Abstract
Purpose
Haemorrhagic cystitis may be due to different etiologies with infectious diseases representing an insidious cause to diagnose. The aim of this narrative review is to provide a comprehensive overview of less common but difficult-to-diagnose causes of infectious haemorrhagic cystitis of bacterial, mycobacterial, and parasitic origin, Moreover, we highlight possible diagnostic tools and currently available treatment options in order to give an updated tool for urologists to use in daily practice.
Patients and Methods
The search engine PubMed was used to select peer-reviewed articles published from 1/Jan/2010 to 31/Aug/2022.
Results
Bacteria, fungal, TB and schistosomiasis are uncommon causes of haemorrhagic cystitis burdened by high morbidity, especially if not promptly diagnosed.
Conclusion
Because haemorrhagic cystitis ranges in severity from mild dysuria associated with pelvic discomfort to severe life-threatening haemorrhage, punctual diagnosis, and immediate treatment are essential to avoid further complications.
Introduction
Cystitis is the inflammation of the urinary bladder; it can be acute or chronic and the degree of severity can range from mild dysuria associated with pelvic discomfort to severe life-threatening haemorrhage.Citation1 Haemorrhagic cystitis, defined as the necrosis of bladder transitional epithelium and associated blood vessels leading to hematuria, may be due different etiologies: it is usually caused by chemical and/or toxin, radiation, as well as mechanical insults; however, infectious diseases (bacterial/mycobacterial, fungal, parasitic or viral) may represent an insidious and difficult-to-diagnose cause of hemorrhagic cystitis.Citation2 In fact, haemorrhagic cystitis can develop in the context of a systemic infectious disease (eg, Cytomegalovirus (CMV), Tuberculosis) or may be isolated to the urinary bladder (eg, uropathogenic Escherichia coli).Citation3,Citation4 While the symptoms are typical (dysuria, frequency and urgency, irritative voiding symptoms, suprapubic pain, and haematuria), they are not specific to a single infectious etiology.Citation5 Indeed, the individual level of immune suppression is related to the infectious agents that can be responsible for hemorrhagic cystitis.Citation6 In this narrative review, we provide a comprehensive overview of less common but difficult-to-diagnose causes of infectious hemorrhagic cystitis of bacterial, mycobacterial, and parasitic origin; moreover, we highlight possible diagnostic tools and currently available treatment options for each pathogen.
Materials and Methods
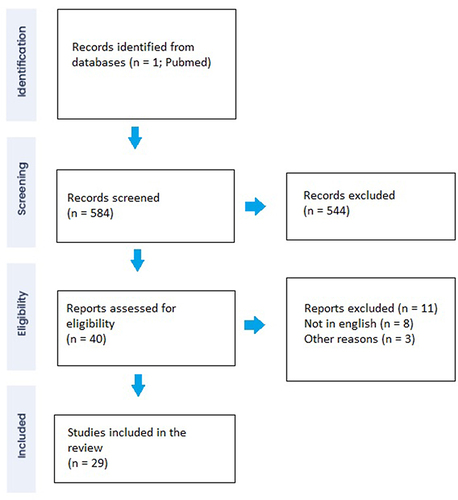
A narrative review was carried out to retrieve the scientific evidence on bacterial, Candida spp, Tuberculosis (TB), and Schistosoma spp. causes of haemorrhagic cystitis. The search engine PubMed was used to select peer-reviewed articles published from 1/Jan/2010 to 31/Aug/2022. The references of the selected manuscripts were carefully assessed to collect articles not included in the primary search by two different authors (NR and GM). The following keywords were used to broaden the spectrum of the article: (haemorrhagic cystitis) AND (infection); (haemorrhagic cystitis) AND (infection). A total of 584 records were found. Based on titles, abstracts, and the content of the full texts, a total of 29 studies were deemed suitable ().
Results
Fungal Infection
Fungal infections of the urinary tract are a relatively rare condition with an incidence of 1–2.2%.Citation7 Candida spp. is the most common organism that can cause urinary tract infections (UTIs) among fungi.Citation8,Citation9 Other fungi include Cryptococcus neoformans, Aspergillus spp., and Mucorales. Blastomyces dermatitidis can cause acute prostatitis, and both B. dermatitidis and Histoplasma capsulatum can be responsible for epididymal-orchitis, but they are not usually involved in hemorrhagic cystitis.Citation9 Fungal infections associated with hemorrhagic cystitis include Candida spp., Aspergillus spp., and Mucorales.
