Abstract
Introduction
Enhanced recovery after surgery (ERAS) protocols aim to optimize patient recovery after major surgery. Our study was to examine the evidence of the effectiveness of interventions designed to improve patient outcomes after radical cystectomy.
Design
Systematic review and meta-analysis.
Data Sources
PubMed, Medline, Embase, Cochrane from January 2005 to January 2021 without language restrictions.
Eligibility Criteria
Randomized and non-randomized controlled studies implementing ERAS measuring its interventions on rates of postoperative complications, 30-day readmission, length of stay (LOS) and bowel function after radical cystectomy.
Data Extraction and Synthesis
Two members of the investigating team independently selected studies and evaluated bias using the Cochrane collaboration tool. Meta-analysis of all comparative studies used inversed-weighted, fixed- effects models and random effects models to pool results. Publication bias was graphically assessed using contour-enhanced funnel plots and the Egger’s test of funnel plot symmetry.
Results
Fifteen studies were included in our meta-analysis; we observed that ERAS decreased the time for the first bowel movement (standardized mean difference [SMD]: –1.30, 95% CI −1.90 to −0.70, P<0.00001) and shortened the length of stay (LOS) ([SMD]: –0.49, 95% CI −0.77 to −0.20, (P < 0.00001)); however, 30-day readmission (risk ratio [RR]: 0.97,95% [CI] 0.73 to 1.28, P=0.52) and the overall postoperative complication rate (risk ratio [RR]: 0.98,95% confidence interval [CI]: 0.88 to 1.09, P= 0.41) showed no significant difference.
Introduction
Bladder cancer has a high incidence and is associated with high morbidity and mortality.Citation1 Radical cystectomy (RC) is the gold standard treatment for high-risk grade non-muscle invasive bladder tumor and muscle invasive bladder cancer (MIBC).Citation2 RC is a complex urological procedure and is associated with poor recovery, often requiring readmission and lengthy hospital stay. Complications are attributed to the patient population who are generally the elderly presenting with comorbidities (eg, coronary artery disease, atherosclerosis, and cerebrovascular accidents). In the past decades, the perioperative management of RC patients has evolved. In the conventional care, some patients were led down a course of interventions that were based on surgical or anesthesia dogma rather than evidence-based studies. Different routines of care such as bowel preparations and preoperative fasting often increase the patient’s physical and emotional stress. The systemic release of stress hormones and inflammatory mediators by the central nervous system can be detrimental after major operations, leading to rapid deconditioning and suboptimal outcomes of patients.Citation3 There is no single element by itself to counteract this physiological cascade but rather a series of care pathways to help minimalize this response subsequently improving the outcome of the surgery. In the late 1990s, the concept of ERAS was first introduced under the name of fast track recovery to study the effect of the surgical stress response on open colorectal surgery.Citation4 It was in 2000 in Denmark that the idea was coined into ERAS evidenced-based perioperative clinical pathways that consider the factors contributing to morbidity and proactively apply measures to reduce or eliminate them.Citation5 The application of evidence-based medicine has helped halve the rate of postoperative complications and reduce LOS.Citation6 In recent years, many studies have recognized and approved the benefits of ERAS, and it began gaining traction in other surgical specialties such as pancreatic, gynecology and urology.Citation7 ERAS pathways for cystectomy patients have tremendous clinical value and several studies have been published on the matter; however, noticeable differences exist in the effect of ERAS protocols and perioperative outcomes.Citation8–Citation21
Considering the lack of consistency in study results, as well as the absence of experimental data from high-quality randomized controlled trials (RCT), we conducted a systematic review and meta-analysis to determine the clinical effectiveness of ERAS pathways versus standard care on various perioperative outcomes of interest after RC.
Methods
Study Selection
In accordance with the PRISMA guidelines 2020 and Cochrane handbook for systemic reviews of interventions,Citation22,Citation23 we conducted a systematic literature search from January 2005 to January 2021 based on databases including PubMed, Medline, Embase and the Cochrane Library without language restrictions. Search terms or keywords used included bladder cancer, enhanced recovery after surgery and radical cystectomy. The following Medical Subject Headings (MESH) terms were used: “Enhanced Recovery After Surgery” [Mesh] OR “Postoperative Care” [Mesh] OR “Recovery of Function” [Mesh] OR “Enhanced Recovery” [tw] OR “fast track protocol*” [tw] OR “eras protocol*” [tw] AND ”Cystectomy” [Mesh] OR “Urinary Bladder Neoplasms” [Mesh] OR “Cystectomy” [tw] OR “Bladder tumor*” [tw] OR “bladder cancer” [tw] OR “Radical cystectomy” [tw] AND “Postoperative Complications”[Mesh] OR “length of stay” [tw] OR “injury” [tw] OR “ileus” [tw] OR “incontinence” [tw] OR “shock” [tw]. We also checked the reference lists of all related articles to ensure literature saturation.
