severe UUI episodes decreased from 2.8 (SD 5.2) to 0.3 (SD 0.4) episodes a day (p=0.017). After implantation, 3 patients had prolonged antibiotic treatment and 3 patients needed prolonged pain treatment for 1 week. In 1 patient, the device was explanted due to pain and swelling suspicious for infection, although tissue cultures did not reveal a bacterial infection.Citation68
Implantable devices are well tolerated and safe without long-term complications. Although pilot studies show promising results, more research is necessary to establish further therapeutic value.
PTNS in children
OAB is also common in the pediatric population aged 6–16 years. If standard treatment options (eg, urotherapy, PMFT, and antimuscarinics) fail, PTNS might be a good option. De Genarro et alCitation69 found that PTNS was well tolerated in 23 children (4–17 years) with refractory lower urinary tract symptoms (LUTS). In 71%, symptoms improved and low scores on the visual analog scale for pain were noted, which decreased even further during the 12-week treatment period.Citation69
Several sham-controlled studies have been conducted using (mainly) transcutaneous stimulation. In a double-blind RCT, 20 children with refractory OAB were given either transcutaneous TNS or sham treatment for 12 consecutive weeks. Pre- and post-urodynamic parameters were compared, and UUI were noted and scored with a range of 0–13 (from good to poor). Clinical results were defined as poor (≤3-point decrease), medium (3–5-point decrease), good (6–8-point decrease), and very good (final score of 0–3). In the PTNS group, very good results were seen in 45% compared to 66% in the sham group, and poor results in 45% versus 33%, respectively. Urodynamic parameters revealed significant improvement of volume voided during urgency (184–265 mL), maximum CMC (215–274 mL), and volume at onset of first DO (48–174 mL). The authors stated that, even though urodynamic data show improvement, subjective data remain the same and the placebo effect plays an important role.Citation70 In a study by Hagstroem et al parasacral stimulation was compared with sham treatment. After 4 weeks of intervention, 61% reported a decrease in incontinence severity versus only 2% in the sham group. However, no differences were seen in maximal and average voided volumes or urodynamic data.Citation71,Citation72 Another group studied the additional effect of parasacral stimulation in patients receiving urotherapy;Citation73 62 children with OAB were randomized either to urotherapy alone or combined with parasacral stimulation. In the standard group, 46% were completely dry versus 67% in the combined group; this was not significant. Furthermore, no differences were seen between both the groups concerning FVC parameters. The authors concluded that parasacral stimulation had no additional effect.Citation73
Long-term outcome seems to be good in children treated with PTNS. In 44 children with LUTS, the cure rate after 1 year was 41% if being treated for OAB and 71% if being treated for dysfunctional voiding both defined according to the International Children’s Continence Society,Citation1 which remained stable after 2 years. Maintenance treatment was necessary in 29% of children with dysfunctional voiding and in 50% of children with OAB.Citation74
Only one study compared PTNS versus parasacral stimulation and found a higher complete resolution of symptoms in the parasacral group versus the PTNS group (71% vs 9%) without significant differences in scores of urgency and UUI. However, because this was not an RCT, the data have to be interpreted with caution.Citation13
Transcutaneous/percutaneous stimulation seems feasible in children with refractory LUTS. However, more trials with larger groups are necessary to determine the actual subjective and objective effect, while current data show conflicting results.
Potential limitations
Because this review has a non-systematic design, there are some limitations. Although a comprehensive search was made to include eligible articles, some potential articles could have been missed. Especially relevant non-English language studies might not have been included. Also, no methodological assessment or data extraction was done to detect heterogeneity or publication bias. Therefore, our conclusion needs to be interpreted with caution.
Conclusion
PTNS can indeed modulate the voiding and storage function of the bladder leading to an overall subjective improvement of symptoms in about 60% of the patients and 47%–56% improvement of FVC parameters with sustainable outcome on the long run. The placebo effect (subjective improvement measured by patients who actually received sham treatment) is about 21% and may be even higher in children. This might be explained by the regular visits, peer-grouping, and/or the weekly attention paid to their problem by the caregivers. PTNS seems not to be cost-effective as a primary treatment compared to antimuscarinics, but is a good treatment option in refractory OAB or when antimuscarinics are not tolerated. Although PTNS is minimally invasive and not costly, it is time consuming. Therefore, new techniques with implants are being explored and show initially promising results.
Disclosure
Dr Heesakkers received grants and personal fees from Bluewind. The authors report no other conflicts of interest in this work.