Candida Species
Epidemiology
Candida spp. is a fungal pathogen that can be part of the healthy human microbiome but can also cause mucosal infections or invasive candidiasis.Citation10 Candida urinary tract infections (UTIs) are less common in the community, whereas they are more frequently reported in hospitalized and immunocompromised patients.Citation8 Literature lacks standardized and generalizable criteria for defining infection and colonization.Citation8 The prevalence of candiduria (≥104 cfu/mL in women and ≥5 × 103 cfu/mL in men) in urine samples from both outpatients and inpatients is high, accounting for 1% of urine cultures positive for any pathogen in hospital laboratories (0.2% of all cultures evaluated).Citation11 The incidence of candiduria can increase up to 22% in intensive care units,Citation12 17% in catheterized patients with spinal cord injury,Citation13 3.4–11% in renal transplant recipients,Citation14,Citation15 and 10% in patients with type 2 diabetes patients.Citation16 However, the clinical significance of candiduria is controversial. In a prospective multicenter observational study, only 2–4% of patients with candiduria had lower urinary tract symptoms (LUTS) or hematuria suggestive of UTIs.Citation17 Some risk factors for Candida urinary tract infections have been identified, including diabetes mellitus, indwelling catheters, renal transplantation, and immunosuppression.Citation8,Citation12,Citation18
Clinical Presentation
Most patients diagnosed with candiduria are asymptomatic and do not need any medical medicalt. If symptoms are present, clinical features are usually indistinguishable from bacterial UTIs.Citation8 Hemorrhagic cystitis due to Candida species is uncommon and usually belongs to high-risk patients such as HIV-infected patients with persistent low CD4+ cell count, hematological, and/or oncological patients with persistent neutropenia.Citation19 Greene et al reported a case of hemorrhagic emphysematous cystitis (EC) secondary to Candida albicans in a patient with agnogenic myeloid metaplasia and treated with prolonged intermittent corticosteroids.Citation20
Tran et al reported a unique case of haemorrhagic Candida glabrata cystitis in an immunocompetent patient after three months of therapy with empagliflozin.Citation21 The patient had a history of type 2 diabetes and neurogenic bladder after transurethral resection of the prostate. The Authors did not provide information on diabetes control. They hypothesized that empagliflozin-induced glycosuria may have played a role in the development of the infection. It is well established that empagliflozin is associated with a higher rate of genital mycotic infectionsCitation22 and in some rare cases has been associated with disruptive clinical conditions, such as Fournier’s gangrene.Citation23
Nonetheless, a pooled analysis of four Phase 3 trials did not demonstrate an increased risk of UTIs for empagliflozin compared with placebo. Therefore, the evidence for an etiologic link between empagliflozin and hemorrhagic cystitis remains controversial.Citation24
Anecdotal cases of coinfections with multiple fungal organisms have been reported in the literature. A rare double fungal infection of the bladder due to Candida albicans and Cladosporium presented with lower urinary tract symptoms (LUTS) and hematuria was reported by Kandaswami et al.Citation20
Diagnosis
Although detection of Candida in urine culture is essential for diagnosis, colony counts do not help to distinguish contamination from colonization or infection.Citation18 Cystoscopic examination may reveal pseudo membranes or pale plaques overlying the urothelium. Ultrasonography or computed tomography may be used in selected cases to detect complications (fungus ball, abscesses, pyelonephritis) or abnormalities of the urinary tract.Citation8 Imaging studies may be recommended to find any risk factor (for example, urinary retention) in case of failure of antifungal therapy.Citation25
Treatment
Fluconazole is usually the drug of choice, pending antifungal susceptibility test (400 mg loading dose, 200 mg (3 mg/kg) daily for 14 days) due to its good penetration in the urine and in the bladder. In patients with Candida spp resistant to fluconazole, liposomal amphotericin B, 3–5 mg/kg daily for 1–7 days, or oral flucytosine, 25 mg/kg 4 times daily for 7–10 days may be considered.Citation8,Citation26 In selected patients (intolerance to systemic therapy, adverse reactions, or azole-resistant organisms) amphotericin B deoxycholate bladder irrigation (50 mg/L sterile water daily for 5 days) may be considered.Citation27
Other Fungal Infections
