Abstract
Brain metastases from prostate cancer typically occur in the more advanced stages of the disease. Clinically, the early diagnosis of visceral disease is crucial, impacting on patient’s management and prognosis. Although magnetic resonance imaging (MRI) is the modality of choice for the detection of brain metastases, it is not routinely performed in the surveillance of prostate cancer patients unless neurological manifestations appear. Prostate-specific membrane antigen (PSMA) is a glycoprotein, a membrane-bound metallopeptidase, overexpressed in more than 90% of prostate cancer cells. This molecular target is a suitable tissue biomarker for prostate cancer functional imaging. We present a case of a 73-year gentleman diagnosed with prostate adenocarcinoma and surgically treated (pT3bN1Mx, Gleason Score of 9) in February 2016. Subsequently, he underwent androgen deprivation therapy because of the occurrence of a bone metastasis. Between 2016 and January 2019 PSA levels were maintained under control. Starting from September 2019, it progressively raised up to 0.85 ng/mL with a doubling time of 3.3 months. Therefore, he performed a [68Ga]Ga-PSMA-11 PET/CT which showed a focal radiopharmaceutical uptake in the right temporal lobe corresponding to the presence of a rounded cystic lesion on brain MRI. The subsequent excisional biopsy diagnosed a prostate adenocarcinoma metastasis. PSMA expression has been reported in brain parenchyma after ischemic strokes and in some brain tumors including gliomas, meningiomas, and neurofibromas. In our case, the lack of symptoms and the relatively low PSA level raised questions about the nature of the lesion, posing the differential diagnosis between brain metastases and primary brain tumor. Finally, our case shows the capability of [68Ga]Ga-PSMA-11 PET/CT to detect metachronous distant brain metastases in a low biochemical recurrent asymptomatic prostate cancer patient, indicating that proper acquisition – from the vertex to thigh – should be always considered, regardless of the PSA level.
Background
Prostate cancer (PCa) is prone to metastasize. Metastases to bones and lymph nodes have been long recognized as the most typical pattern of extra-prostatic tumor spread. Visceral metastases affect mainly patients with advanced castration resistance (CR) disease, typically involving lung, liver, and adrenals.Citation1,Citation2 Brain metastases are rare (<2%),Citation3 and they are typically associated with extracranial metastases in the more advanced stages of the disease (generally when the life expectancy is less than one year).Citation4 The most common neurological manifestations of brain metastases from prostate cancer are non-focal neurologic symptoms related to the intracranial hypertension.Citation5 In some cases, patient do not complain any symptoms.Citation6 Commonly brain metastases occur in the frontal lobes (86% of patients).Citation5,Citation7 Prostate-specific membrane antigen (PSMA) is a glycoprotein, a membrane-bound metallopeptidase, that is overexpressed in over 90% of PCa cells. This molecular target is a suitable tissue biomarker for PCa functional imaging.Citation8 PSMA expression levels progressively increase along with tumor grade and stage, as well as aneuploidy and biochemical recurrent disease. Higher levels of PSMA expression are associated with poorer outcomes and PSMA expression is upregulated when the castrate-resistant phenotype evolves.Citation9,Citation10 [68Ga]Ga-PSMA-11 PET/CT provides the ability to identify and localize prostate cancer cells which overexpressed the target, impacting on patient management.Citation11
Case Presentation
