Abstract
In males with nonobstructive azoospermia, one of the main histopathologic patterns of the testis is Sertoli cell-only syndrome (SCOS), in which no germ cells are present and only Sertoli cells are contained in the seminiferous tubules. There is not any formal treatment for this pathological condition. However, several studies reported the possibility to perform testicular sperm extraction in patients with SCOS, although, according to some authors, sperm retrieval is possible only in the presence of focal spermatogenesis. We report the case of an infertile couple in whom the 30-year-old male was azoospermic. After the diagnosis, the patient underwent multiple bilateral testicular biopsies, which showed a histological pattern corresponding to SCOS. We administered a cycle of hormone stimulation followed by medically assisted procreation procedures to the male patient. Therefore, the male patient was treated with follicle-stimulating hormone gonadotropin for a total of 7 months (150 IU recombinant human follicle stimulating hormone three times per week). After carrying out a new multiple testicular sperm extraction, several spermatozoa were microscopically observed, and it was then possible to perform an intracytoplasmic sperm injection with subsequent embryo transfer of the blastocyst into the wife’s uterus, and so pregnancy was established and developed. Subsequently, the pregnancy resulted in the live birth of a girl.
Introduction
Infertility is a problem that globally affects 15% of couples who have regular unprotected sexual intercourse.Citation1,Citation2 In about 50% of cases of infertility, the exclusive cause is attributed to the female, in 20%−30% of cases to the male, while in the remaining 20%−30% of cases, infertility is the result of a combination of male and female causes.Citation1
Azoospermia has been identified in 15% of infertile males and can be classified as obstructive azoospermia (OA) and nonobstructive azoospermia (NOA).Citation3 OA accounts for 40% of azoospermia cases: in these cases, the endocrine and exocrine functions of the testes are preserved and spermatogenesis in the testis is normal. OA is caused by an obstruction of the male duct system and may occur in any region between the rete testis and the ejaculatory ducts. NOA affects ~1% of the general population, 10% of infertile men, and 60% of azoospermic men.Citation3–Citation5 NOA results from either primary testicular failure, secondary testicular failure, or incomplete or ambiguous testicular failure. In males with NOA, the main histopathological patterns of the testis are the following: hypospermatogenesis, maturation arrest, Sertoli cell-only syndrome (SCOS), incomplete Sertoli cell-only syndrome ([SCOS type II] in which only a few tubules contain germ cells), and complete and homogeneous hyalinization of seminiferous tubules which appear completely acellular.Citation5–Citation7
In the SCOS pattern, no germ cells are present and only Sertoli cells are contained in the seminiferous tubules, but the tubular architecture is not affected by fibrosis. Prevalence of SCOS in azoospermic patients varies between 26.3% and 57.8% of cases.Citation8–Citation12 Many SCOS patients have a normal karyotype and normal secondary male sexual characteristics; other patients have reduced testicular volume and high follicle-stimulating hormone (FSH) levels. Whereas for many of these patients the cause is unknown, for many others, the following associated conditions have been identified: Klinefelter’s syndrome (47,XXY) or mosaic Klinefelter’s syndrome (46,XY/47,XXY); microdeletions in the azoospermia factor (AZF) region of the Y chromosome; hypogonadotropic hypogonadism; cryptorchidism; estrogen treatment; chemotherapy; exposure to chemicals and toxins; history of testicular hypoxia; and chronic alcohol consumption.Citation13–Citation20 Consequently, a number of authors describe a primary and secondary form of SCOS, while other authors prefer to consider the presence of associated conditions. There is not any formal treatment for SCOS.Citation21 Several studies, however, have reported the possibility to perform testicular sperm extraction (TESE) in patients with SCOS, although according to some authors, there is a likelihood of success (sperm retrieval) only in the presence of focal spermatogenesis within a histologic pattern of SCOS.Citation12,Citation22–Citation27 At the moment, only two studies have reported cases of term pregnancy with live birth after TESE in patients with SCOS.Citation26,Citation27
This article is the result of a successful experience of FSH treatment in an SCOS patient, with the appearance of spermatozoa in the seminiferous tubules after about 3 months.