The literature on other fungal organisms as etiologic factors of hemorrhagic cystitis is scarce and only a few case reports are available. Mucorales, Cryptococcus neoformans, Aspergillus fumigatus, and Torulopsis glabrata may be associated with haemorrhagic cystitis.Citation28,Citation29 Muthe et al reported the case of a 60-year-old diabetic woman diagnosed with a mucormycosis infection of the urinary tract presenting with LUTS and recurrent hematuria during SARS-CoV-2 infection.Citation20 Rhizopus species was detected in a fungus-ball-specimen retrieved during cystoscopy in a patient with haemorrhagic cystitis. The patient received intravenous liposomal amphotericin B for 6 weeks followed by posaconazole 300 mg daily for 4 weeks. Complete recovery of symptoms was reported after 10 days. According to the guidelines of the Third European Confederation of Medical Mycology, and the Mycoses Study Group Education and Research Consortium, the first-line therapy for mucormycosis is liposomal amphotericin B (L-AMB) and surgical resection.Citation30 However, despite effective antifungal treatment combined with surgery, mucormycosis maintains a poor prognosis with a mortality rate of 60–90%, particularly in immunocompromised patients.Citation31,Citation32
Bacterial Infection
Epidemiology
Uropathogenic Escherichia coli (UPEC) is by far the most common cause of UTI, accounting for 80% of outpatient infections and 25% of nosocomial infections.Citation33 Among the most common bacterial causes of hemorrhagic cystitis Escherichia coli, Staphylococcus saprophyticus, Proteus spp, and Klebsiella spp can be found.Citation29,Citation34 Non-typhoidal Salmonella (NST)Citation35,Citation36 and Chryseobacterium indologenes are anecdotally reported as bacterial causes of haemorrhagic cystitis.Citation37
Clinical Presentation
Bacterial hemorrhagic cystitis presents with LUTS and hematuria and is indistinguishable from other infectious causes. Chronic and recurrent bacterial cystitis can rarely lead to life-threatening complications, such as spontaneous bladder rupture. Olanipekun et al reported a case of a 57-year-old woman with diabetes mellitus and end-stage renal disease on hemodialysis who presented with bladder necrosis and perforation due to E. coli.Citation38
A rare form of complicated bacterial UTIs is emphysematous cystitis (EC) which is characterized by the presence of gas within the bladder wall and the lumen.Citation39 EC is more frequently reported in elderly women with uncontrolled diabetes mellitus. Other risk factors for EC include recurrent UTIs, neurogenic bladder, and urinary stasis due to bladder outlet obstructions.Citation39 The most common gas-producing bacteria are E. coli (60%) and Klebsiella pneumonia (10–20%).Citation40,Citation41
Clinical symptoms of EC range from asymptomatic conditions (up to 7%) to severe sepsis.Citation39 Gross haematuria occurs in 60% of cases.Citation39,Citation42 The most common manifestation is abdominal pain (80%), whereas pneumaturia is usually less frequent.Citation39
A few anecdotal cases of hemorrhagic cystitis due to less common bacteria have been reported in the literature. Teh et al described the case of an immunocompetent 27-year-old male who presented with gross hematuria and LUTS after a five-month trip through South America. He also complained of sweating, fever, nausea, vomiting, abdominal cramps, and diarrhea. A urine culture at presentation revealed Salmonella Oranienburg. The patient reported complete symptomatic recovery after the administration of intravenous ampicillin and gentamicin and subsequent oral switch to amoxicillin–clavulanate.Citation35 Another case of hemorrhagic cystitis due to NST that caused massive bleeding and shock was described by Na et al.Citation36 He presented with disorientation, high temperature (38°C), pyuria, hematuria, and watery diarrhea. He suffered from not well-controlled diabetes.
Diagnosis
The most important investigation in the evaluation of a patient with bacterial haemorrhagic cystitis is midstream urine culture with antimicrobial susceptibility testing (AST) in order to guide appropriate treatment.Citation43 Ultrasound (US) should be performed to rule out upper urinary tract obstruction or renal stone disease in patients with a history of urolithiasis, renal dysfunction, or high urine pH.Citation44 In patients with persistent hematuria or pathogens other than E. coli, urethrocystoscopy and further imaging, such as a contrast-enhanced computed tomography (CT) scan, or excretory urography, may be recommended to detect underlying conditions and to estimate the severity of infection.Citation1 In case of symptomatic EC, abdominal CT could be useful to assess the presence of ascending emphysematous infections, intraabdominal abscesses, or adjacent neoplastic disease.Citation39
Treatment
Antibiotics, bladder drainage, and treatment of predisposing conditions are the most important aspect of the treatment of hemorrhagic cystitis. Antimicrobial treatment is always guided by AST, while empiric antibiotic treatment should cover Gram negative pathogens and should be guided by local epidemiological resistance assessment.Citation45
