Abstract
Purpose
To investigate the tolerability and the effects of the β-3-adrenoceptor-agonist mirabegron on urinary incontinence and urodynamic parameters in patients with chronic neurogenic detrusor overactivity (NDO).
Patients and Methods
The patient database of a spinal cord injury rehabilitation center in Switzerland was screened for patients with chronic (>12 months) NDO, who had been prescribed mirabegron. Patient characteristics, data regarding bladder management, urinary incontinence and concurrent medication for NDO as well as urodynamic parameters were collected retrospectively. The changes in the urodynamic parameters and the occurrence of urinary incontinence over time were investigated.
Results
The data of 63 patients with a median age of 48 years and a median NDO duration of 8.9 years at the initiation of the mirabegron treatment were analyzed. A median 3.0 and 12.7 months had elapsed from the initiation of the mirabegron therapy to the first and second follow-up evaluation, respectively. The majority of patients (73%) received mirabegron in combination with an established antimuscarinic or onabotulinum toxin therapy. The number of patients suffering from urinary incontinence decreased significantly (p≤0.005) from 60.3% (95% CI 47.2/72.4%) to 38.1% (95% CI 23.6/54.4%). Furthermore, the maximum detrusor pressure during the storage phase was significantly (p≤0.04) lower at the second follow-up evaluation (29.5cmH2O, 95% CI 22/40cmH2O) compared to before the mirabegron treatment (35cmH2O, 95% CI 29/41cmH2O). The bladder capacity and detrusor compliance were significantly (p≤0.005) increased during the mirabegron treatment. No patient had discontinued the mirabegron treatment as a result of side effects.
Conclusion
Mirabegron demonstrated a clinically relevant effect and a good safety profile. Concomitant treatment of NDO with mirabegron may allow reduction in the dose of antimuscarinic medication and thus, improve the long-term persistence of NDO treatment.
Introduction
Neurogenic detrusor overactivity (NDO) affects the majority of individuals with suprasacral spinal cord injury (SCI).Citation1 The first-line therapy for protecting the upper urinary tract and achieving urinary continence is antimuscarinic treatment.Citation1 However, non-compliance as a result of intolerable side effects or insufficient effectiveness are the reasons why antimuscarinic treatment is abandoned for botulinum toxin injections into the detrusor.Citation2 However, the reported long-term compliance rate of botulinum toxin therapy ranges from a low 25%Citation3 to 60%,Citation4,Citation5 as a result of insufficient effectiveness, loss of effectiveness, tolerability issues and the need for repeated injections.Citation3–Citation6
Mirabegron, a selective β-3-adrenoceptor-agonist, is a potential treatment alternative for NDO. The activation of β-3-adrenoceptors causes relaxation of the detrusor smooth musclesCitation7 and thus, improves bladder capacity and compliance without reducing voiding detrusor pressure.Citation8 In individuals with overactive bladder, mirabegron has been shown to significantly reduce micturition frequency as well as episodes of urinary incontinence, urgency and nocturia with a similar efficacy compared to antimuscarinics, but with fewer side effects.Citation9,Citation10 The better tolerability of mirabegron is attributable to the different distribution of β-3-adrenoceptors in the body (bladder, urethra, prostate, gastrointestinal tract) compared to muscarinic receptors (bladder, central nervous system, salivary glands, gastrointestinal tract).Citation11 The distinct target receptors offer the possibility of combining mirabegron and antimuscarinics in order to improve tolerability and maintain efficacy. The combination therapy has been reported to show synergistic effects regarding the improvement of overactive bladder symptoms and good tolerability.Citation10,Citation12
The available data on the effects of mirabegron in the therapy of NDO were collected in diverse populations (eg various neurologic conditions, pediatric patients).Citation13–Citation17 Moreover, some of the available data are based on studies with very small sample sizesCitation13,Citation15 and short follow-up time (ie 4–7 weeks).Citation13–Citation15 We have therefore investigated the effects on urinary incontinence and urodynamic parameters and the tolerability of a stand-alone or add-on mirabegron treatment in patients with chronic (>12 months) NDO as a result of SCI, myelomeningocele (MMC) or multiple sclerosis (MS) over 12 months.