Inclusion Criteria and Exclusion Criteria
Two members of the investigative team (M.P. and Z.J.W.) independently assessed the eligibility of the articles for inclusion in the study and discussed inconsistencies until consensus was obtained. The PICO method was used to define and search for potentially eligible studies.
● P (population): Patients undergoing RC.
● I (intervention): At least one element of ERAS protocols.
● C (comparison): Standard care/conventional therapy/Non-ERAS.
● O (outcomes of interest): At least one of the following; LOS, time to passage of first stool, readmission, overall complications; Clavien–Dindo classification and postoperative ileus (POI),
Studies that met one of the following criteria were excluded:
(1) The inclusion criteria were not met or no outcomes of interest were reported.
(2) Duplicate publications, non-comparative studies, case reports, editorial articles and reviews.
Data Extraction
One reviewer (M.P.) independently screened and extracted data from full-texts, citations, and protocols using standardized data collection forms that contained fields for authors, publication year, country, study design, matching variables (age, gender, body mass index, American society of Anesthesiology score, history of previous surgery, clinical staging, operation type, diversion type, operation time, estimated blood loss), and outcomes of interest. The outcomes of interest were as follows: length of hospital stay, time to first passage of stool, rate of 30-day readmission, and overall complications. The overall incidence of complications was classified within the Clavien–Dindo Classification and postoperative ileus. Disagreements between the two reviewers were resolved through discussion.
Quality Assessment
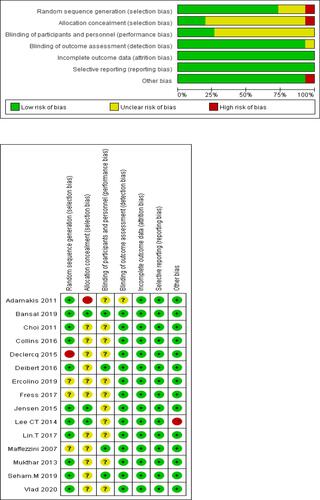
M.P. independently used the Cochrane risk-of-bias tool, RoB tool, in Review Manager software (https://community.cochrane.org/help/tools-and-software/revman-5) to assess the risk of bias of RCTs (), and there were concerns about risk of bias for the majority of studies. The domains of assessment included (1) random sequence generation (selection bias); (2) allocation concealment (selection bias); (3) blinding of participants and personnel (performance bias); (4) blinding of outcome assessment (detection bias); (5) incomplete outcome data (attrition bias); (6) selective reporting (reporting bias); (7) other bias (such as funding sources). Z.J.W. independently rated the level of evidence of the included studies using the Oxford Centre for Evidence-Based Medicine.Citation24
Statistical Analysis
For studies that reported continuous data as median and range, we estimated the mean and standard deviation (SD) using the method described by Wan et al.Citation25 The continuous variables were described as the difference in mean values and the 95% confidence interval (CI) between ERAS and Non-ERAS. The dichotomous outcomes were analyzed by calculating the risk ratio (RR). The meta-analysis was performed using the RevMan 5.4 software.Citation26 An inverse-weighted, fixed-effects meta-analysis was performed. The multiple interventions that constitute ERAS make the assumptions of a fixed-effect meta-analysis (all studies in the meta-analysis share one true effect size across all included studies) unlikely; therefore, the degree of heterogeneity between studies was assessed using the chi-square test (P<0.10) and the I2 statistic with values >50% regarded as being significant heterogeneity. A random-effects meta-analysis was used when significant heterogeneity was found between studies. We performed sensitivity analyses on recent publication 2015 or later and overall high-risk bias. To assess for publication bias, we used the RStudio coding software (RStudio Team. (2020). RStudio: Integrated Development Environment for R. Boston)Citation27 to create contour-enhanced funnel plots and applied the Egger’s test of funnel plot symmetry.Citation28
Characteristics of Included Studies
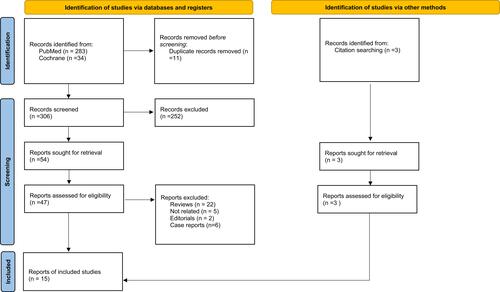
The database search yielded 317 records after duplication removal; we screened 306 records, from which we retrieved 54 full-text records and included 12 studies.Citation8–Citation10,Citation12–Citation17,Citation20,Citation21,Citation29 Moreover, we performed a citation and reference search and included three additional studies.Citation11,Citation18,Citation19 Fifteen studies that met the inclusion criteria were identified (); only 1 studyCitation18 was a retrospective RCT and the 14 others were prospective cohort studies. A total of 1853 participants were included, of which 953 were grouped as ERAS and 900 as control. The characteristics of each study are summarized in . The different ERAS elements between each study are summarized in .