Here, we present a case of a 73-year gentleman diagnosed with prostate adenocarcinoma in February 2016. Baseline serum prostate specific antigen level (PSA) was 9.6 ng/mL. He underwent a robot-assisted laparoscopic radical prostatectomy completed with a bilateral lymphadenectomy. After surgery PSA fall to 0.01 ng/mL. According to the AJCC 8th edition, the PCa was staged as IVA (T3bN1Mx) with a Gleason Score of 9 (4+5). He underwent a [99mTc]Tc-MDP bone scintigraphy which showed a bone metastasis in the sacrum. This finding led to upstage the disease from a stage IVA to a IVB (T3bN1M1b), and he started androgen deprivation therapy (ADT). Between 2016 and January 2019 PSA levels were maintained under control, resulting around 0.01 ng/mL. From September 2019, PSA progressively raised from 0.02 ng/mL up to 0.85 ng/mL in July 2020. The PSA doubling time was of 3.3 months (https://www.mskcc.org) (). The patients were referred to our center to perform a [68Ga]Ga-PSMA-11 PET/CT. Images – from vertex to mid-thigh – were acquired 60 minutes after the i.v. administration of 185 MBq of [68Ga]Ga-PSMA-11, with a Discovery 710 PET/CT (GE Healthcare Milwaukee, Wisconsin, USA), according to the EANM guidelines.Citation12,Citation13 Visual and semiquantitative analysis was performed according to the EANM standardized reporting guidelines v1.0 for PSMA-PET.Citation14 Images showed increased radiopharmaceutical uptake (score of 2/3) in the right temporal lobe (), in a left common iliac lymph node, and in a right external iliac lymph node. The report concluded for miT0, miN1 and suspected – but not definite – CNS miM. At the time of PET/CT, the patient did not experience any neurological symptoms. A brain MRI, requested to characterize the suspected lesion confirmed the presence of a right temporal rounded cystic lesion (42x33x31 mm) which appeared hypointense in T1 and hyperintense in T2, with an inferior and lateral solid part (20 mm of maximum diameter) ()).Citation6,Citation15,Citation16 The patient underwent an excisional biopsy of the brain lesion which documented a prostate adenocarcinoma metastasis with high expression of PSA, PAP, ERG e pan-CK ().
Figure 1 Timeline with main clinical events and patient’s PSA variation from January 2016 to June 2020.
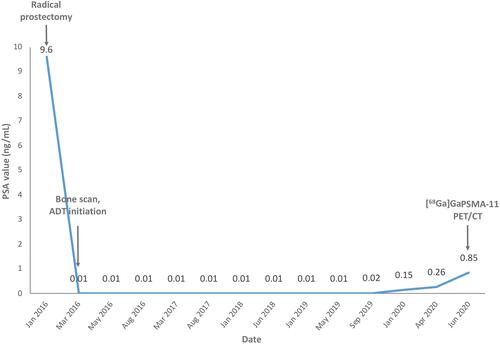
Figure 2 [68Ga]Ga-PSMA-11 PET/CT and MRI findings in the patients showing heterogeneous mass in the right temporal region with central cystic components and peripheral solid components surrounded by peri-lesional edema finally diagnosed as intracranial metastasis due to prostate cancer. (A) [68Ga]Ga-PSMA-11 PET/CT MIP image from vertex to upper tight; (B) trans-axial PET/CT superimposed image showing increased radiopharmaceutical uptake at the right temporal lobe, further confirmed by MRI study; (C) trans-axial ADC map; (D(i), D(ii)) trans-axial contrast-enhanced T1-weighted images; (E(i), E(ii)) trans-axial T2-weighted images; (F(i), F(ii)) trans-axial FLAIR images; (G–J) At histopathological level, the tumor cells presented with rounded nuclei with small nucleoli, forming acinar structures (G), and expressed ERG (nuclear staining) (H), PAP (I) e PSA (J).
![Figure 2 [68Ga]Ga-PSMA-11 PET/CT and MRI findings in the patients showing heterogeneous mass in the right temporal region with central cystic components and peripheral solid components surrounded by peri-lesional edema finally diagnosed as intracranial metastasis due to prostate cancer. (A) [68Ga]Ga-PSMA-11 PET/CT MIP image from vertex to upper tight; (B) trans-axial PET/CT superimposed image showing increased radiopharmaceutical uptake at the right temporal lobe, further confirmed by MRI study; (C) trans-axial ADC map; (D(i), D(ii)) trans-axial contrast-enhanced T1-weighted images; (E(i), E(ii)) trans-axial T2-weighted images; (F(i), F(ii)) trans-axial FLAIR images; (G–J) At histopathological level, the tumor cells presented with rounded nuclei with small nucleoli, forming acinar structures (G), and expressed ERG (nuclear staining) (H), PAP (I) e PSA (J).](/cms/asset/1e794181-c617-452a-8ca1-9e01d3353116/drru_a_12180586_f0002_c.jpg)
After the excisional biopsy, PSA decreased to 0.05 ng/mL. Follow-up brain MRI excluded residual disease ()). Accordingly, the patient was defined as oligometastatic and treated with external beam radiation therapy (EBRT) on pelvic nodal metastases.
