Abstract
Purpose
To determine if switching from one brand of the α1-adrenoceptor antagonist naftopidil (Avishot™) to another brand (Flivas™) under the same conditions causes the same changes in lower urinary tract symptoms (LUTS) and quality of life (QOL) as the perceived placebo effect, and if ambient temperature as a nonspecific factor is related to those changes in benign prostatic hyperplasia (BPH) patients.
Patients and methods
A retrospective study was carried out on 217 BPH patients who had received Avishot™ for more than 6 months and then were switched to Flivas™ at the same dose and timing. The two drugs contain the same principal ingredient and display the same pharmacokinetic properties. The International Prostate Symptom Score (IPSS), QOL score, and average monthly ambient temperature at the patients’ residence area from the Automated Meteorological Data Acquisition System in Japan were used for the evaluation.
Results
A significant change in urinary storage symptoms (P = 0.006), and especially in nighttime frequency (P< 0.001), was observed by switching drugs, suggesting the perceived placebo effect. There was significant improvement of daytime frequency (P< 0.05), nighttime frequency (P< 0.001), storage symptoms (P< 0.001), and total IPSS (P< 0.05) when the magnitude of ambient temperature change from before and 3 months after switching drugs was higher than 10°C, while no significant improvement was noted in any of the parameters examined when the same was lower than 10°C.
Conclusion
The present study showed the nonspecific effect of magnitude of ambient temperature change was involved in the perceived placebo effect on LUTS, especially on storage symptoms, by switching drugs. The nonspecific effect on LUTS with BPH needs to be considered when evaluating subjective treatment efficacy of drugs for LUTS with BPH in routine clinical practice. The present study supports the lifestyle advice “avoid exposing the lower body to cold temperature” or “keep warm when it is cold” for LUTS with BPH.
Introduction
The placebo effect is a common phenomenon in clinical practice and clinical trials for various types of diseases, as shown by Beecher.Citation1 Although the definition of placebo effect varies considerably in the literature, Kaptchuk defined it as the effect seen in patients who have received an intervention that is believed to lack a specific action.Citation2 Ernst and ReschCitation3 and ErnstCitation4 postulated the concepts of “true” and “perceived” placebo effects. The perceived placebo effect is the response observed in the placebo treatment group of randomized controlled trials (RCTs), while the true placebo effect equals the perceived placebo effect minus other nonspecific effects that often determine the outcome not only in the placebo treatment group, but also in the active drug treatment group. It is recognized that RCTs assessing α1-adrenoceptor antagonists (α1 blockers) for lower urinary tract symptoms (LUTS) with benign prostatic hyperplasia (BPH) show high placebo responses of 9%–34%.Citation5,Citation6 On the other hand, nonspecific factors that play a role in other non-specific effects include natural course of disease, regression towards the mean, other time effects such as seasonal effect, and unidentified parallel interventions. In routine urologic practice, urologists are aware that cold ambient temperature as a nonspecific factor exacerbates LUTS with BPH irrespective of the administration of α1 blockers.
Naftopidil, a long-acting α1 blocker with a high affinity for α1D-adrenoceptors,Citation7 is as effective and safe as tamsulosin,Citation8–Citation10 although there are no placebo-controlled RCTs on naftopidil.Citation11 Up to 1999, naftopidil was available in Japan under the brand names Avishot™ (Nippon Organon KK, Osaka, Japan) and Flivas™ (Asahi Kasei Pharma Corp, Tokyo, Japan). The two drugs contained the same principal ingredient and displayed the same pharmacokinetic properties.Citation12 However, in 2008, Asahi Kasei Pharma Corp, acquired all intellectual property rights related to naftopidil. Thereafter, naftopidil has been sold only as Flivas™, and so BPH patients who wanted to continue with naftopidil had to switch from Avishot™ to Flivas™. Although the placebo effect on LUTS has been proven by RCTs,Citation5,Citation6 comparison of the data before and after switching from one brand of naftopidil to another at the same dose and timing would provide additional information as to the perceived placebo effect on LUTS with BPH.