Table 1 Characteristics of Each Study
Results
The majority of studies focused on the length of stay and overall complications. Studies were conducted in diverse country settings including the United States (two), Italy (two) and one each in India, South Korea, Greece, Sweden, Belgium, Canada, Denmark, England, Egypt and Romania. One included study was retrospective,Citation18 13 was prospective randomized control cohort,Citation8–Citation11,Citation13–Citation17,Citation19–Citation21,Citation29 and one1 study was non-randomized prospective cohort.Citation12 Time-to-first defecation was recorded across 10 studies,Citation8–Citation10,Citation14,Citation16,Citation17,Citation19–Citation21 and 30d readmission rate was assessed from 8 studies.Citation9,Citation11,Citation13–Citation15,Citation17,Citation21,Citation29
Length of Stay
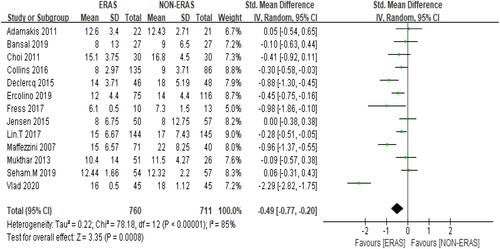
Pooled data analysis from 13 studies,Citation8–Citation12,Citation14,Citation15,Citation17–Citation21,Citation29 showed that the ERAS group was associated with shorter length of stay compared to the Non-ERAS group. SMD= −0.49, 95% CI: −0.77 to −0.20. Heterogeneity: Chi2 = 78.18, df = 12 (P < 0.00001); I2 = 85% () the random-effects model was used. Participants =1471.
Complications
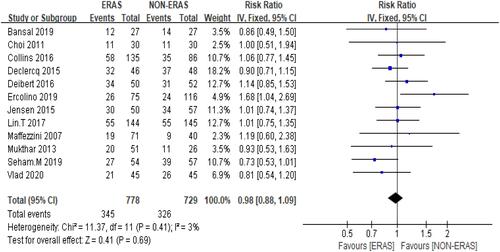
The overall complications were defined as high-grade, low-grade (Clavien–Dindo classification) and POI. The overall complication rate across 12 studiesCitation9–Citation15,Citation17–Citation21 showed that ERAS did not reduce the risk of complications with 44.3% of patients in the ERAS group against 44.79% of patients in the Non-ERAS group. Furthermore, pooled data from the fixed-effects model detected no significant difference between the two groups: RR = 0.98, 95% CI: 0.88–1.09, with low heterogeneity observed between studies: Chi2 = 11.37, df = 11 (P = 0.41); I2 = 3% () participants= 1507.
Bowel Function
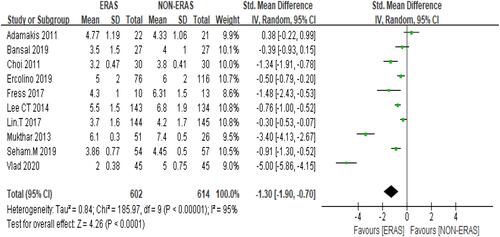
Time-to-first bowel movement was lower in the ERAS group regardless of the diversion type used. Moreover, pooled data from the random-effects model across 10 studies pointed towards a faster return to bowel function in the ERAS group: SMD= −1.30 95% but CI: −1.90 to 0.70. Heterogeneity: Chi2 = 185.97, df = 9 (P < 0.00001); I2 = 95% () participants =1216.