References
- AustinPFBauerSBBowerWThe standardization of terminology of lower urinary tract function in children and adolescents: update report from the standardization committee of the International Children’s Continence SocietyNeurourol Urodyn201635447148125772695
- HaylenBTde RidderDFreemanRMAn International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunctionNeurourol Urodyn201029142019941278
- MilsomICoyneKSNicholsonSKvaszMChenCIWeinAJGlobal prevalence and economic burden of urgency urinary incontinence: a systematic reviewEur Urol2014651799524007713
- CoyneKSWeinANicholsonSKvaszMChenCIMilsomIComorbidities and personal burden of urgency urinary incontinence: a systematic reviewInt J Clin Pract201367101015103324073974
- CoyneKSKvaszMIrelandAMMilsomIKoppZSChappleCRUrinary incontinence and its relationship to mental health and health-related quality of life in men and women in Sweden, the United Kingdom, and the United StatesEur Urol2012611889521831517
- BartoliSAguzziGTarriconeRImpact on quality of life of urinary incontinence and overactive bladder: a systematic literature reviewUrology201075349150019962738
- ZornBHMontgomeryHPieperKGrayMSteersWDUrinary incontinence and depressionJ Urol19991621828410379745
- BowerWFSelf-reported effect of childhood incontinence on quality of lifeJ Wound Ostomy Continence Nurs200835661762119018203
- NataleNKuhnSSiemerSStockleMvon GontardAQuality of life and self-esteem for children with urinary urge incontinence and voiding postponementJ Urol2009182269269819539323
- SampaioASFragaLGSalomaoBAAre lower urinary tract symptoms in children associated with urinary symptoms in their mothers?J Pediatr Urol2017133e1269.e6
- de GroatWCYoshimuraNAnatomy and physiology of the lower urinary tractHandb Clin Neurol20151306110826003239
- de GroatWCGriffithsDYoshimuraNNeural control of the lower urinary tractCompr Physiol20155132739625589273
- BarrosoUJrViterboWBittencourtJFariasTLordeloPPosterior tibial nerve stimulation vs parasacral transcutaneous neuromodulation for overactive bladder in childrenJ Urol2013190267367723422257
- ManriquezVGuzmanRNaserMTranscutaneous posterior tibial nerve stimulation versus extended release oxybutynin in overactive bladder patients. A prospective randomized trialEur J Obstet Gynecol Reprod Biol201619661026645117
- SchreinerLdos SantosTGKnorstMRda Silva FilhoIGRandomized trial of transcutaneous tibial nerve stimulation to treat urge urinary incontinence in older womenInt Urogynecol J20102191065107020458465
- BowerWFMooreKHAdamsRDShepherdRA urodynamic study of surface neuromodulation versus sham in detrusor instability and sensory urgencyJ Urol19981606 Pt 1213321369817339
- FowlerCJGriffithsDde GroatWCThe neural control of micturitionNat Rev Neurosci20089645346618490916
- FerroniMCSlaterRCShenBRole of the brain stem in tibial inhibition of the micturition reflex in catsAm J Physiol Renal Physiol20153093F242F25026017973
- FallMLindstromSElectrical stimulation. A physiologic approach to the treatment of urinary incontinenceUrol Clin North Am19911823934072017820
- LiaoKKChenJTLaiKLEffect of sacral neuromodulation on the spinal nociceptive reflex of patients with idiopathic overactive bladderNeuromodulation2008111505522150991
- BandariJBansalUZhangZNeurotransmitter mechanisms underlying sacral neuromodulation of bladder overactivity in catsNeuromodulation2017201818727730701
- LyonTDFerroniMCKadowBTPudendal but not tibial nerve stimulation inhibits bladder contractions induced by stimulation of pontine micturition center in catsAm J Physiol Regul Integr Comp Physiol20163104R366R37426676253
- TaiCLarsonJAOgaganPDDifferential role of opioid receptors in tibial nerve inhibition of nociceptive and nonnociceptive bladder reflexes in catsAm J Physiol Renal Physiol20123029F1090F109722237803
- LiXLiaoLChenGWangZDengHInvolvement of opioid receptors in inhibition of bladder overactivity induced by sacral neuromodulation in pigs: a possible action mechanismNeurourol Urodyn201617
- BlokBFGroenJBoschJLVeltmanDJLammertsmaAADifferent brain effects during chronic and acute sacral neuromodulation in urge incontinent patients with implanted neurostimulatorsBJU Int20069861238124317034500
- BraunPMBaeznerHSeifCAlterations of cortical electrical activity in patients with sacral neuromodulatorEur Urol2002415562566 discussion 566–56712074800
- Finazzi-AgroERocchiCPachatzCPercutaneous tibial nerve stimulation produces effects on brain activity: study on the modifications of the long latency somatosensory evoked potentialsNeurourol Urodyn200928432032419090588