We conducted a retrospective study on BPH patients to examine if switching from one brand of naftopidil to another at the same dose and timing causes the same changes in LUTS and quality of life (QOL) as the perceived placebo effect, and if ambient temperature is involved in those changes as a nonspecific factor.
Patients and methods
A review of the medical records of the Jichi Medical University Hospital, Tochigi, Japan, showed that, between December 2008 and July 2009, 385 patients with BPH were switched from Avishot™ to Flivas™ (data on file from 2008–2009, Jichi Medical University Hospital). The present retrospective analysis covers 217 of these patients; the other 168 patients were excluded due to receiving other medications for BPH and/or not visiting the hospital or completing the questionnaire (described later) after switching drugs. Patient prostate size according to digital rectal examination ranged from large walnut size to small hen’s egg size. All patients whose LUTS had remained stable under treatment with 50 mg/day or 75 mg/day of Avishot™ for more than 6 months were switched to Flivas™ at the same dose and timing. As naftopidil has been sold only as Flivas™ in Japan since 2008, BPH patients previously treated with Avishot™ who wanted to continue naftopidil treatment had to switch from Avishot™ to Flivas™ at that time.
Before and at 3 months after switching drugs, we evaluated the total International Prostate Symptom Score (IPSS); the scores of individual IPSS items; voiding symptoms (intermittency, weak urinary stream, abdominal straining to void), storage symptoms (nighttime frequency, daytime frequency, urgency); postvoiding symptom (incomplete emptying); and QOL score. Baseline characteristics of the patients are shown in . The average monthly ambient temperatures in degrees Celsius (°C) at the patients’ residence area (longitude 139°E, latitude 36°N, in a temperate zone) during the switch in drugs were obtained from the Automated Meteorological Data Acquisition System (AMeDAS), operated by the Japan Meteorological Agency (http://www.jma.go.jp/jma/indexe.html), and are shown in . In the present study, perceived placebo effect was defined as any significant change of the parameters by switching drugs. The present study was approved by the institutional review board of Jichi Medical University and informed consent was obtained from all patients. The Mann–Whitney U test, Wilcoxon signed rank test, and Spearman rank correlations were used for statistical analysis, and P-values of less than 0.05 were considered statistically significant.
Figure 1 Average monthly ambient temperature around patients’ residential area obtained from AMeDAS. Data is for Oyama city, Tochigi, Japan (longitude 139°E, latitude 36°N, in a temperate zone).
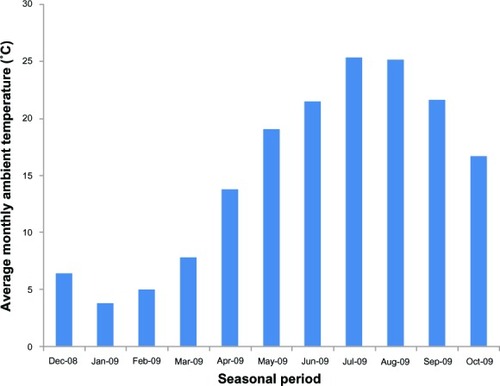
Table 1 Baseline characteristics of study patients
Results
Changes in LUTS and QOL after switching drugs
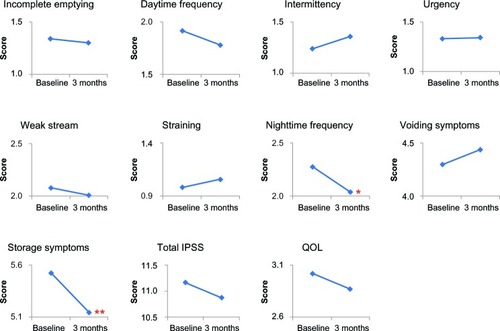
The changes in scores for IPSS items, voiding symptom score, storage symptom score, total IPSS, and QOL score after switching drugs are shown in . Among the IPSS items, nighttime frequency decreased significantly (P < 0.001), while daytime frequency tended to decrease (from 2.0 ± 1.4 to 1.8 ± 1.3; P = 0.097), resulting in the significant improvement of storage symptoms (P = 0.006). Furthermore, QOL scores tended to improve (from 3.0 ± 1.3 to 2.9 ± 1.3; P = 0.052). Thus, switching drugs improved the LUTS, especially the storage symptoms, suggesting the perceived placebo effect on LUTS because the two drugs contain the same principal ingredient and display the same pharmacokinetic properties, as described earlier.