Clinical case
We report the case of an infertile couple in whom the 30-year-old male was azoospermic. The diagnosis of azoospermia was ascertained by at least two semen analyses, following the World Health Organization guidelines.Citation21 The 27-year-old wife, although suffering from polycystic ovary syndrome, did not present any particular problems, and her hormone assays were normal. Physical examination of the man revealed normal secondary male sexual characteristics and the presence of both testes, of normal volume, and with palpable vasa deferentia at the root of the scrotum. His hormone profile, however, was altered: FSH=15 mUI/mL (normal reference range=1.55−9.74 mUI/mL/chemiluminescence) and total testosterone=21.9 mmol/L (normal reference range=4.0−20.0 mUI/mL/chemiluminescence). The luteinizing hormone assay was instead normal: 9.60 mUI/mL (normal reference range=1.5−14.0 mUI/mL/chemiluminescence). Genetic testing was normal with regards to karyotype, genetic mutations of cystic fibrosis transmembrane conductance regulator (CFTR) gene related to congenital bilateral absence of the vas deferens and cystic fibrosis, Y-chromosomal microdeletions, and deletions of the AZF region (AZFa, AZFb, or AZFc).
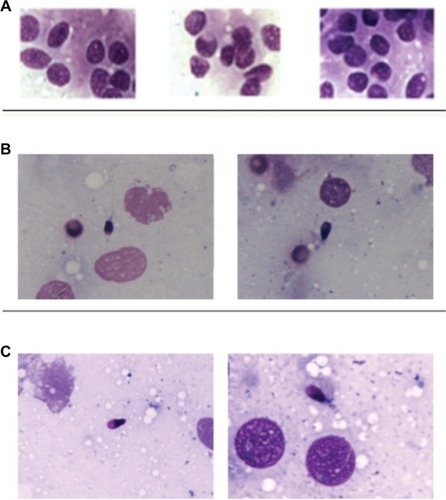
The patient’s medical history revealed no inflammatory episodes or urogenital tract infections. After the diagnosis of azoospermia, the patient underwent multiple bilateral testicular biopsies, which showed a histologic pattern corresponding to SCOS and complete absence of germ cells in both testicles ().
Figure 1 Microscopic observation (100× magnification) before and after FSH treatment.
Abbreviation: FSH, follicle-stimulating hormone.
The couple immediately rejected the idea of using sperm donation; therefore, after having obtained a shared consent (including informed consent) from the couple, we administered a cycle of hormone stimulation for the production of gametes, followed by medically assisted procreation (MAP) procedures to the male patient.
The male patient was thus treated with FSH gonadotropin for 3 months at the following dosage: 150 IU recombinant human FSH (rFSH) injected under the skin three times per week.
The female patient (wife) also underwent stimulation, taking 900 UI of rFSH for each cycle: on the 21st day of her cycle, she took a suppressor drug which inhibited FSH and luteinizing hormone production. In the following cycle, she underwent hormone stimulation from the first to the fifth day of her cycle. Follicular growth was monitored with ultrasound and serum estradiol measurement. When follicles were suitable in number and size, human chorionic gonadotropin drug was administered and the oocyte retrieval was performed within 36 hours.
The patients signed an informed consent containing the aspects of the surgical procedure, the modalities of assisted reproduction, and agreement to the publication of this case report and images.
The study was conducted according to the ethical standards of the Declaration of Helsinki.
Sequence of the MAP procedures
MAP (first cycle)
Sperm retrieval
At the end of the 3-month therapy, a multiple TESE was performed. On microscopic observation (100× phase contrast immersion objective), some spermatozoa were observed ().
Intracytoplasmic sperm injection (ICSI)
Using microscopy, every single spermatozoon was aspirated and injected into the cytoplasm of the six mature oocytes (out of 12 total oocytes) which had previously been retrieved.
Embryo transfer
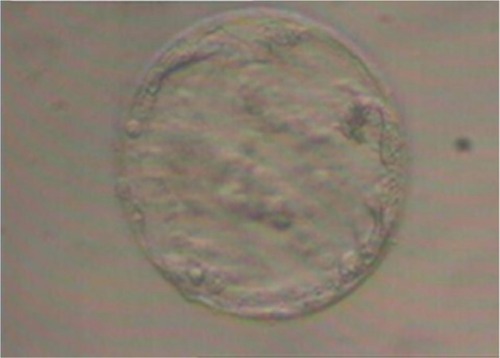
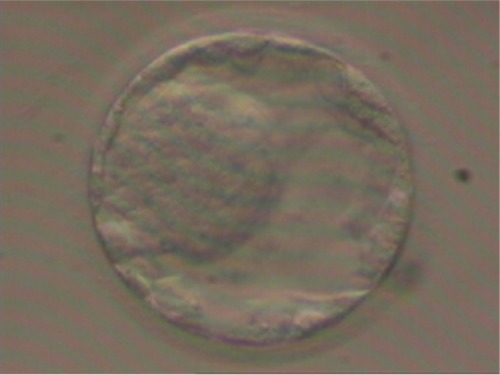
Of the six fertilized oocytes, only three developed into zygotes, from which a single 4BB blastocyst was obtained (according to Gardner’s classification = expanded blastocyst with wall thinned – some loosely grouped cells – a small number of cells forming a cohesive layer), as shown in .Citation28–Citation30
The blastocyst was transferred into the wife’s uterus, but no pregnancy followed.
