Abstract
Benign prostatic hyperplasia and prostate cancer remain the most prevalent urologic health concerns affecting elderly men in their lifetime. Only 20% of benign prostatic hyperplasia and prostate cancer cases coexist in the same zone of the prostate and require a long time for initiation and progression. While the pathogenesis of both diseases is not fully understood, benign prostatic hyperplasia and prostate cancer are thought to have a multifactorial etiology, their incidence and prevalence are indeed affected by age and hormones, and they are associated with chronic prostatic inflammation. At least 20% of all human malignancies arise in a tissue microenvironment dominated by chronic or recurrent inflammation. In prostate malignancy, chronic inflammation is an extremely common histopathologic finding; its origin remains a subject of debate and may in fact be multifactorial. Emerging insights suggest that prostate epithelium damage potentially inflicted by multiple environmental factors such as infectious agents, dietary carcinogens, and hormones triggers procarcinogenic inflammatory processes and promotes cell transformation and disease development. Also, the coincidence of chronic inflammation and tumorigenesis in the peripheral zone has recently been linked by studies identifying so-called proliferative inflammatory atrophy as a possible precursor of prostatic intraepithelial neoplasia and prostate cancer. This paper will discuss the available evidence suggesting that chronic inflammation may be involved in the development and progression of chronic prostatic disease, although a direct causal role for chronic inflammation or infection in prostatic carcinogenesis has yet to be established in humans. Further basic and clinical research in the area, trying to understand the etiology of prostatic inflammation and its signaling pathway may help to identify new therapeutic targets and novel preventive strategies for reducing the risk of developing benign and malignant tumors of the prostate.
Background
In spite of progress in diagnosis and treatment, benign prostatic hyperplasia (BPH) and prostate cancer (CaP) remain the two main prostate pathologies and the two of the most prevalent urologic health concerns affecting men during their lifetime.Citation1
BPH is the most frequent benign neoplasm in aging males and one of the most common chronic conditions in the male population, with a histological prevalence at autopsy of 50% in men aged 50–60 years and of 90% over 80 years old.Citation2 As most chronic diseases, BPH is progressive. If untreated, it often complicates with bladder dysfunction and hypertrophy, possibly leading to acute urinary retention.Citation3–Citation5
CaP is currently the most common nonskin neoplasm and the second leading cause of death among men in the United States and many Western industrialized countries.Citation6 CaP is predominantly a disease in men over 40 years of age and its incidence increases steeply in the seventh decade of life. In 2012, an estimated 241,740 men will be diagnosed with CaP, and it is estimated that 28,170 deaths due to CaP will occur.Citation6 Widespread screenings for prostate-specific antigen, digital rectal examination, and needle biopsy, as well as standard treatment already in clinical use, have enhanced patients’ survival by improving detection of early and localized disease. However, there is still no cure for the advanced and metastatic disease.
Both BPH and CaP are considered chronic diseases with early initiation and slow progression. They require a period of time before they evolve from earlier tissue alterations to clinical onset.Citation7 Both diseases arise in different areas of the prostate, with BPH known to develop in the transitional and the central zones and CaP in the peripheral zone.Citation8 In only approximately 20% of clinical cases, both entities (BPH and CaP) coexist in the same zone.Citation8–Citation10 Although the diseases are not thought to be linked in their etiology, epidemiologic studies have shown that the incidence and prevalence of both diseases rise with increased age, and both conditions are hormone dependent and are associated with prostatic inflammation.Citation11,Citation12
The link between inflammation and carcinogenesis
In the last decade, advanced cancer research has pointed out several cancer-causing factors including infection and inflammation.Citation13–Citation16 The role of infection/inflammation in the initiation and progression of cancer has been an area of intense scientific interest and is usually considered from the perspective that persistent inflammation in the context of chronic infection or tissue injury might promote cell transformation through DNA damage or that tumor cells produce proinflammatory factors that derive chronic inflammation and tumor growth.Citation15,Citation16 Several epidemiologic studies have shown that chronic inflammation secondary to infectious agents, to the exposure of other environmental factors, or to a combination of both is involved in the pathogenesis of about 20% of human cancers, including stomach (Helicobacter pylori), liver (Hepatitis B and C viruses), and colon cancer in patients with inflammatory bowel diseases.Citation17,Citation18 Furthermore, epidemiologic, histopathologic, and molecular pathologic studies suggest that chronic inflammation may be involved in the development and progression of chronic prostatic disease, such BPH and CaP,Citation11–Citation16 although a direct causal role for chronic inflammation or infection in prostatic carcinogenesis has yet to be confirmed and elucidated in humans.