Figure 2 Changes in IPSS and QOL score by switching drugs.
*P < 0.001, **P = 0.006 compared to the baseline by Wilcoxon signed rank test.
Involvement of calendar-based seasons in perceived placebo effect after switching drugs
To examine the possible involvement of the calendar-based seasons in this perceived placebo effect, the patients were divided into eight groups according to the period from baseline to 3 months, as shown in , since there are four seasons in Japan: spring (March, April, May), summer (June, July, August), fall (September, October, November), and winter (December, January, February). There was no significant difference in age, IPSS, and QOL score at baseline among the periods. In the period February–May, significant improvement was observed in total IPSS (P < 0.05), scores for urgency (P < 0.05), nighttime frequency (P < 0.001), and storage symptoms (P < 0.001). In March–June, significant improvement was observed in scores for nighttime frequency (P < 0.01) and storage symptoms (P < 0.05). In June–September, incomplete emptying deteriorated rather significantly (P < 0.05). These results suggest that the nonspecific effect on LUTS in the perceived placebo effect, rather than the true placebo effect, is involved in the changes in LUTS after switching drugs. Since a clear seasonal change in LUTS/QOL was not observed, it can be concluded that the season was not involved in the nonspecific effect in the perceived placebo effect by switching drugs.
Table 2 Changes in IPSS and QOL scores from baseline to 3 months, according to seasonal period
Involvement of ambient temperature in nonspecific effect
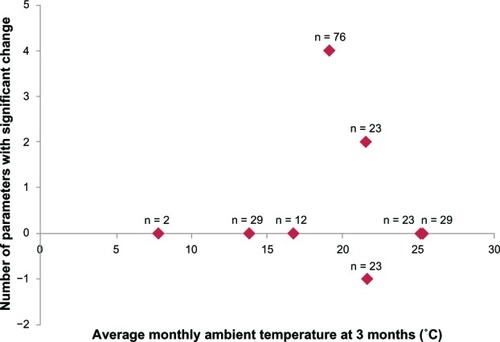
We analyzed the association between the number of parameters with significant change () and the average monthly ambient temperature at 3 months () in each seasonal period to examine the possible involvement of ambient temperature in the nonspecific effect in the perceived placebo effect after switching drugs. For the statistical analysis, one significantly improved parameter was assigned as +1 while one significantly deteriorated parameter was assigned as −1. The results showed no significant association ().
Figure 3 Association between number of parameters with significant change and average monthly ambient temperature at three months.
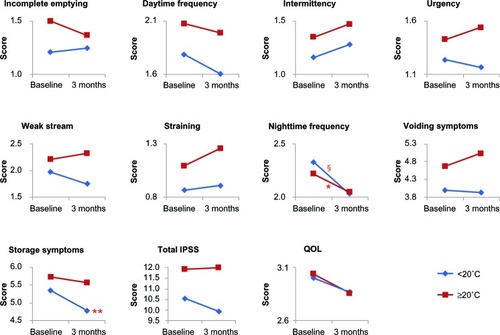
The patients were then divided into two groups according to the average monthly ambient temperature at 3 months in each period higher than 20°C (June, July, August, September) or lower than 20°C (March, April, May, October) (), as the ambient temperature for human thermal comfort is higher than 18°C.Citation13,Citation14 There was no significant difference in IPSS and QOL score at baseline between the two groups (data not shown). In the former group, significant improvement was noted in nighttime frequency (P < 0.05), while in the latter group, there was significant improvement of nighttime frequency (P < 0.001) and storage symptoms (P < 0.01) (). The same analysis was performed for average monthly ambient temperature at baseline; the results were the same for those at 3 months (data not shown). Similar improvement of LUTS in both groups was observed, suggesting that the average monthly ambient temperature itself is not involved in the nonspecific effect on LUTS.
