Abstract
Introduction
Prostate cancer is the most common cancer among men, but overall mortality rates remain low, due to the preponderance of low-risk disease. Over the last decade, there has been a shift toward more conservative management in low-risk prostate cancer, in order to minimize unnecessary intervention. This study aimed to evaluate the number of low-risk radical prostatectomies (RPs) being performed at the Southern Alberta Institute of Urology over a 10-year period.
Methods
We retrospectively reviewed all patients who underwent RP from 2005 to 2014 at our institution. Patients were stratified by D’Amico risk classification and grade group based on 12-core transrectal ultrasound–guided biopsy (TRUS-bx) results. RP findings are reported from February 2005 to October 2014 to describe concordance between TRUS-bx and RPs. Basic descriptive analyses were used for this study.
Results
Over the study period, 2,310 RPs were performed in our institution. Overall, 35.2% of these were performed on men with low-risk prostate cancer. From 2005 to 2014, the proportion of RPs performed for low-risk prostate cancer dropped from 54.0% to 8.9%, and 49.8% of patients who underwent RP for low-risk disease experienced pathologic upgrading, though only 3.8% were upgraded to grade group 3 or greater. Other adverse pathological findings were uniformly low among the low-risk group.
Conclusion
The proportion of patients undergoing RP at our center for low-risk prostate cancer decreased significantly over the 10 years evaluated in this study, reflecting current global trends toward active surveillance in the management of low-risk prostate cancer.
Introduction
Prostate cancer is the most common cancer among men, accounting for 20.7% of the projected 103,100 cancer diagnoses in Canadian men in 2017.Citation1 About one in six men in North America will be diagnosed with prostate cancer in their lifetime, and 80% of men over the age of 80 years have at least microfocal disease.Citation2 Despite its high incidence, the lifetime risk of dying of prostate cancer in North America remains low, at only 2.5%.Citation2 This discrepancy is due to the fact that low-risk prostate cancer accounts for >50% of new diagnoses.Citation2 Prostate cancer is often a relatively indolent disease associated with slow progression, and many affected individuals ultimately die with the disease and not of it.Citation3 Standard interventions for prostate cancer, including radical prostatectomy (RP) and radiotherapy, are associated with significant adverse effects on the sexual, urinary, and bowel function of the patient.Citation2
Over the last 15 years, there has been a widespread shift toward more conservative management of low-risk prostate cancer. One of the foundations of this new paradigm is the concept of active surveillance (AS), in which treatment is deferred in men with low-risk prostate cancer diagnoses until there is evidence of disease progression. Though there are no standardized and uniformly accepted surveillance protocols, monitoring consists of serial prostate-specific antigen (PSA) tests, digital rectal exams, and prostate biopsies.
Numerous prospective randomized trials have been undertaken comparing outcomes of observation or AS vs curative treatment in patients with localized prostate cancer. PIVOT randomized men with localized prostate cancer to either RP or watchful waiting.Citation4 This study found no significant difference in all-cause or prostate-specific mortality between the two arms, especially in men with low-risk disease, and the recently published update with additional follow-up maintained these findings.Citation4,Citation5 The PROTECT trial compared AS to RP and radiotherapy in men with clinically localized prostate cancer. Similarly to PIVOT, no significant difference was observed in all-cause or prostate-specific mortality among the three groups.Citation6 The results of both these prospective randomized trials indicated that men with low-risk prostate cancer can safely avoid the risks of early radical intervention.
Given the growing body of evidence supporting the safety of conservative management for low-risk prostate cancer, its adoption has increased significantly in North America over the past decade and a half. Studies examining large patient cohorts within the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database have found significant increases in the proportion of low-risk and very low-risk prostate cancer patients undergoing noncurative initial management, as opposed to curative intervention.Citation7,Citation8
The PSA era has led to overdiagnosis of prostate cancer, due to the widespread use of prostate needle-core biopsies, which increased detection of “insignificant prostate cancer” representing low-volume/low-grade disease.Citation9 A disease that would have previously gone undiagnosed has become increasingly recognized and treated, often at the expense of patients’ health and quality of life. Therefore, AS has become a clinical necessity and well-accepted and widely utilized management option aimed at mitigating the overtreatment of low-risk prostate cancer. Indeed, Cancer Care Ontario guidelines considered AS the recommended disease-management strategy for most patients with low-risk disease.Citation10 More recently, a 2018 European Association of Urology position article stated that AS should be considered for all men with low-risk prostate cancer who are fit for curative treatment and willing to adhere to the protocol.Citation11 We undertook an institutional retrospective study to assess RP trends over the past decade, evaluate our institutional risk-group stratification, and in particular the proportion of low-risk prostate cancer patients who underwent RP during the study period.