Readmissions
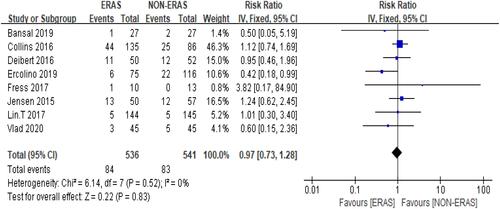
In general, ERAS did not significantly reduce the probability of patients being readmitted after RC. Moreover, 15.7% of the patients in the ERAS group were readmitted within 30 d compared with 15.3% of the patients in the Non-ERAS group. Pooled data from the fixed-effects model showed no significant difference between the two groups’ 30-day readmission rate': RR =0.97, 95% CI: 0.73–1.28, and there was statistical heterogeneity between studies: Chi2 = 6.14, df = 7 (P = 0.52); I2 = 0%, () participants=1077.
Sensitivity Analysis and Publication Bias
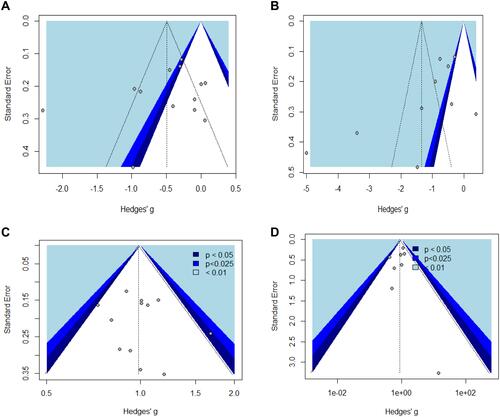
This sensitivity analysis that removes studies with potential bias found no significant changes. To examine small study and publication bias, we used contour-enhanced funnel plots (). Visual inspection of the funnel plots indicates possible bias. To further investigate the possibility of bias, we conducted an Egger test for funnel plot asymmetry, and the results are shown in . With these collective findings, we therefore can conclude that our results are with minimal publication bias.
Table 3 Eggers’ Test
Discussion
The study demonstrated that the implementation of ERAS protocols for patients undergoing radical cystectomy quickens the return of bowel function and shortens the length of hospitalization. No significant difference in readmission and complication rates were noted. Urologists have been slow to adopt ERAS despite evidence from colorectal literature showing ERAS protocols lead to improved outcomes.Citation30,Citation31 The key principles of the ERAS model are evidence-based and include preoperative counseling, preoperative nutrition, omission of mechanical bowel preparation (MBP), limiting preoperative fasting, carbohydrate loading (non-diabetic patients), standardized analgesic regimens, fluid management, prevention of hypothermia and deep venous thrombosis, minimally invasive approach, antimicrobial prophylaxis, prevention of POI, early mobilization and early oral diet.Citation32 The standardized pathways are designed to achieve optimum outcome from major surgeries and provide the necessary measures to attain such goals. Two studies did not omit MBP,Citation11,Citation18 all studies implemented early mobilization, early removal of NG tube, early oral feeding and pain management. The other pathways varied among studies for instance some studies show that ERAS pathways can shorten LOS,Citation10,Citation11,Citation29 whereas others do not;Citation8,Citation15,Citation20 most studies highlight the effect of ERAS pathways on reducing time to recovery of bowel function,Citation9,Citation10,Citation14,Citation17,Citation29 with the exception of oneCitation8; some studies demonstrate that ERAS protocols reduce readmission rates,Citation14,Citation21,Citation33 yet three studies did notCitation11,Citation15,Citation29; some studies concluded that the standardized protocol reduces the overall complicationsCitation9,Citation12,Citation19–Citation21; however some studies showed no change in morbidity.Citation13,Citation14,Citation18 The differences in pathways most certainly raise the question about which elements to universally adopt, but the focus of this study was to assess whether their implementations have any clinical impact. Our study showed the importance in adopting the ERAS protocols to improve perioperative outcomes of RC patients compared to a traditional approach and that the multimodal nature of ERAS is better than individually focusing on a single element within it. A recent umbrella review of 23 meta-analyses across multiple surgical specialties including urology (3) by Zhang et alCitation34 showed strong evidence that ERAS pathways can reduce LOS and cost without increasing morbidity and readmission. Despite these positive results, we need to exercise caution in interpreting these findings, there are limitations that cannot be ignored. The primary limitation is the limited number of RCT studies. Only two studies were blinded, all studies had at least an unclear bias in one domain. Another limitation was that we did not perform a subgroup analysis based on the operation type and diversion type, we grouped all patients implementing the ERAS protocols under one group, which may also introduce biased results. The publication bias was assessed by visual interpretation of funnel plots and Egger’s test for funnel plot symmetry. Lastly, health economics and quality of life were not included in our study due to lack of data. Despite these limitations, pooled data displayed the clinical efficacy of ERAS. Our findings indicated that ERAS protocols on perioperative outcomes of radical cystectomy provide a better improvement of the length of hospital stay and early rehabilitation of gastrointestinal function. Moreover, ERAS protocols did not increase the risk of adverse events, when compared with conventional protocols. These data have important clinical significance and we believe that our study further contributes to the body of evidence that supports the clinical value of ERAS in an effort to improve patients’ outcomes in the cystectomy population.