The definition of inflammation (acute and chronic)
Inflammation is a fundamental physiological process that can arise in any tissue in response to traumatic, infectious, post-ischemic, toxic, or autoimmune injury. In the setting of tissue damage resulting from microbial pathogen infection or other noxious stimuli, these processes lead to eradication of pathogens, clearing of debris, epithelial regeneration, stromal remodeling, and vascularization to heal the wound and restore the normal tissue function. Once the repair is completed, the inflammatory reaction typically subsides. However, if targeted destruction and assisted repair are not properly phased, the immune system becomes deregulated and the infection persists. Inflammation becomes chronic due to persistence of the initiating factors (microbial pathogens or other noxious stimuli) and to a failure of mechanisms required for resolving the inflammatory response. Thus, the chronic inflammation promotes, whether directly or indirectly, an increase in cell proliferation, an enhancement of inflammatory cell recruitment, and excessive production of reactive oxygen and nitrogen species and active proteolytic enzymes, leading to oxidative DNA damage and reduced DNA repair. A microenvironment constituted by all the above inhabits the sustained cell proliferation induced by continued tissue damage, thus predisposing chronic inflammation to neoplasia and malignant transformation.Citation19
Prostatic inflammation
Histologically, the presence of chronic prostatic inflammation is a well-known finding in biopsy and surgical specimens of prostate tissue in patients with or without lower urinary tract symptoms or prostatitis.Citation20,Citation21
Prostatic inflammation, inflammatory immune cells, and BPH
In clinical BPH patients, the most common type of inflammation found is a mild chronic inflammation. Its severity is associated with age and prostate volume in 78% of BPH cases,Citation22–Citation24 and defined by the presence of chronic inflammatory infiltrates composed of T- and B-lymphocytic cells and macrophages.Citation16 Recently, a clinical study done in 282 BPH patients confirmed the nature of the chronic inflammatory infiltrate, which was constituted by cluster of differentiation-3+ (CD3+) T-lymphocytes in 80% of the cases and associated with 52% of B-lymphocytes (CD20+ cells) and 82% of macrophages (CD163+ cells).Citation25 Finally, De Marzo et al described a discrete foci of proliferative glandular epithelium with an appearance of simple atrophy or postatrophic hyperplasia, which occurs in areas associated with chronic inflammation. The key features of this prostatic proliferative lesion are the presence of two distinct cell layers of mononuclear and/or polymorphonuclear inflammatory cells found in both epithelial and stromal compartments and stromal atrophy with a variable amount of fibrosis.Citation26
Prostatic inflammation and CaP
In CaP, chronic inflammation is increasingly discussed as a critical component of tumor carcinogenesis by generating a pathologically conducive microenvironment that may favor the initiation and progression of cancer.Citation16 It is considered as a potential risk factor for many human malignancies including the prostate. Chronic inflammation induces cellular and genomic damage and promotes cellular turnover associated with a sustained inflammatory microenvironment that provides a constant supply of a variety of reactive nitrogen and oxygen species, reactive aldehydes, cytokines, chemokines, and growth factors, which can alter crucial biological processes responsible for maintaining normal cellular homeostasis, leading to uncontrolled proliferative response and genomic instability and risk of CaP development.Citation27–Citation29 Also, the high prevalence of chronic prostatic inflammation and the inflammatory infiltrates found in pathological samples of the prostate isolated from radical prostatectomy specimens, prostate core biopsy, and transurethral prostate specimens has suggested a possible link between chronic inflammation and CaP.Citation30,Citation31 However, it is still unclear whether or not the same population of inflammatory infiltrates promotes both BPH and CaP.
The origin of prostatic inflammation
The etiology of chronic prostatic inflammation remains a large subject of debate. Multiple potential sources exist and include direct infection, urine reflux or corpora amylacea, dietary factors, and hormones, or a combination of two or more of these factors.Citation16
Infectious agents
Many epidemiologic studies have shown that different pathogens including bacteria and viruses could infect and induce an inflammatory response in the prostate.Citation32 These pathogens include sexually transmitted organisms, such as Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, and Treponema pallidum, and nonsexually transmitted bacteria primarily Gram-negative organisms, such as Escherichia coli, known to cause acute and chronic bacterial prostatitis.Citation33–Citation39 These organisms induce severe prostatic inflammation and prostatic abscesses if not treated with antibiotics on time, ie, before they reach the prostate. Also, many viruses such as human herpes simplex virus type-2, human papillomavirus, human cytomegalovirus, and human herpes virus type-8 have been detected in the prostate,Citation40–Citation42 but it still is not clearly known how often these agents infect the prostate and whether or not they elicit an inflammatory response leading to inflammatory lesions in the prostate.
Urine reflux, physical, and chemical trauma
Another etiologic factor involved in the chronic prostatic inflammation is the chemical irritation caused by urine reflux.Citation43 Urine contains many chemical compounds including uric acid that might be very toxic and particularly damaging to the prostate epithelium.Citation44,Citation45 In support of this, a recent work has implicated crystalline uric acid as a “danger signal” released from dying cells for its ability to directly engage the caspase-1-activating cryopyrin (NACHT, leucine-rich repeat, and pyrin domains-containing protein-3) present in the innate immune cells, primarily macrophages. The consequence of this process results in the production of many inflammatory cytokines that can increase the influx of many other inflammatory cells.Citation45 Also, urine reflux in conjunction with infectious agents can function together to increase the severity and the intensity of chronic inflammation in the prostate. In addition, the development of corpora amylacea in the prostate is considered another resource of prostatic inflammation since they are frequently adjacent to the damaged prostatic epithelium and focal inflammatory infiltrates.Citation46
Dietary factors
In addition, epidemiologic studies revealed a link between dietary factors and CaP incidence and mortality.Citation47,Citation48 Long-term exposure to dietary 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine results in prostate carcinomas in male rats, and could induce prostatic inflammation and atrophy before these lesions evolve into prostatic intraepithelial neoplasia (PIN) and cancers.Citation48,Citation49
Hormones
Moreover, hormonal alterations such as estrogen exposure affect the growth and development of the prostate through indirect routes on the hypothalamic–pituitary–gonadal axis or by direct effects mediated by estrogen receptor-α/β, which are primarily expressed by stromal and epithelial cells, respectively.Citation50–Citation54 For example, estrogens given to neonatal rodents induce an “imprinted state,” resulting in a reduction of prostatic growth. This treatment also results in the development of lobe-specific inflammation, hyperplasia, and dysplasia or PIN mediated virtually through estrogen receptor-α.Citation55,Citation56
Immune tolerance
Finally, another potential mechanism of self-perpetuating chronic inflammation in the prostate secondary to all the above-mentioned modes of prostate injury is that damaged prostate epithelial cells might release some antigens that result in a break of the immune tolerance to the prostate.Citation57 Many of these prostatic antigens are not expressed until after puberty when the gland will undergo androgen-stimulated growth and development. This phenomenon is likely to result in a lack of physiological immune tolerance to these antigens. Therefore, when released during the prostate injury, these antigens could prime an immune response resulting in a specific reaction to prostate-restricted antigens. For example, a T-cell immune response to prostate-specific antigen in patients with chronic prostatitis has been reported.Citation57
Taken together all the factors listed above, one or multiple factors in combination induce a chronic epithelial injury that may decrease the barrier function and facilitate the growth of multiple infectious agents, with a chain reaction that further sustains and stimulates the inflammatory response and increases the prostatic inflammatory infiltrates. Whether or not the chronic inflammation has a cause-and-effect relationship with BPH and CaP remains unknown.