Tuberculosis
Epidemiology
According to 2022 WHO Global Tuberculosis (TB) Report, TB cases are increasing across the world, and 10.6 million people fell ill with TB in 2021.Citation46 Pulmonary dis-ease is the most common presentation of TB,Citation47 however between 5% and 45% of disease localization have features of extrapulmonary TB (EPTB), and genito-urinary TB (GUTB) is among the most frequent localization.Citation48 Moreover, between 2% and 20% of cases of pulmonary TB show concurrent genitourinary involvement through haematogenous dissemination of Mycobacterium tuberculosis from the lungs to kidneys, ureters, bladder, prostate, epididymis, urethra, ejaculatory ducts, and genital organs, often well before a diagnosis of pulmonary disease has been established.Citation49,Citation50 Due to its frequently asymptomatic presentation, the prevalence of GUTB is hard to estimate. However, 15–20% of the 10 million new TB diagnoses presented with extrapulmonary TB annually.Citation50 GUTB is most frequently described in middle-aged (40 to 50 years old) and males (2-to-1 ratio).Citation50 Nation-wide TB prevalence, TB-HIV co-infection rates, or individual-specific characteristics, such as immunosuppression, malnutrition, renal transplantation, chronic renal disease and dialysis, liver disease, alcohol or substance abuse, and homelessness may further increase the risk of GUTB.Citation50
Clinical Presentation
Symptoms of GUTB, such as pollakiuria and incontinence, appear when the chronic inflammatory insult to the bladder wall epithelium causes fibrosis of the tissue and ureterovesical junction dysfunction, resulting in reduced bladder capacity (usually less than 100mL).Citation49 Other described symptoms of GUTB are low-back pain (34.4%) and hematuria (35.6% overall, 24.5% in developed countries, 44.3% in developing countries).Citation49 Furthermore, GUTB may cause symptoms only in advanced stages of the diseaseCitation48 when vesicoureteral reflux has already determined chronic renal failure.Citation51
Diagnosis
The gold standard for the diagnosis of GUTB is the identification of the mycobacterium on culture of urine samples; however, this method is often untimely (with from 4to8 weeks required), requires multiple samples (3–6 morning urine samples), with a reported sensitivity varying from 10.7 to 90%.Citation52 Direct microscopy of Ziehl-Neelsen staining of urine samples for the detection of mycobacteria is a rapid test with a specificity of 96.7% and sensitivity of 42.1–52.1%, but it cannot discriminate between Mycobacterium tuberculosis and other mycobacterial species.Citation53 The sensitivity and specificity of off-label use of molecular testing (GenXpert) on urine samples ranged from 89% and 95% in culture-confirmed cases, while to 55% and 99% in clinically diagnosed GUTB cases with a composite reference standard;Citation54–56 although not validated for extrapulmonary samples, this test combines the possibility of an early diagnosis with the detection of genotypic resistance to rifampicin; conversely, a negative test may not exclude GUTB. In the subgroup of people living with HIV (PLWH), lateral flow lipoarabinomannan (LF-LAM) assay on urinary specimens may be considered to consolidate the diagnosis of TB in combination with other methods.Citation57 Radiological testing such as intravenous urography and abdominal computerized tomography scan (CT-scan) may support initial clinical suspect, demonstrating the presence of calyceal irregularities, infundibular stenosis, pseudotumor or renal scarring, renal cavitation, urinary tract calcification, collecting system thickening, stenosis, or dilatation, and contracted bladder:Citation58 these findings have shown sensitivity up to 91.4% for GUTB.Citation52 Invasive procedures (cystoscopy, transurethral biopsy) may reveal the presence of histological alteration compatible with mycobacterial infection and support clinical suspicion when other methods are inconclusive.Citation59
Treatment
The treatment regimen for GUTB is unaltered from the regimen used to treat pulmonary TB and is guided by drug-susceptibility-testing (DST); for drug-susceptible TB (DS-TB) 2 months of rifampicin (R), isoniazid (H), pyrazinamide (Z) and ethambutol (E), followed by 4 months of HR alone are indicated. Resistance patterns may vary and may comprehend first-line drugs as H and R (multi-drug-resistant TB or MDR-TB), or first-line drugs and any second-line drug as fluoroquinolones (FLQ) (pre-extensively DR-TB or pre-XDR-TB) and at least one of bedaquiline (BDQ) and linezolid (LZD) (ex-tensively-drug-resistant TB or XDR-TB); expert specialists in the management of drug-resistant TB (DR-TB) should select the adequate regimen in these cases.Citation60 Surgical interventions to restore adequate bladder capacities, such as augmentation or orthotopic neobladder via devulgarized bowel segments, have been performed in case of severe fibrosis to avoid vesicoureteral reflux and subsequent kidney injury. Late diagnosis of GUTB may result in progression of disease to the point that ablative surgery represents the only available approach.Citation61,Citation62