Author Contributions
Study concept and design: Peerbocus, Wang.
Acquisition of data: Peerbocus.
Analysis and interpretation of data: Peerbocus, Wang.
Critical revision of the manuscript for important intellectual content: Wang.
Statistical analysis: Peerbocus.
Supervision: Wang.
Other: None.
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
Additional information
Funding
References
- Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66(6SUPPL. 1):4–34. doi:10.1016/j.urology.2005.07.062
- Tan WS, Lamb BW, Kelly JD. Complications of radical cystectomy and orthotopic reconstruction. Adv Urol. 2015;2015:1–7. doi:10.1155/2015/323157
- Finnerty CC, Mabvuure NT, Kozar RA, Herndon DN. The surgically induced stress response. J Parenter Enter Nutr. 2013;37:21S–29S. doi:10.1177/0148607113496117
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78(5):606–617. doi:10.1093/bja/78.5.606
- Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183(6):630–641. doi:10.1016/S0002-9610(02)00866-8
- Varadhan KK, Neal KR, Dejong CHC, Fearon KCH, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr. 2010;29(4):434–440. doi:10.1016/j.clnu.2010.01.004
- Ansari D, Gianotti L, Schröder J, Andersson R. Fast-track surgery: procedure-specific aspects and future direction. Langenbecks Arch Surg. 2013;398(1):29–37. doi:10.1007/s00423-012-1006-9
- Adamakis I, Tyritzis SI, Koutalellis G, et al. Early removal of nasogastric tube is beneficial for patients undergoing radical cystectomy with urinary diversion. Int Braz J Urol. 2011;37(1):42–48. doi:10.1590/S1677-55382011000100006
- Bansal D, Nayak B, Singh P, et al. Randomized controlled trial to compare outcomes with and without the enhanced recovery after surgery protocol in patients undergoing radical cystectomy. Indian J Urol. 2020;36(2):95–100. doi:10.4103/iju.IJU_11_20
- Choi H, Kang SH, Yoon DK, et al. Chewing gum has a stimulatory effect on bowel motility in patients after open or robotic radical cystectomy for bladder cancer: a prospective randomized comparative study. Urology. 2011;77(4):884–890. doi:10.1016/j.urology.2010.06.042
- Collins JW, Adding C, Hosseini A, et al. Introducing an enhanced recovery programme to an established totally intracorporeal robot-assisted radical cystectomy service. Scand J Urol. 2016;50(1):39–46. doi:10.3109/21681805.2015.1076514
- Declercq P, De Win G, Van der Aa F, et al. Reduced length of stay in radical cystectomy patients with oral versus parenteral post-operative nutrition protocol. Int J Clin Pharm. 2015;37(2):379–386. doi:10.1007/s11096-015-0072-9
- Deibert CM, Silva MV, RoyChoudhury A, et al. A prospective randomized trial of the effects of early enteral feeding after radical cystectomy. Urology. 2016;96:69–73. doi:10.1016/j.urology.2016.06.045
- Ercolino A, Bianchi FM, Chessa F, et al. Perioperative outcomes of fast track protocol applied to patients treated with radical cystectomy and intestinal urinary diversion: a comparison with standard management in a high-volume center. Eur Urol Suppl. 2019;18(9):e3280–e3281. doi:10.1016/s1569-9056(19)33686-3
- Jensen BT, Petersen AK, Jensen JB, Laustsen S, Borre M. Efficacy of a multiprofessional rehabilitation programme in radical cystectomy pathways: a prospective randomized controlled trial. Scand J Urol. 2015;49(2):133–141. doi:10.3109/21681805.2014.967810
- Lee CT, Chang SS, Kamat AM, et al. Alvimopan accelerates gastrointestinal recovery after radical cystectomy: a multicenter randomized placebo-controlled trial. Eur Urol. 2014;66(2):265–272. doi:10.1016/j.eururo.2014.02.036