Patients and Methods
Patients and Collected Data
This study had been approved by the Ethics Committee Northwestern and Central Switzerland (2018–00417) and all applicable institutional and governmental regulations concerning the ethical use of the data were followed. All data were encrypted and kept confidential.
The patient database of a SCI rehabilitation center in Switzerland was screened for patients with chronic (>12 months) NDO resulting from SCI, MMC or MS, who had been prescribed mirabegron (BetmigaTM, Astellas Pharma, Tokyo, Japan). The diagnosis of NDO had been based on urodynamic studies. Patients who had rejected the further use of their health-related data for retrospective analyses were excluded. Further exclusion criteria were: missing data (ie no urodynamic follow-up examination), non-compliance (ie mirabegron prescribed but not taken), NDO etiology other than SCI, MMC or MS and duration of NDO shorter than 12 months.
Data were collected for the time period from January 2015 to December 2018. Patient characteristics, data regarding bladder management, urinary incontinence and concurrent medication for NDO as well as urodynamic parameters were extracted from electronic and paper patient charts. Standard video-urodynamic examinations had been performed before starting the mirabegron treatment as well as approximately 3 and 12 months after starting the treatment according to the International Continence Society standards.Citation18 Patients presenting for a video-urodynamic examination completed a standardized questionnaire (developed in-house) regarding incontinence, frequency of bladder evacuation and medication. Patients were categorized as continent, if they had used not more than one incontinence pad per day, because this was most commonly a precautionary measure.
Statistical Analyses
The data were calculated as median and 95% confidence interval (CI) or frequency and 95% CI (Clopper-Pearson CI). The changes in the urodynamic parameters and the occurrence of urinary incontinence over time (pre-mirabegron, 3 months and 12 months follow-up) were investigated using the non-parametric (rank-based) analysis of variance of longitudinal data described by Brunner et alCitation19 or Cochran’s Q test, respectively. Post-hoc testing was performed with the Wilcoxon signed-ranks and McNemar’s test. All available data of the included patients were analyzed. There was no imputation of missing data.
The statistical analyses were performed using the SPSS software (Version 25, IBM, Somers, NY, USA) or the R software environment (Version 3.4.0, Copyright 2017, The R Foundation for Statistical Computing). A p-value of ≤0.05 was considered significant.
Results
A total of 92 patients with chronic NDO, who had been prescribed mirabegron were identified. A total of 29 patients (31.5%) were excluded from analysis as a result of missing data (13 patients, 44.8%), non-compliance (8 patients, 27.6%), other NDO etiology (4 patients, 13.8%) and duration of NDO (4 patients, 13.8%). Twenty-one patients had not reached the 12 months follow-up at the time of analysis. Thus, the data of 63 and 42 patients were analyzed for the 3 and 12 months follow-up, respectively.
The characteristics of the evaluated patients are presented in . The median age was 48 years (95% CI 43–56 years) and the median duration of NDO was 8.9 years (95% CI 4.8–12.3 years) at the initiation of the mirabegron therapy. A median 3.0 months (95% CI 2.0–5.0 months) and 12.7 months (95% CI 8.9–15.8 months) had elapsed from the initiation of the mirabegron therapy to the first and second follow-up evaluation, respectively. The majority of the patients (79.4%) was taking 50mg mirabegron. In three patients, the mirabegron dose was increased from 25mg to 50mg, and in three others, the dose was decreased from 50mg to 25mg from the first to the second follow-up evaluation. The indication for the mirabegron treatment was mainly additive (63.5%) therapy of NDO (), ie the established therapy had not been sufficiently effective.
Table 1 The Characteristics of the Evaluated Patients
The concurrent medication for NDO at the three evaluation time points is presented in . The percentage of patients only taking mirabegron for NDO was 30.2% and 21.4% after 3 and 12 months, respectively. There was no noteworthy change in the concurrent medication for NDO after the initiation of the mirabegron treatment.