Schistosomiasis
Epidemiology
Schistosoma haematobium, a trematode with a widespread presence in developing countries’ fresh waters,Citation63 responsible for infecting 250 million people across the world and for 280.000 deaths each year, is a common cause of genito-urinary bleeding.Citation64 However, schistosomiasis may be underdiagnosed in non-endemic countries.Citation65,Citation66
Clinical Presentation
Although mostly asymptomatic, haematuria and dysuria may be present in the early and post-acute phases of schistosomiasis alongside fever, and nonspecific systemic symptoms.Citation67 Hydronephrosis secondary to urinary tract obstruction with or without kidney involvement, bladder calcification, urothelial damage, bladder squamous cell carcinoma, as well as fertility decline may follow the progression of the disease.Citation67
Diagnosis
Although lacking a gold standard definition, the detection of viable eggs through urine filtration microscopy is the mainstay for the diagnosis of schistosomiasis,Citation68 while serological assays may represent a valuable option for screening of imported cases in low-burden countries, with a reported sensitivity ranging from 21.4 to 71.4% and specificity ranging from 48.8% to 94.3%.Citation69,Citation70 Further diagnostic methods, including anti-genic and molecular tests, are under study.Citation71
Treatment
Praziquantel (PZQ) is considered the best available drug for schistosomiasis although its mechanism of action is not fully understood.Citation71,Citation72 In the absence of standardized regimen, a single-day, oral dose of 40 mg/kg of PZQ is indicated by most authors as safe and effective for all Schistosoma spp, either in one or divided in two administrations,Citation71 with a reported cure rate of 77.1% for S. haematobium.Citation73 No correlation between cure rates and dosage of PZQ has been described for S. hematobium,Citation73 how-ever, especially in low-burden settings, higher dosages (up to 80 mg/kg), or a second PZQ dose after 15 days from the first, have been safely and effectively used.Citation71 Reports of decreased PZQ sensitivity in some Schistosoma spp. strains are concerning;Citation72 however, new therapeutic strategies are in development.Citation72,Citation74,Citation75
Conclusion
Bacteria, fungal, TB, and schistosomiasis are uncommon causes of haemorrhagic cystitis burdened by high morbidity, especially if not promptly diagnosed. Due to the paucity of literature and the difficult-to-diagnose presentation, especially in Western countries, urologists are not well aware of which infectious disease agent may be the cause of haemorrhagic cystitis.
Because haemorrhagic cystitis ranges in severity from mild dysuria associated with pelvic discomfort to severe life-threatening haemorrhage, punctual diagnosis, and immediate treatment are essential to avoid further complications.
Disclosure
The authors report no conflicts of interest in this work.
References
- Laguna MP, Albers P, Algaba F. EAU guidelines. Edn. Presented at the EAU Annual Congress Amsterdam 2022. ISBN 978-94-92671-16-5; 2022.
- Haldar S, Dru C, Bhowmick NA. Mechanisms of hemorrhagic cystitis. Am J Clin Exp Urol. 2014;2(3):199–208.
- Mantica G, Ambrosini F, Riccardi N, et al. Genitourinary tuberculosis: a comprehensive review of a neglected manifestation in low-endemic countries. Antibiot Basel Switz. 2021;10(11):1399.
- Furukawa R, Homma H, Inoue T, Horiuchi H, Usui K. Cytomegalovirus hemorrhagic cystitis in a malignant glioma patient treated with temozolomide. Intern Med Tokyo Jpn. 2018;57(20):3047–3050. doi:10.2169/internalmedicine.1005-18
- Visintini E, Visintini C, Venturini M, Palese A. Patients’ experience of haemorrhagic cystitis after Haematopoietic Stem Cell Transplantation: findings from a phenomenological study. Eur J Oncol Nurs. 2021;51:101926. doi:10.1016/j.ejon.2021.101926
- Mori Y, Miyamoto T, Kato K, et al. Different risk factors related to adenovirus- or BK virus-associated hemorrhagic cystitis following allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(3):458–465. doi:10.1016/j.bbmt.2011.07.025
- Ismail M, Hashim H. Complications of fungal cystitis. Curr Bladder Dysfunct Rep. 2013;8(3):212–216. doi:10.1007/s11884-013-0191-x
- Odabasi Z, Mert A. Candida urinary tract infections in adults. World J Urol. 2020;38(11):2699–2707. doi:10.1007/s00345-019-02991-5
- Kauffman CA. Diagnosis and management of fungal urinary tract infection. Infect Dis Clin North Am. 2014;28(1):61–74. doi:10.1016/j.idc.2013.09.004
- World Health Organization (WHO). Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO.