- Lin T, Li K, Liu H, et al. Enhanced recovery after surgery for radical cystectomy with ileal urinary diversion: a multi-institutional, randomized, controlled trial from the Chinese bladder cancer consortium. World J Urol. 2018;36(1):41–50. doi:10.1007/s00345-017-2108-3
- Maffezzini M, Gerbi G, Campodonico F, Parodi D. Multimodal perioperative plan for radical cystectomy and intestinal urinary diversion. I. Effect on recovery of intestinal function and occurrence of complications. Urology. 2007;69(6):1107–1111. doi:10.1016/j.urology.2007.02.062
- Mukhtar S, Ayres BE, Issa R, Swinn MJ, Perry MJA. Challenging boundaries: an enhanced recovery programme for radical cystectomy. Ann R Coll Surg Engl. 2013;95(3):200–206. doi:10.1308/003588413X13511609957579
- Moeen SM, Moeen AM. Usage of intravenous lidocaine infusion with enhanced recovery pathway in patients scheduled for open radical cystectomy: a randomized trial. Pain Physician. 2019;22(2):E71–E80. doi:10.36076/ppj/2019.22.e71
- Vlad O, Catalin B, Mihai H, et al. Enhanced recovery after surgery (ERAS) protocols in patients undergoing radical cystectomy with ileal urinary diversions: a randomized controlled trial. Medicine. 2020;99(27):e20902. doi:10.1097/MD.0000000000020902
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
- Cochrane Handbook for Systematic Reviews of Interventions. Cochrane training. Available from: https://training.cochrane.org/handbook. Accessed May 2, 2021.
- Oxford centre for evidence-based medicine: levels of evidence (March 2009) — Centre for Evidence-Based Medicine (CEBM), University of Oxford. Available from: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009. Accessed May 2, 2021.
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):1–13. doi:10.1186/1471-2288-14-135
- RevMan 5 download. Cochrane training. Available from: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download. Accessed May 2, 2021.
- RStudio. Open source & professional software for data science teams - RStudio. Available from: https://www.rstudio.com/. Accessed May 4, 2021.
- Sterne JAC, Harbord RM. Funnel plots in meta-analysis. Stata J Promot Commun Stat Stata. 2004;4(2):127–141. doi:10.1177/1536867x0400400204
- Frees SK, Aning J, Black P, et al. A prospective randomized pilot study evaluating an ERAS protocol versus a standard protocol for patients treated with radical cystectomy and urinary diversion for bladder cancer. World J Urol. 2018;36(2):215–220. doi:10.1007/s00345-017-2109-2
- Eskicioglu C, Forbes SS, Aarts MA, Okrainec A, McLeod RS. Enhanced recovery after surgery (ERAS) programs for patients having colorectal surgery: a meta-analysis of randomized trials. J Gastrointest Surg. 2009;13(12):2321–2329. doi:10.1007/s11605-009-0927-2
- Lassen K. Consensus review of optimal perioperative care in colorectal surgery. Arch Surg. 2009;144(10):961. doi:10.1001/archsurg.2009.170
- Cerantola Y, Valerio M, Persson B, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: enhanced recovery after surgery (ERAS®) society recommendations. Clin Nutr. 2013;32(6):879–887. doi:10.1016/j.clnu.2013.09.014
- Nayak B, Bansal D, Singh P, Seth A, Nayyar R, Ramachandran R. Randomized controlled trial to compare the length of stay, perioperative outcomes and complications in patients undergoing radical cystectomy with and without the enhanced recovery after surgery (ERAS) protocol in a tertiary care centre in India. Eur Urol Suppl. 2019;18(1):e1326. doi:10.1016/s1569-9056(19)30957-1
- Zhang X, Yang J, Chen X, Du L, Li K, Zhou Y. Enhanced recovery after surgery on multiple clinical outcomes: umbrella review of systematic reviews and meta-analyses. Medicine. 2020;99(29):e20983. doi:10.1097/MD.0000000000020983