- StuxGGeneral standards in acupuncture treatment of chronic painSchmerz199711212612712799831
- MinniBCapozzaNCretiGDe GennaroMCaionePBischkoJBladder instability and enuresis treated by acupuncture and electrotherapeutics: early urodynamic observationsAcupunct Electrother Res199015119251973577
- van VoskuilenACOerlemansDJWeilEHde BieRAvan KerrebroeckPELong term results of neuromodulation by sacral nerve stimulation for lower urinary tract symptoms: a retrospective single center studyEur Urol200649236637216413105
- van KerrebroeckPEvan VoskuilenACHeesakkersJPResults of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical studyJ Urol200717852029203417869298
- McGuireEJZhangSCHorwinskiERLyttonBTreatment of motor and sensory detrusor instability by electrical stimulationJ Urol1983129178796600794
- StollerMLCopelandSMillardRJMurnaghanGFThe efficacy of acupuncture in reversing the unstable bladder in pig-tailed monkeysJ Urol19871374A104A104
- GovierFELitwillerSNittiVKrederKJJrRosenblattPPercutaneous afferent neuromodulation for the refractory overactive bladder: results of a multicenter studyJ Urol200116541193119811257669
- Finazzi-AgroEPettaFSciobicaFPasqualettiPMuscoSBovePPercutaneous tibial nerve stimulation effects on detrusor overactivity incontinence are not due to a placebo effect: a randomized, double-blind, placebo controlled trialJ Urol201018452001200620850833
- PetersKMCarricoDJPerez-MarreroRARandomized trial of percutaneous tibial nerve stimulation versus Sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trialJ Urol201018341438144320171677
- van BalkenMRVandoninckVGisolfKWPosterior tibial nerve stimulation as neuromodulative treatment of lower urinary tract dysfunctionJ Urol2001166391491811490245
- VandoninckVVan BalkenMRFinazzi AgroEPosterior tibial nerve stimulation in the treatment of urge incontinenceNeurourol Urodyn2003221172312478596
- van der PalFvan BalkenMRHeesakkersJPDebruyneFMKiemeneyLABemelmansBLCorrelation between quality of life and voiding variables in patients treated with percutaneous tibial nerve stimulationBJU Int200697111311616336339
- FjorbackMVvan ReyFSvan der PalFRijkhoffNJPetersenTHeesakkersJPAcute urodynamic effects of posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with MSEur Urol2007512464470 discussion 471–46216956713
- KabaySCYucelMKabaySAcute effect of posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with multiple sclerosis: urodynamic studyUrology200871464164518387393
- VandoninckVvan BalkenMRFinazzi AgroEPercutaneous tibial nerve stimulation in the treatment of overactive bladder: urodynamic dataNeurourol Urodyn200322322723212707873
- KlinglerHCPychaASchmidbauerJMarbergerMUse of peripheral neuromodulation of the S3 region for treatment of detrusor overactivity: a urodynamic-based studyUrology200056576677111068296
- YoongWShahPDadswellRGreenLSustained effectiveness of percutaneous tibial nerve stimulation for overactive bladder syndrome: 2-year follow-up of positive respondersInt Urogynecol J201324579579922955253
- van der PalFvan BalkenMRHeesakkersJPDebruyneFMBemelmansBLPercutaneous tibial nerve stimulation in the treatment of refractory overactive bladder syndrome: is maintenance treatment necessary?BJU Int200697354755016469023
- PetersKMCarricoDJWooldridgeLSMillerCJMacDiarmidSAPercutaneous tibial nerve stimulation for the long-term treatment of overactive bladder: 3-year results of the STEP studyJ Urol201318962194220123219541
- Canbaz KabaySKabaySMestanELong term sustained therapeutic effects of percutaneous posterior tibial nerve stimulation treatment of neurogenic overactive bladder in multiple sclerosis patients: 12-months resultsNeurourol Urodyn201736110411026352904
- MacDiarmidSAPetersKMShobeiriSALong-term durability of percutaneous tibial nerve stimulation for the treatment of overactive bladderJ Urol2010183123424019913821
- PreyerOUmekWLamlTPercutaneous tibial nerve stimulation versus tolterodine for overactive bladder in women: a randomised controlled trialEur J Obstet Gynecol Reprod Biol2015191515626073262
- PetersKMMacdiarmidSAWooldridgeLSRandomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trialJ Urol200918231055106119616802
- Vecchioli-ScaldazzaCMorosettiCBerouzAGiannubiloWFerraraVSolifenacin succinate versus percutaneous tibial nerve stimulation in women with overactive bladder syndrome: results of a randomized controlled crossover studyGynecol Obstet Invest201375423023423548260