Methods
Full ethics approval was obtained from the Conjoint Health Research Ethics Board at the University of Calgary. Given that this study was retrospective in nature using deidentified patient information, patient consent for review of medical records was not required. Maintenance of patient-data confidentiality was in accordance with the Declaration of Helsinki. We retrospectively reviewed all patients who underwent RP from February 2005 to October 2014 at our institution. The first major revision of the Gleason grading system for prostatic carcinoma occurred in 2005 at an International Society of Urological Pathology Gleason consensus conference. We used the last transrectal ultrasound–guided biopsy (TRUS-bx) prior to surgical intervention for the analysis. TRUS-bx was performed using a standardized template, which typically included 12 cores sampled from the apex, mid, and base, bilaterally. RPs were also completely sampled. Both TRUS-bx and RPs were analyzed in a centralized genitourinary pathology setting and reported using standardized protocols. Patients were stratified according to TRUS-bx D’Amico risk classifications: low risk, clinical T1c-T2a and PSA <10 and Gleason score 6; intermediate risk, clinical T2b or PSA 10–20 or Gleason score 7; high risk, clinical ≥T2c or PSA ≥20 or Gleason score 8–10. We report the annual number of RPs performed at our institution stratified by TRUS-bx D’Amico risk classification from 2005 to 2014, as well as by grade group. RP findings are reported from February 2005 to October 2014 to describe concordance between TRUS-bx and RP. Basic descriptive analysis was used for this study.
Results
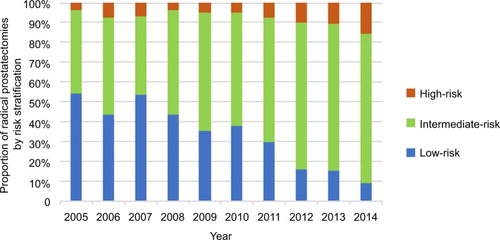
shows the number of RPs performed between 2005 and 2014, stratified by risk category at biopsy. Over the 10-year period, 35.2% of all RPs were performed on men with low-risk prostate cancer. However, there was a downward trend, with procedures for low-risk prostate cancer decreasing from 54.0% of all RPs in 2005 to 8.9% in 2014, as shown in and .
Table 1 Radical prostatectomies performed at our institution 2005–2014 stratified by D’Amico risk classification
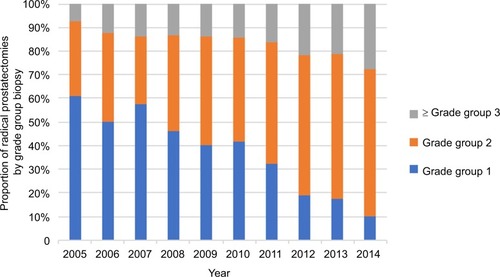
There was a corresponding upward trend in the proportion of procedures performed on intermediate-risk patients, increasing from 42.0% of the total in 2005 to 75.6% in 2014 (). High-risk patients also accounted for an increased proportion of RPs, up from 4.0% in 2005 to 15.6% in 2014 (). demonstrates the pathological grade-group migration that has taken place over the 10-year period. Grade group 1 on biopsy accounted for 60.8% of RPs in 2005 compared to 10.0% in 2014. Conversely, clinically significant prostate cancer (≥ grade group 2) on biopsy increased from 39.2% to 90.0% of RPs.
shows pathological findings on RP, stratified by risk category at biopsy. Of note, although 405 (49.8%) patients who underwent RP for low-risk prostate cancer had upgrading of their disease on final pathology, 374 (46.0%) of these were upgraded to favorable intermediate (grade group 2), with only 3.8% upgraded to grade group 3 or greater. Overall, in the low-risk patient group, 7.6% of patients were stage pT3 (6.6% pT3a and 1.0% pT3b). Of the 543 low-risk RP patients in whom lymph nodes were assessed, only two (0.4%) had nodal disease. Lymphovascular invasion was present in 1.8% of the low-risk group.