Discussions
We report the case in which [68Ga]Ga-PSMA-11 PET/CT identified a suspected brain lesion in an asymptomatic patient with biochemical recurrence from PCa and relative low PSA level (0.85 ng/mL). The lesion was confirmed to be a brain metastasis from prostate cancer. The [68Ga]Ga-PSMA-11 PET/CT EAMN Guidelines recommend to acquire images from the base of the skull to the mid-thigh, possibly extending the coverage to the skull and/or the entire lower extremity in presence of symptoms or disseminated disease.Citation13 Our patient was completely asymptomatic, and the level of PSA did not support the hypothesis of a disseminated disease. However, if we did not include the skull in the field of view, the patient would be misdiagnosed and the diagnosis of brain metastases would be delayed until symptoms appear, possibly resulting in an unfavorable outcome. Indeed, although post-hoc studies of enzalutamide trials in the pre- and post-chemotherapy mCRPC setting demonstrate a certain degree of response, PCa patients with visceral metastases invariably have a worse prognosis than patients with bone-only metastases, and the unfavorable outcome related to visceral metastases is shown also in the oligometastatic subgroup.Citation17–Citation20 PSMA imaging is recommended – for its proved ability to positively influence the subsequent treatment strategy – by the European Association of Urology (EAU) guidelines on PCa for any case of biochemical recurrence after radical prostatectomy (PSA > 0.2mg/mL), as in the case of our patient. Indeed, in this clinical setting, PSMA-PET has proved to outperform other radiotracers, such as choline or fluciclovine.Citation21,Citation22 However, despite the PSMA expression is predominant in PCa, it is not specific and can be found in other benign conditions and malignant tissues, especially in tumor-associated endothelial cells in neovasculature.Citation23,Citation24 In particular, PSMA overexpression has been reported in brain parenchyma after ischemic strokes and in some brain tumors such as gliomas, meningiomas, paragangliomas and neurofibromas.Citation25,Citation26 As mentioned above, brain metastases from prostate cancer are rare, and typically occurred in the more advanced stages of the disease, in the context of a disseminated systemic symptomatic disease.Citation3,Citation4,Citation27 Early diagnosis of visceral disease – and particularly brain parenchymal involvement – is crucial, possibly positively impacting on patient’s management and prognosis.Citation28
MRI is the modality of choice for the detection of brain metastases. However, it is not routinely performed in the surveillance of PCa patients unless neurological manifestations appear.Citation16 Intracranial metastases originating from prostate cancer may variably manifest, and can appear as hemorrhagic lesions (as in the case of metastases from melanoma, renal cell carcinoma, breast and thyroid cancer) or non-hemorrhagic lesions. On contrast-enhanced MRI, intracranial metastases from prostate cancer appear as well-defined lesions, surrounded by edema with an enhancement pattern that varies from purely solid to ring-like and from mixed cystic to solid.Citation6 In our patient, MRI showed a mixed cystic intracranial non-hemorrhagic lesion ().
In our case, the lack of symptoms and the relatively low PSA level (0.85 ng/mL at the time of PET/CT), raised questions about the nature of the lesion, posing the differential diagnosis between a brain metastases and a primary brain tumor. Indeed, literature shows that brain PCa metastases typically occur in patients suffering from advanced PCa with markedly elevated PSA values.Citation6,Citation29,Citation30 In our patient multimodal [68Ga]Ga-PSMA-11 PET/CT together with brain MRI findings were suggestive of brain metastases, anticipating any neurological symptoms eventually impacting on the natural history of the disease. The subsequent surgical treatment determined a biochemical drop in PSA level. Taking advantages of the experience gained in this case, we underline the importance of appropriate imaging acquisition protocols covering the whole-body (from the top of the head to the thigh), and highlight the noteworthiness to evaluate brain parenchyma even in the low PSA cases avoiding misregistration due to movements artifacts. Such lesson on [68Ga]Ga-PSMA-11 along with the proper education on its strengths and pitfalls is timely and necessary, given the promise for successfully treated mCRPC patients with radioligand therapy.Citation31 Effective treatment options for PCa patients suffering from visceral metastases, are among the new therapeutic challenges in PCa for both survival length and quality of life.