Figure 4 Changes in IPSS and QOL score by average monthly ambient temperature at three months after switching drugs.
*P < 0.05, **P < 0.01, §P < 0.001 compared to the baseline in each group by Wilcoxon signed rank test.
Involvement of magnitude of ambient temperature change in nonspecific effect
We analyzed the association between the number of parameters with significant change () and the magnitude of change in average monthly ambient temperature from baseline to 3 months () in each seasonal period to examine the possible involvement of the magnitude of change in average monthly ambient temperature in the nonspecific effect in the perceived placebo effect by switching drugs. For the statistical analysis, one significantly improved parameter was assigned as +1 while one significantly deteriorated parameter was assigned as −1. As shown in , a significant association was observed (P = 0.0322); namely, the greater the magnitude of change in average monthly ambient temperature from baseline to 3 months, the greater the number of significant parameters in each period. We then divided the patients into two groups according to magnitude of change in average monthly ambient temperature from baseline to 3 months higher than 10°C (February–May, March–June, April–July) or lower than 10°C (December–March, January–April, May– August, June–September, July–October) to have a similar number of patients in each group for the statistical analysis (). There was no significant difference in IPSS and QOL score at the baseline between the two groups (data not shown). As shown in , in the former group, there was significant improvement in daytime frequency (P < 0.05), nighttime frequency (P < 0.001), storage symptoms (P < 0.001), and total IPSS (P < 0.05). On the other hand, in the latter group, no significant improvement was noted in any of the parameters examined. The periods of February– May and March–June in which significant improvement of LUTS was observed () were those with the two highest magnitudes of ambient temperature change among the periods (). On the other hand, incomplete emptying deteriorated somewhat in the periods of June–September and July–October () when the magnitude of ambient temperature change was lower than 0°C (). These results suggest that the magnitude of ambient temperature change is involved in the nonspecific effect in the perceived placebo effect by switching drugs.
Figure 5 Magnitude of changes in average monthly ambient temperature from baseline to three months around patients’ residential area obtained from AMeDAS.
Abbreviation: AMeDAS, automated meteorological data acquisition system.
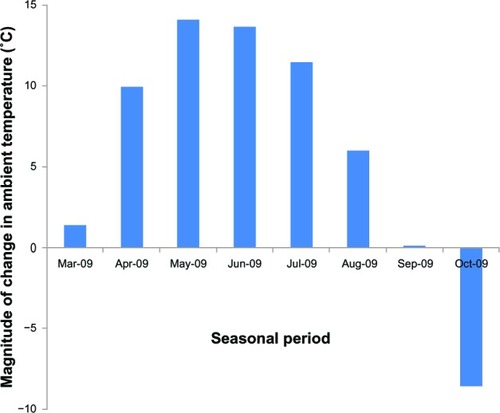
Figure 6 Association between number of parameters with significant change and magnitude of changes in average monthly ambient temperature.
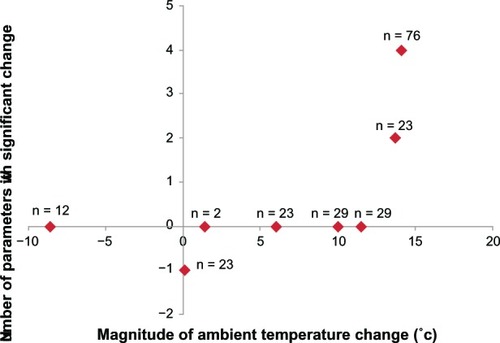
Figure 7 Changes in IPSS and QOL score by magnitude of changes in average monthly ambient temperature from baseline to three months.