BPH and inflammatory mediators
In BPH, chronic inflammation may cause cytokine release from the inflammatory cells and prostatic tissue injury induced by the increase of oxygen demand of prostatic proliferating cells.Citation58,Citation59 Also, cytokines, growth factors, and inflammatory mediators released by the inflammatory cells may interact not only with the immune effectors cells but also with the stromal and epithelial prostatic cells, resulting in a prostatic tissue injury.Citation60 Multiple investigations done in this area have shown that lymphocyte-derived growth factors impact the prostatic stromal cell growth.Citation61 These inflammatory infiltrates are chronically activated and responsible for the release of cytokines, mostly interleukin-2 (IL-2), interferon-γ, and tumor growth factor-β (TGF-β), which may support the fibromuscular growth in BPH.Citation58,Citation59 Once initiated, dendritic cells induce, sustain, and regulate the infiltrates’ T-cell responses and their activities contribute to the maintenance and progression of immune inflammatory infiltrates in the aging prostatic tissue.Citation61,Citation62
During chronic inflammation observed in BPH tissue, an upregulation of different proinflammatory cytokines has also been reported including IL-15 and interferon-γ in stromal cells, IL-17 in infiltrating T-cells, and IL-8 in epithelial cells.Citation58–Citation61 These proinflammatory cytokines, more specifically IL-17, released by adjacent inflammatory cells induce cyclooxygenase-2 (COX-2) expression in the BPH epithelial cells and is associated with an increased proliferative rate (growth and survival of prostatic cells).Citation63 IL-17 in conjunction with functional IL-23, a heterodimeric protein produced by activated dendritic cells, monocytes, and macrophages, and through the IL-17/IL-23 pathway promotes the inflammation response in the prostatic tissue.Citation64–Citation67
In BPH stromal cells, Penna et al recently showed that both interferon-y and IL-17, produced by the activated alloantigen-specific CD4+ T-cells, induce the production of both IL-6 (a potent autocrine growth factor) and IL-8 (a paracrine inducer of fibroblast growth factor-2), which are the key growth factors of epithelial and stromal prostate cells.Citation68 These results are consistent with a possible link between the T-cell autoimmune response induced by stromal prostatic cells and prostate hyperproliferation.Citation68 Furthermore, it has been shown that TGF-β regulates stromal cell proliferation and differentiation in BPH and it is a key factor for androgen control of prostatic growth.Citation61,Citation69
Another source of inflammatory mediators is the local hypoxia which induces low levels of reactive oxygen species, which in turn promotes neovascularization and angiogenesis. Also, as a response to hypoxia, prostatic stromal cells upregulate the secretion of multiple vascular endothelial growth factors such as fibroblast growth factor-2, fibroblast growth factor-7, TGF-β, and IL-8 that can determine the prostatic growth rate.Citation58,Citation67
Although there is still no evidence of a causal relationship between chronic inflammation and BPH, the idea that prostatic inflammation may play an important role in BPH development and progression is intriguing. The inflammatory infiltrate-mediated T-cell activity results in stimulation of stromal and epithelial cell proliferation that is sustained by an autoimmune mechanism and the prostatic tissue injury. The subsequent chronic process of repetitive wound healing induced by chronic inflammation ends up by evolving the simple micronodular hyperplasia into a macroscopic nodular enlargement that gradually translates and progresses into the clinical entity of BPH nodules.
The association between CaP and chronic prostatic inflammation
In CaP, several key observations have supported the postulated relationship between chronic inflammation and prostate carcinogenesis.
Sexual transmitted diseases and CaP
Epidemiologic studies have shown, perhaps due to inflammation-induced oncogenesis, that sexually transmitted diseases play a role in the initiation and progression of CaP.Citation70,Citation71 An increased risk of CaP among patients with a history of clinical or symptomatic prostatitis has been reported.Citation72–Citation75 Also, several meta-analyses have demonstrated an increased risk of CaP among patients affected with syphilis, gonorrhea, and human papillomavirus infection.Citation76,Citation77 Finally, other indicators including number of sexual partners, age of first intercourse, sexual behavior, and frequency of sex have been reported with CaP risk.Citation76–Citation80
Cytokine network and CaP
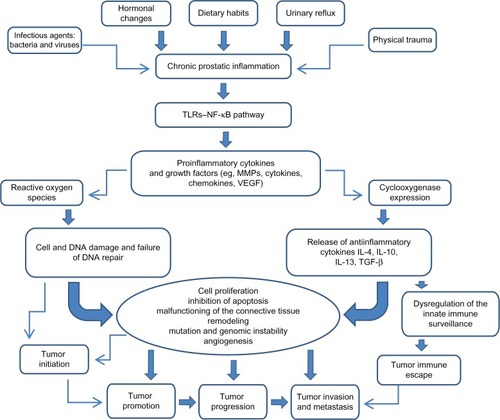
The cytokine network () including proinflammatory and anti-inflammatory cytokines is very useful not only for assessment of prostatic inflammation but also for early cancer detection and prognosis.Citation81,Citation82
Figure 1 Impact of chronic prostatic inflammation and inflammatory mediators on tumor initiation and progression.