Table 2 The Concurrent Medication for Neurogenic Detrusor Overactivity in the Evaluated Patients
The percentage of patients suffering from urinary incontinence significantly (p≤0.005) decreased from 60.3% to 33.3% and 38.1% at the first and second follow-up evaluation, respectively (). Furthermore, the mirabegron treatment resulted in significantly decreased maximum detrusor storage pressure (p=0.04) () as well as increased maximum cystometric capacity (p=0.005) and detrusor compliance (p=0.0001) both at the first and second follow-up evaluation ().
Figure 1 The maximum detrusor pressure during the storage phase before (pre-mirabegron) and during mirabegron treatment (3 and 12 months follow-up) in the evaluated patients. *Significantly (p=0.04) different from pre-mirabegron value.
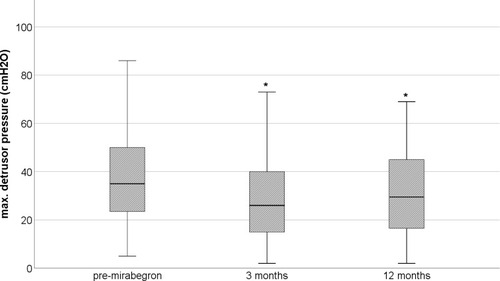
Table 3 Urodynamic and Clinical Data Before and During Mirabegron Therapy
At the first follow-up, the mirabegron treatment had been stopped in five patients as a result of insufficient effectiveness. These patients received onabotulinum toxin injections into the detrusor muscle. At the 12 months follow-up, the mirabegron treatment had been stopped in one patient who switched to permanent drainage via suprapubic catheterization. There was no other change regarding the method of bladder evacuation in the investigated individuals during the follow-up. No patient had discontinued the mirabegron treatment as a result of side effects. Four patients had reported side effects during mirabegron treatment, such as dry skin, tachycardia, headache and stomach pain. Apart from the patient complaining of stomach pain, all others were taking antimuscarinics concomitantly.
Discussion
The number of patients suffering from urinary incontinence was significantly lower during mirabegron treatment over 12 months in patients with chronic (>12 months) NDO. Furthermore, the mirabegron treatment resulted in significantly decreased maximum detrusor storage pressure as well as increased maximum cystometric capacity and detrusor compliance. No patient had discontinued the mirabegron treatment as a result of side effects.
In the present investigation, the proportion of patients suffering from urinary incontinence had decreased by 36.8% at the 12 months follow-up. Wollner and PannekCitation13 have reported a 55% reduction in daily incontinence episodes during mirabegron treatment in patients with NDO. Furthermore, they have observed a significant reduction in the number of daily bladder evacuations.Citation13 In the present investigation, there was no change in the number of daily bladder evacuations during mirabegron treatment. However, the number of daily evacuations prior to the mirabegron treatment was already identical to the number during mirabegron treatment in the previous report, where the number of daily evacuations decreased from 8.1 to 6.4.Citation13 In individuals with non-neurogenic overactive bladder, mirabegron has also been reported to significantly reduce the number of daily incontinence episodes and micturition.Citation9,Citation10
After 12 months of mirabegron treatment, maximum detrusor storage pressure had significantly decreased compared to the baseline value (from 35cmH2O to 29.5cmH2O/-16%). Furthermore, there was a significant increase in maximum cystometric capacity (from 330mL to 420mL/+27%) and detrusor compliance (from 30mL/cmH2O to 63mL/cmH2O/+110%). The improvement in detrusor compliance was also the most prominent change (+59-61%) in other studies with patients affected by NDO.Citation13,Citation14 However, some investigators did not observe significant changes in maximum detrusor storage pressure and bladder capacity after mirabegron treatment.Citation14,Citation15 Whereas othersCitation13 have reported a significant decrease in detrusor storage pressure during (−66%) and increase in bladder capacity (+15%). In individuals with non-neurogenic overactive bladder, Matsukawa et alCitation8 observed a significant improvement in maximum detrusor storage pressure (−27%) and bladder capacity (+19%) without a reduction in voiding detrusor pressure.