*P < 0.05, §P < 0.001 compared to the baseline in each group by Wilcoxon signed rank test.
Discussion
The present study showed that switching from Avishot™ to Flivas™ induced significant changes in LUTS, especially in storage symptoms, suggesting the perceived placebo effect. These changes in LUTS after switching drugs would be due to the nonspecific effect in the perceived placebo effect rather than the true treatment effect of naftopidil or the switching of drugs itself because the extent of changes in LUTS was different among the periods when the drug was switched. In addition, the present study has shown for the first time that magnitude of ambient temperature change was a nonspecific factor that influences LUTS in BPH.
It is well known that cold temperature is a stress factor that can induce various physiological responses, such as increase in blood pressure;Citation15,Citation16 thus, cold temperature might affect bladder function, resulting in the change in LUTS. A questionnaire study showed that feeling colder or warmer is one of the reasons for nighttime frequency in BPH patients.Citation17 In experimental rat studies, cold temperature is shown to enhance the activation of the hypothalamic–pituitary–adrenal axisCitation18 and increase the secretion of urinary epinephrine,Citation19 which is one of the neurotransmitters that could modulate LUTS.Citation20,Citation21 Furthermore, in experimental studies with conscious rats, a sudden drop in environmental temperature induced detrusor overactivityCitation22,Citation23 and partially changed the micturition pattern via α1-adrenoceptors.Citation23 These reports support our results that storage symptoms were influenced by the nonspecific effect of the magnitude of ambient temperature change. Other factors, such as increased insensible water loss in warm and hot seasons, which leads to decreased urinary frequency as a result of decreased urine volume during nighttime, might be a factor in our results, although frequency–volume charts were not included in the present retrospective study.
Seasonal (summer versus winter) variation in LUTS has been investigated by means of a community-based questionnaire in three different climatic regions of Japan: subarctic (Hokkaido), temperate (Kyoto), and subtropical (Okinawa).Citation24 Storage symptoms, including daytime frequency, nighttime frequency, and urgency, improved in summer compared to winter, or deteriorated in winter compared to summer; the magnitude of ambient temperature change between two seasons in three regions (summer and winter in subarctic, temperate, and subtropical regions) was higher than 10°C. Furthermore, this tendency was marked in the subtropical and temperate zones but was not observed in the subarctic zone, suggesting that the variation in storage symptoms depended on the region in which the study was performed. The present result that the magnitude of change in average monthly temperature higher than 10°C was involved in the variation in storage symptoms is consistent with the study described above,Citation24 seemingly due, in part, to the fact that the present study was performed in the temperate zone. Considering that (1) nighttime frequency is an indoor event; (2) low ambient temperature does not always lead to variation in LUTS;Citation24 (3) indoor temperature is affected not only by ambient temperature but by various other factors, such as type of heating and housing; and (4) a sudden drop in environmental temperature induces detrusor overactivity as shown by experimental animal studies,Citation22,Citation23 suggests that indoor temperature rather than ambient temperature might be appropriate as a nonspecific factor for LUTS at the individual level irrespective of the region. Further studies are needed to elucidate the involvement of indoor temperature in the variation of LUTS.
In routine urologic practice, urologists are aware that cold weather exacerbates LUTS with BPH irrespective of the administration of α1 blockers. The guideline by the Japanese Urological Association (JUA) recommends that males with LUTS avoid exposing the lower body to cold temperature.Citation25 However, there is little high-quality evidence that provides reliable information on the influence of ambient temperature on LUTS in BPH patients.Citation25–Citation27 Our results support the lifestyle advice “avoid exposing the lower body to cold temperature” or “keep warm when it is cold:”Citation25 improvement of LUTS was observed when magnitude of ambient temperature change was higher than 10°C; incomplete emptying was worse in the periods of June–September and July–October () when the magnitude of ambient temperature change was lower than 0°C ().