Abbreviations: IL, interleukin; MMP, matrix metalloproteinase; NF-κB, nuclear factor-κB; TGF-β, transforming growth factor-β; TLR, toll-like receptor; VEGF, vascular endothelial growth factor.
IL-1
IL-1 is a proinflammatory cytokine that promotes the growth and progression of several solid tumors.Citation83 IL-1β is required but not sufficient for metastasis of both B16 melanoma cells and human CaP cells in vivo, whereas IL-1α is required for angiogenesis in a model of mammary carcinoma.Citation83
IL-6
Another proinflammatory cytokine IL-6, involved in the crosstalk between CaP cells and inflammatory cells, promotes the malignant processes and induces apoptosis and angiogenesis.Citation84–Citation86 Also, it has been shown that IL-6 enhances cell proliferation and acts as a survival molecule for many prostate tumor cell lines such as PC3, LNCaP, and DU145.Citation16,Citation26 Clinically, elevated levels of IL-6 were found in the serum of patients with CaP metastatic disease, which was associated with poor disease prognosis.Citation87
IL-17
IL-17, secreted by CD4 T-cells, promotes the migration of endothelial cells and induces fibroblasts to upregulate proangiogenic factors such as vascular endothelial growth factor, macrophage inflammatory protein-2, prostaglandins, and nitric oxide involved in angiogenesis and in vivo growth of tumor cells.Citation88 Furthermore, Steiner et al have shown that 58% of human malignant prostate tissues have an increased level of IL-17 messenger ribonucleic acid and both prostate tumor cells and prostate stromal cells treated with IL-17 in vitro have an increase in messenger ribonucleic acid and protein expression of both IL-6 and IL-8.Citation64 These data suggest that IL-17 acts directly on the prostate tumor cells and promotes their growth and metastasis, or indirectly by increasing the level of inflammatory cytokines and growth factors released locally in the prostate.
IL-8
Human IL-8, an inflammatory chemokine, promotes tumor cell growth and the progression of human solid tumors; this includes CaP, due largely to its ability to regulate the expression of matrix metalloproteinases (MMPs).Citation89 Numerous studies have demonstrated a correlation between MMPs, IL-8, and CaP. Increased levels of IL-8, MMP-2, and MMP-9 were associated with high Gleason scores and metastatic disease. Also, a high level of IL-8 leads to an increase in MMP-9 expression, which in turn may directly increase the tumor grade and metastasis in CaP patients.Citation89,Citation90
TNF-α
TNF-α, another pro-inflammatory cytokine, plays a role in many solid tumors’ growth. Elevated serum levels of both TNF-α and IL-6 have been shown to correlate with advanced metastatic disease and decreased survival in CaP patients.Citation87 Additionally, TNF-α upregulates αvβ6 expression, leading to increase in MMP-9 expression involved in extracellular matrix degradation, tumor progression, and metastasis in vitro and in vivo.Citation91,Citation92
TGF-β
TGF-β, a multifunctional cytokine, has been shown to increase the survival and proliferation of transformed prostate epithelial cells and is found at elevated levels in the serum of human CaP patients with metastatic disease.Citation93,Citation94 Loss of TGF-β type I and II receptors on transformed human prostate epithelial cells correlates inversely with tumor grade and may allow escape from TGF-β-mediated growth regulation. Furthermore, TGF-β activates the transcriptional factor nuclear factor-κB and directly increases tumor cell survival.Citation93–Citation95
Chemokines and CaP
Chemokines are also involved in human prostate epithelial cells, growth and survival. It has been shown that these prostate epithelial cells produce a high level of macrophage chemotactic protein-1, which through the chemokine (C—C motif) receptor-2 and the phosphatidylinositol 3-kinase modulates the proliferation and invasiveness of prostate tumor cells in vitro and in vivo and promotes metastasis in the prostate.Citation96 Other studies have shown that chemokine (C—C motif) ligand-5 and its receptor chemokine (C—C motif) receptor-5 expressed on CaP cell surfaces may function as an autocrine factor and activate many cellular responses involved in cancer initiation, invasion, and progression.Citation97,Citation98
Polymorphisms of proinflammatory genes and CaP
Polymorphisms of several cytokines genes such as TNF-β1, IL-1α/β, IL-8, IL-10, and chemokine (C—C motif) receptor-5 can influence not only the inflammation and the immune response but are also mostly associated with susceptibility to CaP, as observed in a large number of case–control studies, twin studies, and segregation analysis.Citation66 It is widely hypothesized that the interactions of cytokine network genes, additively or epistatically, determine the individual risk for CaP as well as for BPH, which is also described as an immune-mediated inflammatory disease.