Therapy with rFSH (150 IU three times per week) was, therefore, prolonged in the male patient, in view of a new TESE and subsequent ICSI. The female patient (wife) repeated the same initial stimulation protocol.
After 4 months of gonadotropin therapy, a new MAP attempt was made.
MAP (second cycle)
Sperm retrieval
After carrying out a new multiple TESE, several spermatozoa were observed microscopically ().
ICSI
Every spermatozoon was aspirated and injected into the cytoplasm of the six mature oocytes (out of 14 total oocytes) which had previously been retrieved.
Embryo transfer
Of the six fertilized oocytes, four developed into zygotes. From them a single blastocyst was obtained, but this time with improved characteristics: grade 4AA = expanded blastocyst with wall thinned – many thickened cells – many cells forming a cohesive layer (according to Gardner’s classification), as shown in .Citation28–Citation30
Fifteen days after the embryo transfer, the wife was pregnant.
Discussion
This is the first article in the literature describing in detail the case of a patient suffering from SCOS who, after treatment with FSH, produced sperm cells, which were then retrieved using TESE. As a result, it was then possible to perform an ICSI with subsequent embryo transfer of the blastocyst into the wife’s uterus and establishment and development of pregnancy, which was uneventful and resulted in the live birth of a girl. In the literature, we found studies in which, in SCOS cases, after recovery of sperm cells through TESE, cases of “ongoing pregnancies” were reported after embryo implantation following ICSI.Citation22,Citation23,Citation26,Citation27,Citation31 However, only two of these studies reported that the pregnancies had reached term with the live birth of a baby.Citation26,Citation27 The appearance of sperm cells in the seminiferous tubules after treatment with gonadotropin has also been reported in the literature.Citation8,Citation39
In their literature review 10 years ago, Donoso et al found that microdissection-TESE (or micro-TESE) can lead to higher percentages of sperm retrieval compared to traditional TESE, especially in patients with SCOS;Citation40 the same study observed that TESE procedures with multiple biopsies make it possible to increase the possibility of sperm retrieval compared to fine needle testicular aspiration, particularly in cases of SCOS. It must be noted that Mulhall et al did not find, in general, any significant difference in the percentage of sperm retrieval between the two methods, except in cases with mean testicular volumes of 10 mL or less: in those cases, instead, microdissection-TESE showed better results.Citation41
Currently, there is no consensus on administering gonadotropin to infertile patients with NOA with the aim of increasing the possibility of sperm retrieval; this is partly due to the fact that many patients with SCOS already present baseline FSH serum levels that are higher than normal.Citation42 Furthermore, various studies have shown that in patients with NOA and SCOS, after TESE, there is a greater likelihood of sperm retrieval where FSH blood levels are higher.Citation43–Citation45
The gonadotropin we used in this case was human rFSH, specifically follitropin alpha, which, from the point of view of biologic activity, is biosimilar to (likewise human recombinant) follitropin beta.Citation46–Citation48 From the point of view of biogenetic engineering, in follitropin alpha, two separate vectors, one for each subunit, are used to make up the master cell bank for an FSH-producing cell line, while follitropin beta uses a single vector containing the coding sequences of both subunit genes.Citation49,Citation50
Recombinant follitropin alpha, from the point of view of its therapeutic effect, is interchangeable with urinary gonadotropin (urofollitropin, purified human-derived FSH).Citation51–Citation53
Conclusion
When the histopathologic profile of the azoospermic patient’s testicular biopsy corresponds to SCOS, performance of multiple TESE along with intracytoplasmic sperm injection is certainly indicated as first-line treatment. Multiple TESE is justified, since a focal spermatogenesis site might not coincide with the site of a single testicular sampling. In our case, treatment with FSH gonadotropin was able to lead to the production of sperm cells, which were absent in the first testicular biopsy. The second ICSI cycle and embryo transfer then led to therapeutic success, with the establishment and development of pregnancy and the live birth of a girl.
Acknowledgments
Luca Paulis (Graduate Pharmacist) is the son of Gianni Paulis. Luca collaborates in his father’s studies as in other past articles.
Disclosure
The authors report no conflicts of interest in this work.