Table 2 Radical prostatectomy pathological outcomes stratified by transrectal ultrasound–guided biopsy results
Discussion
We found a clear downward trend in the proportion of RPs performed on men with low-risk prostate in our center (54% in 2005 compared to 8.9% in 2014). This is similar to the overall trends observed in North America and Europe, which show decreasing rates of curative intervention for men with low-risk prostate cancer. One European multicenter study found that the percentage of patients who had undergone RP and were subsequently found to have localized Gleason 6 disease decreased from 46% in 2000 to 8% in 2015.Citation12 Similarly, a recent study comparing RP trends at Copenhagen University Hospital and Stanford University Hospital found that the proportion of RPs performed on low-risk prostate cancer patients decreased between 2000 and 2013 in both institutions (26.5%–16.6% in Denmark and 41.5%–27.9% at Stanford).Citation13 Finally, a recent large study involving over 125,000 Veterans’ Affairs patients with low-risk prostate cancer in the US found that radical management in men >65 years of age decreased from 65% to 21% between 2005 and 2015.Citation14
The decreasing rates of definitive intervention for men with low-risk prostate cancer has been offset by the widespread adoption of AS. An analysis of very low-risk prostate cancer patients in the NCDB found that among patients eligible for AS, the percentage of those enrolled in AS increased from 11.6% in 2010 to 27.3% in 2013.Citation8 Another study looking at both the SEER database and the NCDB found that noncurative initial management (watchful waiting or AS) rose from 21% to 32% in SEER and 13% to 20% in NCDB for patients with low-risk prostate cancer.Citation7 Finally, the Veterans’ Affairs study previously mentioned largely attributed the decrease in radical treatment for low-risk prostate cancer to significant uptake of AS, with AS rates increasing from 3% to 41% in men aged >65 years from 2005 to 2015 and from 4% to 39% in men <65 years of age.Citation14
It is however evident that although AS rates have been increasing, this still represents an underutilized tool in the management of low-risk prostate cancer.Citation15 The Veterans’ Affairs study highlighted that AS rates in Europe remain considerably higher than in the US: as high as 74% in Sweden in 2014.Citation14 Because our data were limited to patients who had undergone RP and did not include other management options, we cannot directly comment on our institutional rate of AS. However, the observed downward trend of the proportion of RPs performed in low-risk patients in our institution follows the global trend of noncurative management for patients with low-risk disease. The fact that that almost 10% of all RPs performed at our institution were done in patients with low-risk disease at the conclusion of the study period may also indicate that there is still room to increase further the number of patients managed by AS. This is further supported by the fact that >50% of the low-risk patients who underwent RP were not upgraded on subsequent pathology of the RP specimen. Furthermore, adverse findings on final pathology, namely pT3 disease, positive node status, and lymphovascular invasion, were uniformly low in the low-risk group. Many of these patients would thus have likely been good candidates for AS.
There are a number of potential barriers that may potentially inhibit further increases of watchful waiting or AS for low-risk prostate cancer and maintain the persistence of definitive treatment of this generally indolent type of disease. The main obstacle is certainly the risk of disease progression that is expected in situations of conservative cancer management. The extended follow-up of PIVOT found that disease progression was more common among men assigned to observation than those assigned to surgery.Citation5 Disease progression of all types occurred in 68.4% of observation patients vs 40.9% of surgery patients. Patients under observation also had a 4.5% higher absolute risk of metastasis than surgery patients.Citation5 Moreover, this disparity is likely underrepresenta-tive of the true difference, because of the significant crossover between the study arms: 21% of patients in the surgery arm had never had an RP, and 20% of patients in the observation group ultimately received definitive treatment.Citation5 AS was also shown to be associated with higher rates of disease progression in the PROTECT trial, which showed that the incidence of overall clinical progression and metastatic disease was significantly higher in the AS group (22.9 vs 8.9 per 1,000 person-years for overall clinical progression, and 6.3 vs 2.4 per 1,000 person-years for metastatic disease).Citation6 Therefore, interventions for low-risk prostate cancer, whether by RP or radiotherapy, are inevitably associated with lower rates of disease progression. Nonetheless, the higher disease-progression rates seen with conservative management in both the PIVOT and PROTECT trials did not translate into higher mortality. In PROTECT, there was no significant difference in prostate cancer–specific mortality among the AS, RP, and radiotherapy groups (10-year survival of 98.8%, 99.0%, and 99.6%, respectively).Citation6 Similarly, there was no significant difference in all-cause mortality among these same groups (10.9, 10.1, and 10.3 deaths per 1,000 person-years, respectively).Citation6 These findings are corroborated by the two largest prospective AS cohorts (Sunnybrook Health Sciences Center and Johns Hopkins University). Only 0.15% of patients in the Johns Hopkins series had died of prostate cancer at 15 years, and the cancer-specific survival rate of the Sunnybrook cohort was 98% at 10 years.Citation11
Despite the higher rate of disease progression in AS compared to intervention, there is no associated increased risk of death. However, when assessing barriers to conservative management of low-risk prostate cancer, it is also as important to examine patients’ perception of risk as it is to examine the actual risk. Ultimately, it is the patient who decides how their disease will be managed. Indeed, it has been shown that there is significant discrepancy between perceived and actual risk associated with different modalities of prostate cancer treatment. One study showed that patients who had undergone RP estimated the mortality risk of AS at 50.9% compared to 17.8% for RP.Citation17 These results demonstrate that failure in patient education remains one of the major barriers in the uptake of AS. The psychological impact of the conservative management of prostate cancer compared to active intervention represents an additional patient-related barrier to AS. It is reasonable to suspect that patients who are living with cancer will have increased anxiety compared to those who have undergone curative treatment. Indeed, there were early reports of increased levels of anxiety and depression in patients on watchful waiting,Citation18 as well as of patients foregoing watchful waiting because of fear of possible consequences.Citation19 However, two recent systematic reviews found that AS posed no greater risk of negative psychological impact than active treatment.Citation20,Citation21 Moreover, patient regret has been reported to be increased in patients undergoing RP and radiotherapy compared to watchful waiting (15.0%, 16.6%, and 8.2%, respectively).Citation22 AS, however, has not been shown to be associated with increased regret compared to intervention.Citation23 Overall, patients with prostate cancer considering AS should be advised that they would not be at increased risk of negative psychological impact.