COX-2 and CaP
There is emerging evidence on the key role of COX-2 in prostate carcinogenesis.Citation99 COX-2 is considered a promoter of proliferation in CaP and its expression is associated with reactive oxygen species production and genomic damage induced by chronic inflammation.Citation28 Its rapid induction results in enhanced synthesis of prostanoids at the tumor site with several procarcinogenic effects including direct stimulation of prostate tumor growth and inhibition of immune surveillance in the prostate. Several reports have shown that COX-2, an early-response gene induced by a variety of cytokines and growth factors, is involved in invasion and angiogenesis in vitro and in vivo and is upregulated in many human malignancies including CaP.Citation28,Citation67,Citation98 This upregulation is seen throughout the tumorigenic process from early hyperplasia to metastatic disease,Citation100–Citation102 and has been described in many clinical cases with evolution of proliferative inflammatory atrophy (PIA) and PIN.Citation103
Genetic and epigenetic instability and CaP risk
The common genetic and epigenetic instabilities in CaP include a strong representation of genes that encode proteins with critical functions in the host in response to infection, inflammation, and oxidative stress; their mutation may reduce the possibility of preventing carcinogenesis.Citation30 These genes include but are not limited to phosphatase and tensin analog protein (PTEN), macrophage scavenger receptor (MSR1), Ribonuclease L (RNASEL), and Toll-like receptor 4 (TLR4). Recognition of their ligands determines a cascade of events associated with the activation of IL-1 receptor followed by the activation of the master inflammatory transcriptional regulator factor nuclear factor-κB and proinflammatory genes.Citation66
PIA as a precursor of CaP
PIA, the proliferative glandular epithelium with the morphological appearance of simple atrophy, occurs in association with chronic inflammation and it is thought to be a possible precursor of CaP. This lesion arises as a consequence of regenerative proliferation of the prostatic epithelial cell in response to inflammatory injury. It is considered as a precursor of high-grade PIN and CaP.Citation26,Citation30 Furthermore, it is often observed in proximity to high-grade PIN, and the morphologic transitions between PIA and high-grade PIN occur within the same acini or prostatic duct with an expression change of an antioxidant enzyme involved in the detoxification of carcinogens and inflammatory oxidants in prostate cells. This enzyme, glutathione S-transferase P1, is considered a signal of cellular stress and it is overexpressed in PIA and increases in chronic prostatic inflammation.Citation16 Glutathione S-transferase P1 inactivation, mostly by hypermethylation, is associated with high-grade PIN and CaP and may increase prostate cells’ susceptibility to additional genomic damage induced by inflammatory oxidant or nutritional carcinogens, with consequent selective growth and proliferation.Citation16
Role of hormones in CaP
A growing body of evidence supporting the important role of estrogens in human CaP is accumulating, although mechanisms underlying the implication of estrogens in prostate carcinogenesis remain totally unspecified. Both in vitro studies and in vivo animal models have suggested that androgens and estrogens play an important role in the development and/or progression of cancer.Citation104 For instance, long-term administration of testosterone and estradiol induce a high-incidence risk of rat prostate adenocarcinoma. High levels of estrogen in the presence of testosterone induce an early (4 weeks) prostate-specific inflammatory response and a later development of prostate carcinomas (nearly 50 weeks) in Noble rats, suggesting that estrogen-induced early inflammatory events are a prerequisite for the onset of CaP.Citation16,Citation105 Additionally, chronic exposure of Wistar rats to estradiol and dihydrotestosterone results in an early upregulation of IL-1β, IL-6, and inducible nitric oxide synthase, later accompanied by an increase in IL-4 and IL-5 expression that occurred irrespective of the presence of inflammatory cells and resembled a type-2 helper response.Citation106 Lastly, estrogens neonatally administered to rodents induce an imprinted state called developmental estrogenization, resulting in the development of lobe-specific inflammation, hyperplasia, and/or PIN and dysplasia.Citation107
Chronic inflammation as a possible link between BPH and CaP
A growing body of evidence suggests that chronic inflammation is a common condition in the human prostate and it could be initiated by several known or unknown stimuli that would determine the proinflammatory status in the prostatic microenvironment. The inflammatory infiltrates mostly composed by leukocytes are responsible for the secretion of cytokines involved in the paracrine and autocrine regulation of prostatic stromal and epithelial cell growth. As in the context of chronic inflammation and proinflammatory cytokine expression, the activity of IL-6, IL-8, IL-15, and IL-17 has been considered influential in the development of both diseases (BPH and CaP) (), although further confirmatory studies are needed.
Table 1 Inflammatory mediators involved in benign prostatic hyperplasia and prostate cancer pathogenesis
So far, it can be hypothesized that chronic prostatic inflammation could be considered one of the possible conditions associated with BPH, CaP, or both. Further research on inflammatory responses within the prostate is needed to improve knowledge on the mechanisms involved in the interaction among inflammatory infiltrates, prostatic stroma, and prostatic epithelium. More clarification is also needed to elucidate whether or not chronic prostatic inflammation could be considered the starting point for the development of benign and malignant proliferative disease of the prostate. With this in mind, there is a need to improve the capability to define the type of, and quantify, asymptomatic prostatic inflammation. Research into the relationship among BPH, CaP, and chronic prostatic inflammation may benefit from improving clinical imaging for the diagnosis of individual conditions and from a better histologic characterization of the spatial distribution of inflammatory infiltrates, BPH nodules, and preneoplastic and neoplastic lesions of the prostate.
Conclusion
While the pathogenesis of both diseases, BPH and CaP, is not fully understood and several mechanisms seem to be involved in their initiation and progression, a growing body of evidence suggests the important role of inflammatory infiltrates and their mediators in the development of chronic prostatic diseases. Chronic prostatic inflammation, a common condition in human prostates, should not be considered only as an occasional histologic finding in prostate specimens but as a possible link between BPH and CaP. It may result from the immunologic response of different pathogens that induce prostatic tissue damage and subsequent chronic processes of repetitive wound healing, and it may have a role in BPH growth and its progression toward dysplasia and cancer. Further basic and clinical research in the area and trying to understand the etiology of prostatic inflammation and its pathway may help to identify new therapeutic targets and novel strategies for reducing the risk of developing benign and malignant tumors of the prostate.