- RaiBPCodyJDAlhassoAStewartLAnticholinergic drugs versus non-drug active therapies for non-neurogenic overactive bladder syndrome in adultsCochrane Database Syst Rev201212CD00319323235594
- StewartFGameiroLFEl DibRGameiroMOKapoorAAmaroJLElectrical stimulation with non-implanted electrodes for overactive bladder in adultsCochrane Database Syst Rev201612CD01009827935011
- SancaktarMCeyhanSTAkyolIThe outcome of adding peripheral neuromodulation (Stoller afferent neuro-stimulation) to anti-muscarinic therapy in women with severe overactive bladderGynecol Endocrinol2010261072973220210697
- KarademirKBaykalKSenBSenkulTIseriCErdenDA peripheric neuromodulation technique for curing detrusor overactivity: Stoller afferent neurostimulationScand J Urol Nephrol200539323023316118096
- SoutoSCReisLOPalmaTPalmaPDenardiFProspective and randomized comparison of electrical stimulation of the posterior tibial nerve versus oxybutynin versus their combination for treatment of women with overactive bladder syndromeWorld J Urol201432117918423749315
- ShayaFTBlumeSGuAZyczynskiTJumadilovaZPersistence with overactive bladder pharmacotherapy in a Medicaid populationAm J Manag Care2005114 SupplS121S12916161385
- van BalkenMRVergunstHBemelmansBLPrognostic factors for successful percutaneous tibial nerve stimulationEur Urol200649236036516359781
- BeusterienKKennellyMJBridgesJFAmosKWilliamsMJVasavadaSUse of best-worst scaling to assess patient perceptions of treatments for refractory overactive bladderNeurourol Urodyn20163581028103326370222
- HashimHBeusterienKBridgesJFAmosKCardozoLPatient preferences for treating refractory overactive bladder in the UKInt Urol Nephrol201547101619162726347077
- LeongRKde WachterSGJooreMAvan KerrebroeckPECost-effectiveness analysis of sacral neuromodulation and botulinum toxin A treatment for patients with idiopathic overactive bladderBJU Int2011108455856421166750
- BertapelleMPVotteroMPopoloGDSacral neuromodulation and botulinum toxin A for refractory idiopathic overactive bladder: a cost-utility analysis in the perspective of Italian Healthcare SystemWorld J Urol20153381109111725218855
- StaskinDRPetersKMMacDiarmidSShoreNde GroatWCPercutaneous tibial nerve stimulation: a clinically and cost effective addition to the overactive bladder algorithm of careCurr Urol Rep201213532733422893501
- MartinsonMMacDiarmidSBlackECost of neuromodulation therapies for overactive bladder: percutaneous tibial nerve stimulation versus sacral nerve stimulationJ Urol2013189121021623174264
- AndrewsBJReynardJMTranscutaneous posterior tibial nerve stimulation for treatment of detrusor hyperreflexia in spinal cord injuryJ Urol2003170392612913738
- van der PalFvan BalkenMRHeesakkersJPDebruyneFMBemelmansBLImplant-driven tibial nerve stimulation in the treatment of refractory overactive bladder syndrome: 12-month follow-upNeuromodulation20069216317122151641
- JanssenDAFaragFHeesakkersJPUrgent-SQ implant in treatment of overactive bladder syndrome: 9-year follow-up studyNeurourol Urodyn201332547247523070697
- van BredaHMMartensFMTrompJHeesakkersJPA new implanted posterior tibial nerve stimulator for the treatment of overactive bladder syndrome: 3-month results of a novel therapy at a single centerJ Urol2017198120521028189576
- De GennaroMCapitanucciMLMastracciPSilveriMGattiCMosielloGPercutaneous tibial nerve neuromodulation is well tolerated in children and effective for treating refractory vesical dysfunctionJ Urol200417151911191315076308
- BoudaoudNBinetALineAManagement of refractory overactive bladder in children by transcutaneous posterior tibial nerve stimulation: a controlled studyJ Pediatr Urol2015113138 e131138 e11025979217
- HagstroemSMahlerBMadsenBDjurhuusJCRittigSTranscutaneous electrical nerve stimulation for refractory daytime urinary urge incontinenceJ Urol20091824 Suppl2072207819695629
- BorchLRittigSKamperisKMahlerBDjurhuusJCHagstroemSNo immediate effect on urodynamic parameters during transcutaneous electrical nerve stimulation (TENS) in children with overactive bladder and daytime incontinence – a randomized, double-blind, placebo-controlled studyNeurourol Urodyn201617
- SillenUArwidssonCDoroszkiewiczMEffects of transcutaneous neuromodulation (TENS) on overactive bladder symptoms in children: a randomized controlled trialJ Pediatr Urol20141061100110524881806
- CapitanucciMLCamanniDDemelasFMosielloGZaccaraADe GennaroMLong-term efficacy of percutaneous tibial nerve stimulation for different types of lower urinary tract dysfunction in childrenJ Urol20091824 Suppl2056206119695611