There are certain clinical scenarios in which patients with low-risk prostate cancer may be appropriately managed with definitive therapy, such as RP. This includes patients with low-risk prostate cancer who suffer from severe lower-urinary-tract symptoms secondary to bladder-outlet obstruction. Additionally, some patients with low-risk prostate cancer on AS who are found to have a Prostate Imaging Reporting and Data System 5 lesion on multiparametric magnetic resonance imaging (mpMRI) may wish to proceed straight to RP before a confirmatory biopsy, given the high probability of clinically significant prostate cancer and the risks and discomfort of TRUS-bx. Though we strongly feel that pathological confirmation is a diagnostic gold standard and should be recommended in all instances prior to surgery, an RP in these patients may be considered. Finally, advances in robotic nerve-sparing RP have led to improved functional outcomes while maintaining oncological control in properly selected patients.Citation16 However, despite the reduced morbidity of these techniques, surgery is never without risk. Regardless of the surgical technique and degree of nerve sparing, RP carries the risk of significant consequences, both functional (eg, incontinence, erectile dysfunction) and perioperative (eg, deep-vein thrombosis, pulmonary embolism, myocardial infection). Therefore, for the majority of patients with low-risk prostate cancer, the risk of surgery outweighs the potential benefit, even using novel nerve-sparing techniques.
In order for AS to be fully utilized, strategies and techniques must be developed to overcome barriers to its uptake. Regarding the risk of clinical progression, technologies are being studied in order to mitigate the increased risk in AS compared to curative treatment. One such tool is mpMRI, which is being increasingly used in the diagnosis and surveillance of patients with prostate cancer. mpMRI–TRUS fusion biopsy has been shown to offer increased detection of intermediate- and high-risk disease than standard 12-core TRUS-bx.Citation24,Citation25 Furthermore, novel biomarkers in addition to the traditional total PSA also show promise for improved patient assessment for AS candidacy. One example is the 4K Panel, a panel of four kallikreins: total PSA, free PSA, intact PSA, and human kallikrein 2. This panel has been shown to provide improved prediction of high-grade prostate cancer, and can also accurately reclassify patients already on AS.Citation26,Citation27 These new technologies can help ensure that patients with clinically significant prostate cancer are not missed while on AS. It is also worth noting that although widespread support for AS has generally been restricted to the low-risk prostate cancer patient population, there is emerging evidence to support use of AS for the management of favorable intermediate-risk prostate cancer. For example, a recent study found that patients on AS with intermediate-risk prostate cancer with Gleason 6 disease had similar metastasis-free survival compared to those with low-risk prostate cancer.Citation28 Another recent review article laid out comprehensive guidelines for assessing intermediate-risk patients that should be deemed eligible for AS, including <10%–20% Gleason 4 pattern on biopsy, low PSA density, and favorable molecular markers, if available.Citation29
Conclusion
The proportion of patients undergoing RP at our institution who had low-risk prostate cancer decreased dramatically over the study period, likely due to an increase in the uptake of AS in this patient population. Further evaluation of the rates of AS and barriers to its uptake should be undertaken, in order further to reduce the unnecessary treatment of patients with low-risk prostate cancer.
Acknowledgments
The abstract of this study was presented at the 2018 CUA Annual Meeting.
Disclosure
The authors report no conflicts of interest in this work.