Disclosure
The author reports no conflicts of interest in this work.
References
- De MarzoAMCoffeyDSNelsonWGNew concepts in tissue specificity for prostate cancer and benign prostatic hyperplasiaUrology1999533 Suppl 3a293910094098
- McVaryKTBPH: epidemiology and comorbiditiesAm J Manag Care200612Suppl 5S122S12816613526
- FitzpatrickJMThe natural history of benign prostatic hyperplasiaBJU Int200697Suppl 23616507045
- FitzpatrickJMKirbyRSManagement of acute urinary retentionBJU Int200697Suppl 2162016507048
- RoehrbornCGPathology of benign prostatic hyperplasiaInt J Impot Res200820Suppl 3S11S1819002119
- SiegelRNaishadhamDJemalACancer statistics, 2012CA Cancer J Clin2012621102922237781
- AlcarazAHammererPTubaroAScroderFHCastroRIs there evidence of a relationship between benign prostatic hyperplasia and prostate cancer? Findings of a literature reviewEur Urol200955486487319027219
- McNealJENormal histology of the prostateAm J Surg Pathol19881286196332456702
- McNealJENormal anatomy of the prostate and changes in benign prostatic hypertrophy and carcinomaSemin Ultrasound CT MR1988953293342483527
- McNealJERedwineEAFreihaFSStameyTAZonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spreadAm J Surg Pathol198812128979063202246
- SfanosKSDe MarzoAMProstate cancer and inflammation: the evidenceHistopathology201260119921522212087
- ChughtaiBLeeRTeAKaplanSRole of inflammation in benign prostatic hyperplasiaRev Urol201113314715022110398
- WagenlehnerFMElkahwajiJEAlgabaFThe role of inflammation and infection in the pathogenesis of prostate carcinomaBJU Int2007100473373717662075
- OmabeMEzeaniMInfection, inflammation and prostate carcinogenesisInfect Genet Evol20111161195119821397049
- De MarzoAMNakaiYNelsonWGInflammation, atrophy, and prostate carcinogenesisUrol Oncol200725539840017826659
- De MarzoAMPlatzEASutcliffeSInflammation in prostate carcinogenesisNat Rev Cancer20077425626917384581
- AmesBNGoldLSWillettWCThe causes and prevention of cancerProc Natl Acad Sci U S A19959212525852657777494
- GiovannucciEMedical history and etiology of prostate cancerEpidemiol Rev200123115916211588842
- AlbiniASpornMBThe tumour microenvironment as a target for chemopreventionNat Rev Cancer20077213914717218951
- RobertGSalagierskiMSchalkenJAde la TailleAInflammation and benign prostatic hyperplasia: cause or consequence?Prog Urol2010206402407 French20538203
- SchattemanPHHoekxLWyndaeleJJJeurisWVan MarckEInflammation in prostate biopsies of men without prostatic malignancy or clinical prostatitis: correlation with total serum PSA and PSA densityEur Urol200037440441210765070
- FibbiBPennaGMorelliAAdoriniLMaggiMChronic inflammation in the pathogenesis of benign prostatic hyperplasiaInt J Androl201033347548819508330
- NickelJCRoehrbornCGO’LearyMPBostwickDGSomervilleMCRittmasterRSExamination of the relationship between symptoms of prostatitis and histological inflammation: baseline data from the REDUCE chemoprevention trialJ Urol20071783 Pt 189690017632164
- NickelJCRoehrbornCGO’LearyMPBostwickDGSomervilleMCRittmasterRSThe relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trialEur Urol20085461379138418036719
- RobertGDescazeaudANicolaiewNInflammation in benign prostatic hyperplasia: a 282 patients’ immunohistochemical analysisProstate200969161774178019670242
- De MarzoAMMarchiVLEpsteinJINelsonWGProliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesisAm J Pathol199915561985199210595928
- NelsonWGDe MarzoAMDeWeeseTLIsaacsWBThe role of inflammation in the pathogenesis of prostate cancerJ Urol20041725 Pt 2S6S1115535435
- PalapattuGSSutcliffeSBastianPJProstate carcinogenesis and inflammation: emerging insightsCarcinogenesis20052671170118115498784
- De MarzoAMMeekerAKZhaSHuman prostate cancer precursors and pathobiologyUrology2003625 Suppl 1556214607218
- NelsonWGDe MarzoAMIsaacsWBProstate cancerN Engl J Med2003349436638112878745
- De MarzoAMNelsonWGIsaacsWBEpsteinJIPathological and molecular aspects of prostate cancerLancet2003361936195596412648986
- KleinEASilvermanRInflammation, infection, and prostate cancerCurr Opin Urol200818331531918382242
- SutcliffeSSexually transmitted infections and risk of prostate cancer: review of historical and emerging hypothesesFuture Oncol2010681289131120799875
- PelouzePSGonorrhea in the Male and Female: A Book for PractitionersPhiladelphia, PAWB Saunders1935
- HandsfeldHHLipmanTOHarnischJPTroncaEHolmesKKAsymptomatic gonorrhea in men. Diagnosis, natural course, prevalence and significanceN Engl J Med197429031171234202519
- PolettiFMediciMCAlinoviAIsolation of Chlamydia trachomatis from the prostatic cells in patients affected by nonacute abacterial prostatitisJ Urol198513446916934032572
- GardnerWAJrCulbersonDEBennettBDTrichomonas vaginalis in the prostate glandArch Pathol Lab Med198611054304322421689
- ThomsonLSyphilis of the prostateAm J Syphilis19204323341
- BushmanWEtiology of ProstatitisLeporHProstatic DiseasesPhiladelphia, PAWB Saunders2000550557
- StricklerHDGoedertJJSexual behavior and evidence for an infectious cause of prostate cancerEpidemiol Rev200123114415111588840