Although data analysis demonstrated a significant improvement in clinical and urodynamic outcomes, mirabegron treatment had been stopped in 5 of 63 (8%) patients after two months, because of insufficient effectiveness and switched to onabotulinum toxin injections into the detrusor muscle. In individuals with non-neurogenic overactive bladder, the reported rate of mirabegron treatment withdrawal as a result of ineffectiveness is higher (16–19%).Citation20,Citation21 In contrast to antimuscarinic treatment, botulinum toxin injections seem to be superior to mirabegron treatment in improving symptoms of overactive bladder.Citation22,Citation23 Treatment failure may have resulted from lower effectiveness of mirabegron when used as a second-line therapy compared to first-line therapy.Citation24
The majority of patients (88%) had been prescribed mirabegron as a second-line treatment and concomitantly to antimuscarinics, α1-adrenergic receptor blocker or onabotulinum toxin detrusor injections. There was no noteworthy change in the concurrent medication for NDO after the initiation of the mirabegron treatment. However, the additive therapy with mirabegron averted the need to increase the dose of antimuscarinics or to resort to botulinum toxin detrusor injections. In individuals with non-neurogenic overactive bladder, the combination therapy of mirabegron with antimuscarinics has been reported to have synergistic effects.Citation10,Citation12,Citation25 The design of the present investigation was not suitable to detect any potential synergistic effects of antimuscarinics and mirabegron.
The reported side effects of mirabegron are primarily cardiovascular complications (tachycardia, hypertension and cardiac arrhythmia), gastrointestinal symptoms (constipation, diarrhea, abdominal pain) and headache. However, meta-analyses have not demonstrated an increased risk of cardiovascular complications for mirabegron compared to placebo.Citation9–Citation11,Citation26 Furthermore, the incidence of antimuscarinic side effects is lower for mirabegron, and thus, tolerability is better compared to antimuscarinics.Citation10,Citation26,Citation27 In the present investigation, four patients (6%) had reported side effects, such as tachycardia, stomach pain or headache. However, no patient had discontinued the mirabegron treatment as a result of these side effects. Three of the four patients, who had reported side effects, were treated with antimuscarinics concomitantly. It is therefore uncertain whether these side effects were caused by mirabegron or the antimuscarinic drugs. Wollner and PannekCitation13 have reported one patient (7%) suffering from constipation during mirabegron treatment. Krhut et alCitation14 also observed mirabegron-related side effects in one patient (3%).
The retrospective design pertains to the limitations of the present study. However, the present data represent the real-world clinical effects of mirabegron in individuals with NDO and are relevant for assessing the merit of future prospective studies. As a result of the retrospective study design, the occurrence of side effects may have been underestimated. However, clinically relevant side effects would have been reported and documented reliably.
The treatment with mirabegron was effective in patients with chronic NDO during a follow-up of 12 months. Furthermore, there were only few side effects and no patient abandoned the treatment because of these side effects. Thus, mirabegron demonstrated a clinically relevant effect and a good safety profile. The potential cardiovascular effects of mirabegron are a concern in individuals with a neurologic lesion at the sixth thoracic level or above. However, there were only two patients with tetraplegia (5.6%) who had experienced side effects, such as tachycardia or headache. These side effects were not severe enough to necessitate a treatment stop. Concomitant treatment with mirabegron may avert the need to increase or even to reduce the dose of antimuscarinic medicationCitation25 and thus, improve the long-term persistence of NDO treatment.Citation20,Citation28
Conclusion
Mirabegron demonstrated a clinically relevant effect and a good safety profile. Concomitant treatment of NDO with mirabegron may allow reduction in the dose of antimuscarinic medication and thus, improve the long-term persistence of NDO treatment.
Disclosure
The authors declare no conflicts of interest.