References
- SharlipIDJarowJPBelkerAMBest practice policies for male infertilityFertil Steril200277587388212009338
- MartinezGDanielsKChandraAFertility of men and women aged 15–44 years in the United States: National Survey of Family Growth, 2006–2010Natl Health Stat Report201251128
- WosnitzerMGoldsteinMHardyMPReview of AzoospermiaSpermatogenesis20144e2821825105055
- WillottGMFrequency of azoospermiaForensic Sci Int19822019107095683
- McLachlanRIRajpert-De MeytsEHoei-HansenCEde KretserDMSkakkebaekNEHistological evaluation of the human testis–approaches to optimizing the clinical value of the assessment: mini reviewHum Reprod200722121616887924
- OstadMLiottaDYeZSchlegelPNTesticular sperm extraction for nonobstructive azoospermia: results of a multibiopsy approach with optimized tissue dispersionUrology19985246926969763095
- CastiglioniMColpiEMScroppoFIColpiGMThe Infertile Male-5: Management of Non-Obstructive AzoospermiaBertolottoMTrombettaCScrotal PathologyBerlin HeidelbergSpringer-Verlag2012249261
- ShiraishiKOhmiCShimabukuroTMatsuyamaHHuman chorionic gonadotrophin treatment prior to microdissection testicular sperm extraction in non-obstructive azoospermiaHum Reprod201227233133922128297
- ParikhURGoswamiHMDeliwalaKJShahAMTesticular biopsy in male infertility (Study of 80 cases)Int J Pathol20111121
- Lekshmi AmmalPKrishna DasSTesticular Biopsy in Male InfertilityJMSCR2017542061620619
- RashedMRagabNShalabyARagabWPatterns of testicular histopathology in men with primary infertilityInt J Pathol20075219
- FrancoGScarselliFCascianiVA novel stepwise micro-TESE approach in non obstructive azoospermiaBMC Urol20161612027176005
- StouffsKGheldofATournayeHSertoli Cell-Only Syndrome: Behind the Genetic ScenesBiomed Res Int20162016619130726925412
- PaulisGChromosomic causes of infertilityCavalliniGBerettaGClinical Management of Male InfertilityNew York, NYSpringer20146377
- ForestaCFerlinAGarollaAHigh frequency of well-defined Y-chromosome deletions in idiopathic Sertoli cell-only syndromeHum Reprod19981323023079557827
- BellinghamMMcKinnellCFowlerPAFoetal and post-natal exposure of sheep to sewage sludge chemicals disrupts sperm production in adulthood in a subset of animalsInt J Androl201235331732922150464
- FisherJSMacphersonSMarchettiNSharpeRMHuman ‘testicular dysgenesis syndrome’: a possible model using in-utero exposure of the rat to dibutyl phthalateHum Reprod20031871383139412832361
- GatYGornishMPerlowAAzoospermia and Sertoli-cell-only syndrome: hypoxia in the sperm production site due to impairment in venous drainage of male reproductive systemAndrologia201042531432120860630
- NistalMJimenezFPaniaguaRSertoli cell types in the Sertoli-cell-only syndrome: relationships between Sertoli cell morphology and etiologyHistopathology19901621731802182507
- PajarinenJTKarhunenPJSpermatogenic arrest and ‘Sertoli cell-only’ syndrome–common alcohol-induced disorders of the human testisInt J Androl19941762922997744508
- World Health OrganizationWHO Laboratory Manual for the Examination and Processing of Human SemenFifth editionWorld Health Organization2010
- BerookhimBMPalermoGDZaninovicNRosenwaksZSchlegelPNMicrodissection testicular sperm extraction in men with Sertoli cell-only testicular histologyFertil Steril201410251282128625441063
- ModarresiTHosseinifarHDaliri HampaAPredictive factors of successful microdissection testicular sperm extraction in patients with presumed sertoli cell-only syndromeInt J Fertil Steril20159110711225918598
- RamasamyRYaganNSchlegelPNStructural and functional changes to the testis after conventional versus microdissection testicular sperm extractionUrology20056561190119415922422
- TalasHYamanOAydosKOutcome of repeated micro-surgical testicular sperm extraction in patients with non-obstructive azoospermiaAsian J Androl20079566867317712484
- GulUTuruncTHaydardedeogluBYayciogluOKuzgunbayBOzkardesHSperm retrieval and live birth rates in presumed Sertoli-cell-only syndrome in testis biopsy: a single centre experienceAndrology201311475123258629
- YildirimMEKocAKaygusuzICThe association between serum follicle-stimulating hormone levels and the success of microdissection testicular sperm extraction in patients with azoospermiaUrol J20141141825182825194084
- GardnerDKLaneMStevensJSchlenkerTSchoolcraftWBBlastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transferFertil Steril20007361155115810856474