- ZambranoAKalantariMSimoneauAJensenJLVillarrealLPDetection of human polyomaviruses and papillomaviruses in prostatic tissue reveals the prostate as a habitat for multiple viral infectionsProstate200253426327612430138
- SamantaMHarkinsLKlemmKBrittWJCobbsCSHigh prevalence of human cytomegalovirus in prostatic intraepithelial neoplasia and prostatic carcinomaJ Urol20031703998100212913758
- KirbyRSLoweDBultitudeMIShuttleworthKEIntra-prostatic urinary reflux: an aetiological factor in abacterial prostatitisBr J Urol19825467297317150931
- IsaacsJTProstatic structure and function in relation to the etiology of prostatic cancerProstate1983443513666866850
- MartinonFPetrilliVMayorATardivelATschoppJGout-associated uric acid crystals activate the NALP3 inflammasomeNature2006440708123724116407889
- DrachenbergCBPapadimitriouJCProstatic corpora amylacea and crystalloids: similarities and differences on ultrastructural and histochemical studiesJ Submicrosc Cytol Pathol19962821411508964038
- SugimuraTWakabayashiKNakagamaHNagaoMHeterocyclic amines: mutagens/carcinogens produced during cooking of meat and fishCancer Sci200495429029915072585
- KnizeMGFeltonJSFormation and human risk of carcinogenic heterocyclic amines formed from natural precursors in meatNutr Rev200563515816515971410
- BorowskyADDingleyKHUbickETurteltaubKWCardiffRDDevere-WhiteRInflammation and atrophy precede prostatic neoplasia in a PhIP-induced rat modelNeoplasia20068970871516984728
- CoffeyDSSimilarities of prostate and breast cancer: evolution, diet, and estrogensUrology2001574 Suppl 1313811295592
- HarkonenPLMakelaSIRole of estrogens in development of prostate cancerJ Steroid Biochem Mol Biol200492429730515663993
- GilleranJPPutzODeJongMThe role of prolactin in the prostatic inflammatory response to neonatal estrogenEndocrinology200314452046205412697713
- HuangLPuYAlamSBirchLPrinsGSEstrogenic regulation of signaling pathways and homeobox genes during rat prostate developmentJ Androl200425333033715064308
- NaslundMJStrandbergJDCoffeyDSThe role of androgens and estrogens in the pathogenesis of experimental nonbacterial prostatitisJ Urol19881405104910533172358
- HuangLPuYAlamSBirchLPrinsGSThe role of Fgf10 signaling in branching morphogenesis and gene expression of the rat prostate gland: lobe-specific suppression by neonatal estrogensDev Biol2005278239641415680359
- PrinsGSBirchLCouseJFChoiIKatzenellenbogenBKorachKSEstrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor α: studies with αERKO and βERKO miceCancer Res200161166089609711507058
- PonniahSArahIAlexanderRBPSA is a candidate self-antigen in autoimmune chronic prostatitis/chronic pelvic pain syndromeProstate2000441495410861757
- BrigantiACapitanioUSuardiNBenign prostatic hyperplasia and its aetiologiesEur Urol Suppl2009813865871
- AbdollahFBrigantiASuardiNMetabolic syndrome and benign prostatic hyperplasia: evidence of a potential relationship, hypothesized etiology, and preventionKorean J Urol201152850751621927696
- RobertGDescazeaudAAlloryYVacherotFde la TailleAShould we investigate prostatic inflammation for the management of benign prostatic hyperplasia?Eur Urol Suppl2009813879886
- KramerGMittereggerDMarbergerMIs benign prostatic hyperplasia (BPH) an inflammatory disease?Eur Urol20075151202121617182170
- JahnischHFusselSKiesslingADendritic cell-based immunotherapy for prostate cancerClin Dev Immunol2010201051749321076523
- KramerGMarbergerMCould inflammation be a key component in the progression of benign prostatic hyperplasia?Curr Opin Urol2006161252916385197
- SteinerGENewmanMEPaiklDExpression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostateProstate200356317118212772186
- SteinerGEStixUHandisuryaACytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissueLab Invest20038381131114612920242
- CarusoCBalistreriCRCandoreGPolymorphisms of pro-inflammatory genes and prostate cancer risk: a pharmacogenomic approachCancer Immunol Immunother200958121919193319221747
- WangLYangJRYangJYLiuZTChronic inflammation in benign prostatic hyperplasia: implications for therapyMed Hypotheses20087051021102317935901
- PennaGFibbiBAmuchasteguiSHuman benign prostatic hyperplasia stromal cells as inducers and targets of chronic immunemediated inflammationJ Immunol200918274056406419299703
- DescazeaudAWeinbreckNRobertGTransforming growth factor β-receptor II protein expression in benign prostatic hyperplasia is associated with prostate volume and inflammationBJU Int20111082 Pt 2E23E2820840324
- SutcliffeSSexually transmitted infections and risk of prostate cancer: review of historical and emerging hypothesesFuture Oncol2010681289131120799875
- SutcliffeSPlatzEAInflammation and prostate cancer: a focus on infectionsCurr Urol Rep20089324324918765120
- VastoSCarrubaGCandoreGItalianoEDi BonaDCarusoCInflammation and prostate cancerFuture Oncol20084563764518922121
- DennisLKLynchCFTornerJCEpidemiologic association between prostatitis and prostate cancerUrology2002601788312100928
- RobertsROBergstralhEJBassSELieberMMJacobsenSJProstatitis as a risk factor for prostate cancerEpidemiology2004151939914712152