References
- Blok B, Padilla-Fernandez B, Pannek J, et al. Non-oncology guidelines: neuro-urology. Guidelines Eur Assoc Urology. 2019 Available from https://uroweb.org/guideline/neuro-urology. Accessed 2019.
- Cheng T, Shuang WB, Jia DD, et al. Efficacy and safety of onabotulinumtoxin A in patients with neurogenic detrusor overactivity: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2016;11(7):e0159307. doi:10.1371/journal.pone.015930727463810
- Rahnama’i MS, Marcelissen TAT, Brierley B, Schurch B, de Vries P. Long-term compliance and results of intravesical botulinum toxin A injections in male patients. Neurourol Urodyn. 2017;36(7):1855–1859. doi:10.1002/nau.2319628084637
- Leitner L, Guggenbuhl-Roy S, Knupfer SC, et al. More than 15 years of experience with intradetrusor onabotulinumtoxin A injections for treating refractory neurogenic detrusor overactivity: lessons to be learned. Eur Urol. 2016;70(3):522–528. doi:10.1016/j.eururo.2016.03.05227106070
- Joussain C, Popoff M, Phe V, et al. Long-term outcomes and risks factors for failure of intradetrusor onabotulinumtoxin A injections for the treatment of refractory neurogenic detrusor overactivity. Neurourol Urodyn. 2018;37(2):799–806. doi:10.1002/nau.2335228745807
- Pannek J, Gocking K, Bersch U. Long-term effects of repeated intradetrusor botulinum neurotoxin A injections on detrusor function in patients with neurogenic bladder dysfunction. BJU Int. 2009;104(9):1246–1250. doi:10.1111/j.1464-410X.2009.08600.x19426192
- Sidaway P. Urinary incontinence: novel insights into the mechanism of action of mirabegron on human bladder smooth muscle. Nat Rev Urol. 2015;12(6):304. doi:10.1038/nrurol.2015.93
- Matsukawa Y, Takai S, Funahashi Y, Yamamoto T, Gotoh M. Urodynamic evaluation of the efficacy of mirabegron on storage and voiding functions in women with overactive bladder. Urology. 2015;85(4):786–790. doi:10.1016/j.urology.2015.01.00225709050
- Sebastianelli A, Russo GI, Kaplan SA, et al. Systematic review and meta-analysis on the efficacy and tolerability of mirabegron for the treatment of storage lower urinary tract symptoms/overactive bladder: comparison with placebo and tolterodine. Int J Urol. 2018;25(3):196–205. doi:10.1111/iju.1349829205506
- Kelleher C, Hakimi Z, Zur R, et al. Efficacy and Tolerability of Mirabegron Compared with Antimuscarinic Monotherapy or Combination Therapies for Overactive Bladder: a Systematic Review and Network Meta-analysis. Eur Urol. 2018;74(3):324–333. doi:10.1016/j.eururo.2018.03.02029699858
- Bhide AA, Digesu GA, Fernando R, Khullar V. Mirabegron - a selective beta3-adrenoreceptor agonist for the treatment of overactive bladder. Res Rep Urol. 2012;4:41–45. doi:10.2147/RRU.S2893024199179
- Gratzke C, van Maanen R, Chapple C, et al. Long-term safety and efficacy of mirabegron and solifenacin in combination compared with monotherapy in patients with overactive bladder: a randomised, multicentre phase 3 study (SYNERGY II). Eur Urol. 2018;74(4):501–509. doi:10.1016/j.eururo.2018.05.00529866467
- Wollner J, Pannek J. Initial experience with the treatment of neurogenic detrusor overactivity with a new beta-3 agonist (mirabegron) in patients with spinal cord injury. Spinal Cord. 2016;54(1):78–82. doi:10.1038/sc.2015.19526503222
- Krhut J, Borovicka V, Bilkova K, et al. Efficacy and safety of mirabegron for the treatment of neurogenic detrusor overactivity-Prospective, randomized, double-blind, placebo-controlled study. Neurourol Urodyn. 2018;37(7):2226–2233. doi:10.1002/nau.2356629603781