Conclusion
Our case shows the capability of [68Ga]Ga-PSMA-11 PET/CT to detect metachronous distant brain metastases in low biochemical recurrent asymptomatic PCa patient. Acquisition from the vertex to thigh, to include the evaluation to the whole brain parenchyma in the field of view, should be considered regardless of the PSA level.
Institutional Approval
The patient was studied within a clinical trial (EudraCT number: 2018-004458-14) approved by AIFA and the Comitato Etico Regione Toscana Area Vasta Nord Ovest. In addition, the patient provided a specific written informed consent for the utilization of both case details and imaging data here presented within this specific publication; however, no further specific institutional board approved was requested.
Disclosure
Prof. Dr. Paola Anna Erba report grants from AIRC, during the conduct of the study; grants from AAA, personal fees, non-financial support from GE, royalties from Springer, personal fees, non-financial support from Philogen outside the submitted work. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Iwamoto H, Izumi K, Kadono Y, et al. Incidences of visceral metastases from prostate cancer increase after progression of castration-resistant status. J Clin Oncol. 2018;36(6_suppl):291. doi:10.1200/JCO.2018.36.6_suppl.291
- Pezaro C, Omlin A, Lorente D, et al. Visceral disease in castration-resistant prostate cancer. Eur Urol. 2014;65(2):270–273. doi:10.1016/j.eururo.2013.10.055
- Craig J, Woulfe J, Sinclair J, et al. Isolated brain metastases as first site of recurrence in prostate cancer: case report and review of the literature. Curr Oncol. 2015;22(6):e493–7. doi:10.3747/co.22.2542
- Gzell CE, Kench JG, Stockler MR, et al. Biopsy-proven brain metastases from prostate cancer: a series of four cases with review of the literature. Int Urol Nephrol. 2013;45(3):735–742. doi:10.1007/s11255-013-0462-7
- Tremont-Lukats IW, Bobustuc G, Lagos GK, et al. Brain metastasis from prostate carcinoma: the M. D. Anderson Cancer Center experience. Cancer. 2003;98(2):363–368. doi:10.1002/cncr.11522
- Ross MI, Bird N, Mendichovszky IA, et al. Neurologically asymptomatic cerebral oligometastatic prostate carcinoma metastasis identified on [Ga]Ga-THP-PSMA PET/CT. EJNMMI Res. 2020;10(1):108. doi:10.1186/s13550-020-00696-0
- Hatzoglou V, Patel GV, Morris MJ, et al. Brain metastases from prostate cancer: an 11-year analysis in the MRI era with emphasis on imaging characteristics, incidence, and prognosis. J Neuroimaging. 2014;24(2):161–166. doi:10.1111/j.1552-6569.2012.00767.x
- Gordon IO, Tretiakova MS, Noffsinger AE, et al. Prostate-specific membrane antigen expression in regeneration and repair. Mod Pathol. 2008;21(12):1421–1427. doi:10.1038/modpathol.2008.143
- Seifert R, Seitzer K, Herrmann K, et al. Analysis of PSMA expression and outcome in patients with advanced prostate cancer receiving. Theranostics. 2020;10(17):7812–7820. doi:10.7150/thno.47251
- Aggarwal R, Wei X, Kim W, et al. Heterogeneous flare in prostate-specific membrane antigen positron emission tomography tracer uptake with initiation of Androgen pathway blockade in metastatic prostate cancer. Eur Urol Oncol. 2018;1(1):78–82. doi:10.1016/j.euo.2018.03.010
- Perera M, Papa N, Roberts M, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. 2020;77(4):403–417. doi:10.1016/j.eururo.2019.01.049
- Van den Wyngaert T, Strobel K, Kampen WU, et al. The EANM practice guidelines for bone scintigraphy. Eur J Nucl Med Mol Imaging. 2016;43(9):1723–1738. doi:10.1007/s00259-016-3415-4