- Colodner R, Nuri Y, Chazan B, Raz R. Community-acquired and hospital-acquired candiduria: comparison of prevalence and clinical characteristics. Eur J Clin Microbiol Infect Dis. 2008;27(4):301–305. doi:10.1007/s10096-007-0438-6
- Álvarez-Lerma F, Nolla-Salas J, León C, et al. Candiduria in critically ill patients admitted to intensive care medical units. Intensive Care Med. 2003;29(7):1069–1076. doi:10.1007/s00134-003-1807-y
- Goetz LL, Howard M, Cipher D, Revankar SG. Occurrence of candiduria in a population of chronically catheterized patients with spinal cord injury. Spinal Cord. 2010;48(1):51–54. doi:10.1038/sc.2009.81
- Delgado J, Calvo N, Gomis A, et al. Candiduria in renal transplant recipients: incidence, clinical repercussion, and treatment indication. Transplant Proc. 2010;42(8):2944–2946. doi:10.1016/j.transproceed.2010.08.019
- Safdar N, Slattery WR, Knasinski V, et al. Predictors and outcomes of candiduria in renal transplant recipients. Clin Infect Dis. 2005;40(10):1413–1421. doi:10.1086/429620
- Esmailzadeh A, Zarrinfar H, Fata A, Sen T. High prevalence of candiduria due to non- albicans Candida species among diabetic patients: a matter of concern? J Clin Lab Anal. 2018;32(4):e22343. doi:10.1002/jcla.22343
- Kauffman CA, Vazquez JA, Sobel JD, et al. Prospective multicenter surveillance study of funguria in hospitalized patients. Clin Infect Dis. 2000;30(1):14–18. doi:10.1086/313583
- Padawer D, Pastukh N, Nitzan O, et al. Catheter-associated candiduria: risk factors, medical interventions, and antifungal susceptibility. Am J Infect Control. 2015;43(7):e19–e22. doi:10.1016/j.ajic.2015.03.013
- Duong DT, Goodman HS. Eosinophilic cystitis caused by Candida glabrata: a case report. Urol Case Rep. 2019;26:100970. doi:10.1016/j.eucr.2019.100970
- Greene MH. Emphysematous cystitis due toClostridium perfringens and Candida albicans in Two patients with hematologic malignant conditions. Cancer. 1992;70(11):2658–2663. doi:10.1002/1097-0142(19921201)70:11<2658::AID-CNCR2820701115>3.0.CO;2-B
- Tran L, Thomas M, Harvey J, Sampath R, Rose R. 1700. A rare case of candida glabrata hemorrhagic cystitis with empagliflozin use. Open Forum Infect Dis. 2019;6(Supplement_2):S622–S623. doi:10.1093/ofid/ofz360.1564
- Levine MJ. Empagliflozin for type 2 diabetes mellitus: an overview of Phase 3 clinical trials. Curr Diabetes Rev. 2017;13(4). doi: 10.2174/1573399812666160613113556
- Khokhar F, Hernandez C, Mahapatra R. Fournier’s gangrene in an HIV-positive patient on empagliflozin for the treatment of diabetes mellitus. Cureus. 2022. doi:10.7759/cureus.26083
- Kim G, Gerich JE, Salsali A, et al. Empagliflozin (EMPA) increases genital infections but not urinary tract infections (UTIs) in pooled data from four pivotal Phase III trials. Diabetes. 2013;62(Suppl 1):LB21 (Abstract 74–LB).
- Thomas L, Tracy CR. Treatment of fungal urinary tract infection. Urol Clin North Am. 2015;42(4):473–483. doi:10.1016/j.ucl.2015.05.010
- D’Amico MJ, Foss H, Uhr A, Rudnick B, Kloniecke E, Gomella LG. Hemorrhagic cystitis: a review of the literature and treatment options. Can J Urol. 2022;29(5):11276–11283.
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1–e50.
- Muthe MM, Shirsath SD, Shirsath RD, Firke VP. A rare case of isolated vesical mucormycosis in a patient with COVID-19 pneumonitis. Indian J Radiol Imaging. 2022;32(03):408–410. doi:10.1055/s-0042-1744137
- Manikandan R, Kumar S, Dorairajan LN. Hemorrhagic cystitis: a challenge to the urologist. Indian J Urol. 2010;26(2):159–166. doi:10.4103/0970-1591.65380
- Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405–e421. doi:10.1016/S1473-3099(19)30312-3
- Lanternier F, Sun HY, Ribaud P, Singh N, Kontoyiannis DP, Lortholary O. Mucormycosis in organ and stem cell transplant recipients. Clin Infect Dis. 2012;54(11):1–8. doi:10.1093/cid/cis195
- Bader MS, Hawboldt J, Brooks A. Management of complicated urinary tract infections in the era of antimicrobial resistance. Postgrad Med. 2010;122(6):7–15. doi:10.3810/pgm.2010.11.2217
- Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Am J Med. 2002;113(1):14–19. doi:10.1016/S0002-9343(02)01055-0
- Sabih A, Leslie SW. Complicated urinary tract infections. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. Available from: http://www.ncbi.nlm.nih.gov/books/NBK436013/. Accessed July 26, 2023.