- Welk B, Hickling D, McKibbon M, Radomski S, Ethans K. A pilot randomized-controlled trial of the urodynamic efficacy of mirabegron for patients with neurogenic lower urinary tract dysfunction. Neurourol Urodyn. 2018;37(8):2810–2817. doi:10.1002/nau.2377430168626
- Park JS, Lee YS, Lee CN, Kim SH, Kim SW, Han SW. Efficacy and safety of mirabegron, a beta3-adrenoceptor agonist, for treating neurogenic bladder in pediatric patients with spina bifida: a retrospective pilot study. World J Urol. 2019;37(8):1665–1670. doi:10.1007/s00345-018-2576-030511212
- Soebadi MA, Hakim L, Van der Aa F, De Ridder D. Real-life data on mirabegron in neurogenic bladder dysfunction. Urol Int. 2019;103(2):195–201. doi:10.1159/00050034931096260
- Rosier P, Schaefer W, Lose G, et al. International continence society good urodynamic practices and terms 2016: urodynamics, uroflowmetry, cystometry, and pressure-flow study. Neurourol Urodyn. 2017;36(5):1243–1260. doi:10.1002/nau.2312427917521
- Brunner E, Domhof S, Langer F. Nonparametric Analysis of Longitudinal Data in Factorial Experiments. New York: Wiley; 2002.
- Shen YC, Wang HJ, Chuang YC. Efficacy and persistence of low-dose mirabegron (25 mg) in patients with overactive bladder: analysis in a real-world urological practice. Int Urol Nephrol. 2018;50(7):1219–1226. doi:10.1007/s11255-018-1907-929882001
- Nozawa Y, Kato D, Tabuchi H, Kuroishi K. Safety and effectiveness of mirabegron in patients with overactive bladder in a real-world clinical setting: a japanese post-marketing study. LUTS. 2018;10(2):122–130. doi:10.1111/luts.1214827860325
- Freemantle N, Ginsberg DA, McCool R, et al. Comparative assessment of onabotulinumtoxinA and mirabegron for overactive bladder: an indirect treatment comparison. BMJ Open. 2016;6(2):e009122. doi:10.1136/bmjopen-2015-009122
- Drake MJ, Nitti VW, Ginsberg DA, et al. Comparative assessment of the efficacy of onabotulinumtoxinA and oral therapies (anticholinergics and mirabegron) for overactive bladder: a systematic review and network meta-analysis. BJU Int. 2017;120(5):611–622. doi:10.1111/bju.1394528670786
- Serati M, Leone Roberti Maggiore U, Sorice P, Cantaluppi S, Finazzi Agro E, Ghezzi F. Is mirabegron equally as effective when used as first- or second-line therapy in women with overactive bladder? Int Urogynecol J. 2017;28(7):1033–1039. doi:10.1007/s00192-016-3219-x27942790
- Herschorn S, Chapple CR, Abrams P, et al. Efficacy and safety of combinations of mirabegron and solifenacin compared with monotherapy and placebo in patients with overactive bladder (SYNERGY study). BJU Int. 2017;120(4):562–575. doi:10.1111/bju.1388228418102
- Chen HL, Chen TC, Chang HM, et al. Mirabegron is alternative to antimuscarinic agents for overactive bladder without higher risk in hypertension: a systematic review and meta-analysis. World J Urol. 2018;36(8):1285–1297. doi:10.1007/s00345-018-2268-929556972
- Torimoto K, Matsushita C, Yamada A, et al. Clinical efficacy and safety of mirabegron and imidafenacin in women with overactive bladder: a randomized crossover study (the MICRO study). Neurourol Urodyn. 2017;36(4):1097–1103. doi:10.1002/nau.2305027265880
- Wada N, Watanabe M, Banjo H, et al. Long-term persistence with mirabegron in a real-world clinical setting. Int J Urol. 2018;25(5):501–506. doi:10.1111/iju.1355829651798