- GardnerDKSurreyEMinjarezDLeitzAStevensJSchoolcraftWBSingle blastocyst transfer: a prospective randomized trialFertil Steril200481355155515037401
- YangZLiuJCollinsGSSelection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot studyMol Cytogenet2012512422551456
- De CrooIVan der ElstJEveraertKDe SutterPDhontMFertilization, pregnancy and embryo implantation rates after ICSI in cases of obstructive and non-obstructive azoospermiaHum Reprod20001561383138810831574
- KalsiJThumMYMuneerAAbdullahHMinhasSIn the era of micro-dissection sperm retrieval (m-TESE) is an isolated testicular biopsy necessary in the management of men with non-obstructive azoospermia?BJU Int2012109341842421883824
- Abdel RaheemAGaraffaGRushwanNTesticular histopathology as a predictor of a positive sperm retrieval in men with non-obstructive azoospermiaBJU Int2013111349249922583840
- HusseinAEvaluation of diagnostic testis biopsy and the repetition of testicular sperm extraction surgeries in infertility patientsFertil Steril20131001889323582438
- TournayeHVerheyenGNagyPAre there any predictive factors for successful testicular sperm recovery in azoospermic patients?Hum Reprod199712180869043908
- SuLMPalermoGDGoldsteinMVeeckLLRosenwaksZSchlegelPNTesticular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia: testicular histology can predict success of sperm retrievalJ Urol199916111211610037381
- TalasHYamanOAydosKOutcome of repeated micro-surgical testicular sperm extraction in patients with non-obstructive azoospermiaAsian J Androl20079566867317712484
- KitamuraMNishimuraKMiuraHRetrieval and cryopreservation of testicular spermHinyokika Kiyo200046858759011019382
- RogozaAZwalińskiMBrzóskaBEmerichJSuccessful treatment of azoospermia in sertoli cell only syndrome: a case reportGinekol Pol19986964985019695371
- DonosoPTournayeHDevroeyPWhich is the best sperm retrieval technique for non-obstructive azoospermia? A systematic reviewHum Reprod Update200713653954917895238
- MulhallJPGhalySWAvivNAhmedAThe utility of optical loupe magnification for testis sperm extraction in men with nonobstructive azoospermiaJ Androl200526217818115713823
- AttiaAMAbou-SettaAMAl-InanyHGGonadotrophins for idiopathic male factor subfertilityCochrane Database Syst Rev20138CD00507123970458
- RamasamyRLinKGosdenLVRosenwaksZPalermoGDSchlegelPNHigh serum FSH levels in men with nonobstructive azoospermia does not affect success of microdissection testicular sperm extractionFertil Steril200992259059318973887
- ColpiGMColpiEMPiediferroGMicrosurgical TESE versus conventional TESE for ICSI in non-obstructive azoospermia: a randomized controlled studyReprod Biomed Online200918331531919298728
- BohringCSchroeder-PrintzenIWeidnerWKrauseWSerum levels of inhibin B and follicle-stimulating hormone may predict successful sperm retrieval in men with azoospermia who are undergoing testicular sperm extractionFertil Steril20027861195119812477511
- HarlinJCsemiczkyGWramsbyHFriedGRecombinant follicle stimulating hormone in in-vitro fertilization treatment-clinical experience with follitropin alpha and follitropin betaHum Reprod200015223924410655291
- TulppalaMAhoMTuuriTComparison of two recombinant follicle-stimulating hormone preparations in in-vitro fertilization: a randomized clinical studyHum Reprod199914112709271510548606
- de MoraFFauserBCJMBiosimilars to recombinant human FSH medicines: comparable efficacy and safety to the original biologicReprod Biomed Online2017351818628462793
- HowlesCMGenetic engineering of human FSH (Gonal-F)Hum Reprod Update1996221721919079412
- OlijveWde BoerWMuldersJWvan WezenbeekPMMolecular biology and biochemistry of human recombinant follicle stimulating hormone (Puregon)Mol Hum Reprod1996253713829238705
- van WelyMKwanIBurtALRecombinant versus urinary gonadotrophin for ovarian stimulation in assisted reproductive technology cyclesCochrane Database Syst Rev2011162CD005354
- BakerVLFujimotoVYKettelLMClinical efficacy of highly purified urinary FSH versus recombinant FSH in volunteers undergoing controlled ovarian stimulation for in vitro fertilization: a randomized, multicenter, investigator-blind trialFertil Steril20099141005101118367182
- SohrabvandFSheikhhassaniSBagheriMComparison of highly purified urinary versus recombinant FSH: Effect on ART outcomes in polycystic ovary syndromeIran J Reprod Med201210322923625242998