- CohenRJShannonBAMcNealJEShannonTGarrettKLPropionibacterium acnes associated with inflammation in radical prostatectomy specimens: a possible link to cancer evolution?J Urol200517361969197415879794
- HayesRBPotternLMStricklerHSexual behaviour, STDs and risks for prostate cancerBr J Cancer200082371872510682688
- StricklerHDGoedertJSexual behavior and evidence for an infectious cause of prostate cancerEpidemiol Rev200123114415111588840
- DennisLKDawsonDVMeta-analysis of measures of sexual activity and prostate cancerEpidemiology2002131727911805589
- TaylorMLMainousAG3rdWellsBJProstate cancer and sexually transmitted diseases: a meta-analysisFam Med200537750651215988645
- RosenblattKAWicklundKGStanfordJLSexual factors and the risk of prostate cancerAm J Epidemiol2001153121152115811415949
- HochreiterWWNadlerRBKochAEEvaluation of the cytokines interleukin 8 and epithelial neutrophil activating peptide 78 as indicators of inflammation in prostatic secretionsUrology20005661025102911113752
- FujitaKEwingCMSokollLJCytokine profiling of prostatic fluid from cancerous prostate glands identifies cytokines associated with extent of tumor and inflammationProstate200868887288218361406
- VoronovEShouvalDSKrelinYIL-1 is required for tumor invasiveness and angiogenesisProc Natl Acad Sci U S A200310052645265012598651
- CuligZPuhrMInterleukin-6: a multifunctional targetable cytokine in human prostate cancerMol Cell Endocrinol20123601–2525821664423
- MalinowskaKNeuwirtHCavarrettaITInterleukin-6 stimulation of growth of prostate cancer in vitro and in vivo through activation of the androgen receptorEndocr Relat Cancer200916115516919011039
- CuligZSteinerHBartschGHobischAInterleukin-6 regulation of prostate cancer cell growthJ Cell Biochem200595349750515838876
- MichalakiVSyrigosKCharlesPWaxmanJSerum levels of IL-6 and TNF-α correlate with clinicopathological features and patient survival in patients with prostate cancerBr J Cancer200490122312231615150588
- NumasakiMFukushiJOnoMInterleukin-17 promotes angiogenesis and tumor growthBlood200310172620262712411307
- InoueKSlatonJWEveBYInterleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancerClin Cancer Res2000652104211910815938
- UeharaHTroncosoPJohnstonDExpression of interleukin-8 gene in radical prostatectomy specimens is associated with advanced pathologic stageProstate2005641404915651067
- ScottKAArnottCHRobinsonSCTNF-α regulates epithelial expression of MMP-9 and integrin αvβ6 during tumour promotion. A role for TNF-α in keratinocyte migration?Oncogene200423416954696615273742
- ThomasGJLewisMPHartIRMarshallJFSpeightPMαvβ6 integrin promotes invasion of squamous carcinoma cells through up-regulation of matrix metalloproteinase-9Int J Cancer200192564165011340566
- ParkJILeeMGChoKTransforming growth factor-β1 activates interleukin-6 expression in prostate cancer cells through the synergistic collaboration of the Smad2, p38-NF-κB, JNK, and Ras signaling pathwaysOncogene200322284314433212853969
- LuTBurdelyaLGSwiatkowskiSMSecreted transforming growth factor β2 activates NF-κB, blocks apoptosis, and is essential for the survival of some tumor cellsProc Natl Acad Sci U S A2004101187112711715118089
- LuSDongZCharacterization of TGF-β-regulated interleukin-8 expression in human prostate cancer cellsProstate2006669996100416541418
- LuYCaiZXiaoGLiuYCCR2 expression correlates with prostate cancer progressionJ Cell Biochem2007101367668517216598
- VadayGGPeehlDMKadamPALawrenceDMExpression of CCL5 (RANTES) and CCR5 in prostate cancerProstate200666212413416161154
- KonigJESengeTAllhoffEPKonigWAnalysis of the inflammatory network in benign prostate hyperplasia and prostate cancerProstate200458212112914716737
- HussainTGuptaSMukhtarHCyclooxygenase-2 and prostate carcinogenesisCancer Lett2003191212513512618325
- FujitaHKoshidaKKellerETCyclooxygenase-2 promotes prostate cancer progressionProstate200253323224012386924
- EdwardsJMukherjeeRMunroAFWellsACAlmushatatABartlettJMHer2 and COX2 expression in human prostate cancerEur J Cancer2004401505514687789
- Aparicio GallegoGDiaz PradoSJimenez FonsecaPGarcia CampeloRCassinello EspinosaJAnton AparicioLMCyclooxygenase-2 (COX-2): a molecular target in prostate cancerClin Transl Oncol200791169470218055324
- WangWBerghADamberJEChronic inflammation in benign prostate hyperplasia is associated with focal upregulation of cyclooxygenase-2, Bcl-2, and cell proliferation in the glandular epitheliumProstate2004611607215287094
- RisbridgerGPBiancoJJEllemSJMcPhersonSJOestrogens and prostate cancerEndocr Relat Cancer200310218719112790781
- TamNNLeavIHoSMSex hormones induce direct epithelial and inflammation-mediated oxidative/nitrosative stress that favors prostatic carcinogenesis in the noble ratAm J Pathol200717141334134117717140
- HarrisMTFeldbergRSLauKMLazarusNHCochraneDEExpression of proinflammatory genes during estrogen-induced inflammation of the rat prostateProstate2000441192510861753
- PrinsGSHuangLBirchLPuYThe role of estrogens in normal and abnormal development of the prostate glandAnn N Y Acad Sci2006108911317261752