- Teh J, Quinlan M, Bolton D. Salmonella Oranienburg haemorrhagic cystitis in an immunocompetent young male. JMM Case Rep. 2017;4(8):e005105. doi:10.1099/jmmcr.0.005105
- Na SK, Jung HK, Kim YS, et al. Hemorrhagic cystitis with massive bleeding from nontyphoidal Salmonella infection: a case report. Kidney Res Clin Pract. 2013;32(2):84–86. doi:10.1016/j.krcp.2013.04.005
- Mehta R, Pathak A. Emerging chryseobacterium indologenes infection in Indian neonatal intensive care units: a case report. Antibiotics. 2018;7(4):109. doi:10.3390/antibiotics7040109
- Olanipekun T, Effoe V, Turner J, Flood M. Bladder necrosis and perforation in end-stage renal disease and recurrent urinary tract infection: a rare medical emergency. Int J Crit Illn Inj Sci. 2019;9(2):101–104. doi:10.4103/IJCIIS.IJCIIS_72_18
- Amano M, Shimizu T. Emphysematous cystitis: a review of the literature. Intern Med. 2014;53(2):79–82. doi:10.2169/internalmedicine.53.1121
- Mokabberi R, Ravakhah K. Emphysematous urinary tract infections: diagnosis, treatment and survival (Case Review Series). Am J Med Sci. 2007;333(2):111–116. doi:10.1097/00000441-200702000-00009
- Bjurlin MA, Hurley SD, Kim DY, et al. Clinical outcomes of nonoperative management in emphysematous urinary tract infections. Urology. 2012;79(6):1281–1285. doi:10.1016/j.urology.2012.02.023
- Yoshida K, Murao K, Fukuda N, Tamura Y, Ishida T. Emphysematous cystitis with diabetic neurogenic bladder. Intern Med. 2010;49(17):1879–1883. doi:10.2169/internalmedicine.49.3247
- Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625–663.
- van Nieuwkoop C, Hoppe BPC, Bonten TN, et al. Predicting the need for radiologic imaging in adults with febrile urinary tract infection. Clin Infect Dis. 2010;51(11):1266–1272. doi:10.1086/657071
- Wagenlehner FME, Hoyme U, Kaase M, Fünfstück R, Naber KG, Schmiemann G. Uncomplicated Urinary Tract Infections. Dtsch Ärztebl Int. 2011. doi:10.3238/arztebl.2011.0415
- World Health organization (WHO). Global Tuberculosis Report 2022. Geneva: World Health Organization; 2022. Licence: cc bY-Nc-sa 3.0 iGo.
- Gow J. Genitourinary tuberculosis. In: Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Campbell’s Urology. 7th ed. Philadelphia, PA: WB Saunders Company; 1998:807–836.
- Kulchavenya E. Extrapulmonary tuberculosis: are statistical reports accurate? Ther Adv Infect Dis. 2014;2(2):61–70. doi:10.1177/2049936114528173
- Figueiredo AA, Lucon AM, Srougi M, Schlossberg D. Urogenital tuberculosis. Microbiol Spectr. 2017;5(1). doi:10.1128/microbiolspec.TNMI7-0015-2016
- Muneer A, Macrae B, Krishnamoorthy S, Zumla A. Urogenital tuberculosis - epidemiology, pathogenesis and clinical features. Nat Rev Urol. 2019;16(10):573–598. doi:10.1038/s41585-019-0228-9
- Figueiredo AA, Lucon AM, Gomes CM, Srougi M. Urogenital tuberculosis: patient classification in seven different groups according to clinical and radiological presentation. Int Braz J Urol. 2008;34(4):422–32; discussion 432. doi:10.1590/S1677-55382008000400004
- Hemal AK, Gupta NP, Rajeev TP, Kumar R, Dar L, Seth P. Polymerase chain reaction in clinically suspected genitourinary tuberculosis: comparison with intravenous urography, bladder biopsy, and urine acid fast bacilli culture. Urology. 2000;56(4):570–574. doi:10.1016/S0090-4295(00)00668-3
- Moussa OM, Eraky I, El-Far MA, Osman HG, Ghoneim MA. Rapid diagnosis of genitourinary tuberculosis by polymerase chain reaction and non-radioactive DNA hybridization. J Urol. 2000;164(2):584–588. doi:10.1016/S0022-5347(05)67427-7
- World Health Organization (WHO). The use of next-generation sequencing technologies for the detection of mutations associated with drug; 2018. Available from: https://apps.who.int/iris/bitstream/handle/10665/274443/WHO-CDS-TB-2018.19-eng.pdf. Accessed August 3, 2021.
- World Health Organization (WHO). Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detec-Tion of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF Assay for the Diagnosis of Pul-Monary and Extrapulmonary TB in Adults and Children: Policy Update. Geneva, Switzerland: World Health Organization; 2013.