References
- Canadian Cancer SocietyCanadian Cancer statistics 2017Can Cancer Soc 201720171132
- KlotzLActive surveillance: patient selectionCurr Opin Urol201323323924423548978
- GaristoJDKlotzLActive surveillance for prostate cancer: how to do it right | cancer network | the Oncology JournalOncology2017 Available from: http://www.cancernetwork.com/oncology-journal/active-surveillance-prostate-cancer-how-do-it-rightAccessed October 27, 2017
- WiltTJBrawerMKJonesKMRadical prostatectomy versus observation for localized prostate cancerN Engl J Med2012367320321322808955
- WiltTJJonesKMBarryMJFollow-up of prostatectomy versus observation for early prostate cancerN Engl J Med2017377213214228700844
- HamdyFCDonovanJLLaneJA10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancerN Engl J Med2016375151415142427626136
- WeinerABPatelSGEtzioniREggenerSENational trends in the management of low and intermediate risk prostate cancer in the United StatesJ Urol201519319510225106900
- ParikhRRKimSSteinMNHafftyBGKimIYGoyalSTrends in active surveillance for very low-risk prostate cancer: do guidelines influence modern practice?Cancer Med20176102410241828925011
- TrpkovKYilmazABismarTAMontironiR“Insignificant” prostate cancer on prostatectomy and cystoprostatectomy: variation on a theme “low-volume/low-grade” prostate cancer?BJU Int2010106330431520653654
- ChenRCRumbleRBLoblawDAActive surveillance for the management of localized prostate cancer (Cancer care Ontario guideline): American Society of clinical oncology clinical practice guideline endorsementJCO2016341821822190
- BrigantiAFossatiNCattoJWFPosition paper active surveillance for low-risk prostate cancer: the European Association of Urology position in 2018Eur Urol201874335736829937198
- van den BerghRGandagliaGTilkiDTrends in radical prostatectomy risk group distribution in a european multicenter analysis of 28 572 patients: towards tailored treatmentEur Urol Focus Epub201788
- LoftMDBergKDKjaerATemporal trends in clinical and pathological characteristics for men undergoing radical prostatectomy between 1995 and 2013 at Rigshospitalet, Copenhagen, Denmark, and Stanford University Hospital, United StatesClin Genitourin Cancer201711227554586
- LoebSByrneNMakarovDVLeporHWalterDUse of conservative management for low-risk prostate cancer in the Veterans Affairs integrated health care system from 2005–2015JAMA201831921223129800017
- HoffmanKENiuJShenYPhysician variation in management of low-risk prostate cancerJAMA Intern Med201417491450145925023650
- MartiniACumarasamySHainesKGIIITewariAKAn updated approach to incremental nerve sparing for robot-assisted radical prostatectomyBJU Int Epub20181221
- KendelFHelbigLNeumannKPatients’ perceptions of mortality risk for localized prostate cancer vary markedly depending on their treatment strategyInt J Cancer2016139474975327038059
- DaleWBilirPHanMMeltzerDThe role of anxiety in prostate carcinoma: a structured review of the literatureCancer2005104346747815959911
- HolmboeESConcatoJTreatment decisions for localized prostate cancer: asking men what’s importantJ Gen Intern Med2000151069470111089712
- BellarditaLValdagniRvan den BerghRHow does active surveillance for prostate cancer affect quality of life? A systematic reviewEur Urol201567463764525454617
- CarterGCloverKBrittonBWellbeing during active surveillance for localised prostate cancer: a systematic review of psychological morbidity and quality of lifeCancer Treat Rev2015411466025467109
- HoffmanRMLoMClarkJATreatment decision regret among long-term survivors of localized prostate cancer: results from the prostate cancer outcomes studyJ Clin Oncol201735202306231428493812
- HurwitzLMCullenJKimDJLongitudinal regret after treatment for low- and intermediate-risk prostate cancerCancer2017123214252425828678408
- SiddiquiMMRais-BahramiSTurkbeyBComparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancerJAMA2015313439039725626035
- ElkjærMCAndersenMHHøyerSPedersenBGBorreMProstate cancer: in-bore magnetic resonance guided biopsies at active surveillance inclusion improve selection of patients for active treatmentActa Radiol201859561962628747132
- ParekhDJPunnenSSjobergDDA multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancerEur Urol201568346447025454615
- LinDWNewcombLFBrownMDEvaluating the four kallikrein panel of the 4Kscore for prediction of high-grade prostate cancer in men in the Canary prostate active surveillance studyEur Urol201772344845427889277
- MusunuruHBYamamotoTKlotzLActive surveillance for intermediate risk prostate cancer: survival outcomes in the Sunnybrook experienceJ Urol201619661651165827569437
- KlotzLActive surveillance for intermediate risk prostate cancerCurr Urol Rep201718108028801885