- Fendler WP, Eiber M, Beheshti M, et al. Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44(6):1014–1024. doi:10.1007/s00259-017-3670-z
- Ceci F, Oprea-Lager DE, Emmett L, et al. E-PSMA: the EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur J Nucl Med Mol Imaging. 2021;48(5):1626–1638. doi:10.1007/s00259-021-05245-y
- Fink KR, Fink JR. Imaging of brain metastases. Surg Neurol Int. 2013;4(Suppl 4):S209–19. doi:10.4103/2152-7806.111298
- Deike-Hofmann K, Thünemann D, Breckwoldt MO, et al. Sensitivity of different MRI sequences in the early detection of melanoma brain metastases. PLoS One. 2018;13(3):e0193946. doi:10.1371/journal.pone.0193946
- Bourlon MT, Flaig TW. Visceral metastases in prostate cancer: an underestimated and understudied subgroup. Oncology (Williston Park). 2014;28(11):980–986. doi:10.46883/ONC.2014.2811.0980
- Buelens S, De Bleser E, Dhondt B, et al. Importance of metastatic volume in prognostic models to predict survival in newly diagnosed metastatic prostate cancer. World J Urol. 2019;37(12):2565–2571. doi:10.1007/s00345-018-2449-6
- Alumkal JJ, Chowdhury S, Loriot Y, et al. Effect of visceral disease site on outcomes in patients with metastatic castration-resistant prostate cancer treated with enzalutamide in the PREVAIL Trial. Clin Genitourin Cancer. 2017;15(5):610–617.e3. doi:10.1016/j.clgc.2017.02.007
- Pond GR, Sonpavde G, de Wit R, et al. The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. Eur Urol. 2014;65(1):3–6. doi:10.1016/j.eururo.2013.09.024
- Mottet N, Cornford P, van den Bergh R, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. EAU Guidelines. Edn. Presented at the EAU Annual Congress Amsterdam; 2020.
- Morigi JJ, Stricker PD, van Leeuwen PJ, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56(8):1185–1190. doi:10.2967/jnumed.115.160382
- Sheikhbahaei S, Afshar-Oromieh A, Eiber M, et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur J Nucl Med Mol Imaging. 2017;44(12):2117–2136. doi:10.1007/s00259-017-3780-7
- Ristau BT, O’Keefe DS, Bacich DJ. The prostate-specific membrane antigen: lessons and current clinical implications from 20 years of research. Urol Oncol. 2014;32(3):272–279. doi:10.1016/j.urolonc.2013.09.003
- Noto B, Vrachimis A, Schäfers M, et al. Subacute stroke mimicking cerebral metastasis in 68Ga-PSMA-HBED-CC PET/CT. Clin Nucl Med. 2016;41(10):e449–51. doi:10.1097/RLU.0000000000001291
- Bertagna F, Albano D, Cerudelli E, et al. Potential of radiolabeled PSMA PET/CT or PET/MRI diagnostic procedures in gliomas/glioblastomas. Curr Radiopharm. 2020;13(2):94–98. doi:10.2174/1874471012666191017093721
- Bhambhvani HP, Greenberg DR, Srinivas S, et al. Prostate cancer brain metastases: a single-institution experience. World Neurosurg. 2020;138:e445–e449. doi:10.1016/j.wneu.2020.02.152
- Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: report of the advanced prostate cancer consensus conference 2019. Eur Urol. 2020;77(4):508–547. doi:10.1016/j.eururo.2020.01.012
- Chan M, Hsiao E, Turner J. Cerebellar metastases from prostate cancer on 68Ga-PSMA PET/CT. Clin Nucl Med. 2017;42(3):193–194. doi:10.1097/RLU.0000000000001526
- Yin C, Ho B, Chan L, et al. Asymptomatic prostate cancer brain metastases on 68Ga-PSMA PET/CT. Clin Nucl Med. 2019;44(6):e382–e384. doi:10.1097/RLU.0000000000002548
- Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patient with metastatic castration-resistant prostate cancer (TheraP): a randomized, open-label, Phase 2 trial. Lancet. 2021;397(10276):797–804. doi:10.1016/S0140-6736(21)00237-3