- Chen K, Malik AA, Nantasenamat C, et al. Clinical validation of urine-based Xpert® MTB/RIF assay for the diagnosis of urogenital tuberculosis: a systematic review and meta-analysis. Int J Infect Dis. 2020;95:15–21.
- World Health Organization (WHO). The Use of Lateral Flow Urine Lipoarabinomannan Assay (LF-LAM) for the Diagnosis and Screening of Active Tuberculosis in People Living with HIV: Policy Guidance. World Health Organization; 2015. Available from: https://apps.who.int/iris/handle/10665/193633. Accessed July 26, 2023.
- Figueiredo AA, Lucon AM, Arvellos AN, et al. A better understanding of urogenital tuberculosis pathophysiology based on radiological findings. Eur J Radiol. 2010;76(2):246–257. doi:10.1016/j.ejrad.2009.05.049
- Shapiro AL, Viter VI. Цистоскопия и эндовезикальная биопсия при туберкулезе почек [Cystoscopy and endovesical biopsy in renal tuberculosis]. Urol Nefrol. 1989;1:12–15. Russian.
- World Health Organization (WHO). Consolidated Guidelines on Tuberculosis. Module 4: Treatment - Drug-Susceptible Tuberculosis Treatment. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO.
- Figueiredo AA, Lucon AM, Junior RF, Srougi M. Epidemiology of urogenital tuberculosis worldwide: urogenital tuberculosis epidemiology. Int J Urol. 2008;15(9):827–832. doi:10.1111/j.1442-2042.2008.02099.x
- de Figueiredo AA, Lucon AM, Srougi M. Bladder augmentation for the treatment of chronic tuberculous cystitis. Clinical and urodynamic evaluation of 25 patients after long term follow-up. Neurourol Urodyn. 2006;25(5):433–440. doi:10.1002/nau.20264
- Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet Lond Engl. 2014;383(9936):2253–2264. doi:10.1016/S0140-6736(13)61949-2
- LoVerde PT. Schistosomiasis. Adv Exp Med Biol. 2019;1154:45–70.
- Riccardi N, Nosenzo F, Peraldo F, et al. Increasing prevalence of genitourinary schistosomiasis in Europe in the Migrant Era: neglected no more? PLoS Negl Trop Dis. 2017;11(3):e0005237. doi:10.1371/journal.pntd.0005237
- Mantica G, Van der Merwe A, Terrone C, et al. Awareness of European practitioners toward uncommon tropical diseases: are we prepared to deal with mass migration? Results of an international survey. World J Urol. 2020;38(7):1773–1786. doi:10.1007/s00345-019-02957-7
- Chiamah OC, Ubachukwu PO, Anorue CO, Ebi S. Urinary schistosomiasis in Ebonyi State, Nigeria from 2006 to 2017. J Vector Borne Dis. 2019;56(2):87–91. doi:10.4103/0972-9062.263721
- World Health Organization (WHO). Guideline on Control and Elimination of Human Schistosomiasis. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO.
- Comelli A, Riccardi N, Canetti D, et al. Delay in schistosomiasis diagnosis and treatment: a multicenter cohort study in Italy. J Travel Med. 2020;27(1):taz075. doi:10.1093/jtm/taz075
- Kinkel HF, Dittrich S, Bäumer B, Weitzel T. Evaluation of eight serological tests for diagnosis of imported schistosomiasis. Clin Vaccine Immunol. 2012;19(6):948–953. doi:10.1128/CVI.05680-11
- Cucchetto G, Buonfrate D, Marchese V, et al. High-dose or multi-day praziquantel for imported schistosomiasis? A systematic review. J Travel Med. 2019;26(7):taz050. doi:10.1093/jtm/taz050
- Ndamse CC, Masamba P, Kappo AP. Bioorganometallic compounds as novel drug targets against schistosomiasis in Sub-Saharan Africa: an alternative to praziquantel? Adv Pharm Bull. 2022;12(2):283–297. doi:10.34172/apb.2022.029
- Zwang J, Olliaro PL, Jones MK. Clinical efficacy and tolerability of praziquantel for intestinal and urinary schistosomiasis-a meta-analysis of comparative and non-comparative clinical trials. PLoS Negl Trop Dis. 2014;8(11):e3286. doi:10.1371/journal.pntd.0003286
- Molehin AJ, McManus DP, You H. Vaccines for human schistosomiasis: recent progress, new developments and future prospects. Int J Mol Sci. 2022;23(4):2255. doi:10.3390/ijms23042255
- Rennar GA, Gallinger TL, Mäder P, et al. Disulfiram and dithiocarbamate analogues demonstrate promising antischistosomal effects. Eur J Med Chem. 2022;242:114641. doi:10.1016/j.ejmech.2022.114641