The present study raises important issues for the evaluation of clinical trials for BPH. Namely, the treatment effect could be overestimated if patients are enrolled in a cold season and evaluated in a warm season, while it could be underestimated if patients are enrolled in a warm season and evaluated in a cold season in short-term uncontrolled trials. In short-term, nonrandomized controlled trials, the difference in the distribution of entry timing in each group (control group or active drug treatment group) could lead to overestimation or underestimation of treatment effect. The same would be true in routine clinical practice. Thus, consideration must be given to the nonspecific effect by ambient temperature change when interpreting the changes in LUTS in those clinical trials and in routine clinical practice.
The limitations of the present study include the retrospective nature; relatively small number of patients examined; comparison of different populations in different periods, lack of frequency–volume charts; lack of four enrollment periods (August–November, September–December, October–January, November–February); and insufficient background information on the patients. Despite these limitations, the present study provides evidence that the nonspecific effect of magnitude of ambient temperature change was involved in the perceived placebo effect on LUTS, especially on storage symptoms, by switching drugs. The nonspecific effect on LUTS in BPH needs to be considered when evaluating subjective treatment efficacy of the drugs for LUTS in BPH in routine clinical practice. In addition, the present study supports the lifestyle advice “avoid exposing the lower body to cold temperature” or “keep warm when it is cold”Citation25 for LUTS with BPH.
Disclosure
The authors report no conflicts of interest in this work.
References
- BeecherHKThe powerful placeboJ Am Med Assoc1955159171602160613271123
- KaptchukTJPowerful placebo: the dark side of the randomised controlled trialLancet1998351172217259734904
- ErnstEReschKLConcept of true and perceived placebo effectsBMJ19953115515537663213
- ErnstEPlacebo: new insights into an old enigmaDrug Discov Today20071241341817467578
- MoyadMAThe placebo effect and randomized trials: analysis of conventional medicineUrol Clin North Am200229112513312109340
- van LeeuwenJHCastroRBusseMBemelmansBLThe placebo effect in the pharmacologic treatment of patients with lower urinary tract symptomsEur Urol200650344045216753253
- TakeiRIkegakiIShibataKTsujimotoGAsanoTNaftopidil, a novel alpha1-adrenoceptor antagonist, displays selective inhibition of canine prostatic pressure and high affinity binding to cloned human alpha1-adrenoceptorsJpn J Pharmacol19997944745410361884
- IkemotoIKiyotaHOhishiYUsefulness of tamsulosin hydrochloride and naftopidil in patients with urinary disturbances caused by benign prostatic hyperplasia: a comparative, randomized, two-drug crossover studyInt J Urol2003101158759414633083
- GotohMKamihiraOKinukawaTOnoYOhshimaSOrigasaHTokai Urological Clinical Trial GroupComparison of tamsulosin and naftopidil for efficacy and safety in the treatment of benign prostatic hyperplasia: a randomized controlled trialBJU Int200596458158616104914
- NishinoYMasueTMiwaKTakahashiYIshiharaSDeguchiTComparison of two alpha1-adrenoceptor antagonists, naftopidil and tamsulosin hydrochloride, in the treatment of lower urinary tract symptoms with benign prostatic hyperplasia: a randomized crossover studyBJU Int200697474775116536766
- GarimellaPSFinkHAMacdonaldRWiltTJNaftopidil for the treatment of lower urinary tract symptoms compatible with benign prostatic hyperplasiaCochrane Database Syst Rev2009CD00736019821408
- NakashimaMKanamaruMUematsuTPhase I clinical study of naftopidil (KT-611), a new alpha1-adrenoceptor blockerJ Clin Therap Med199286381 Japanese
- CollinsKJLow indoor temperatures and morbidity in the elderlyAge Ageing19861542122203751747
- ZhangLEmuraKNakaneYA proposal of optimal floor surface temperature based on survey of literatures related to floor heating environment in JapanAppl Human Sci19981726166
- RoseGSeasonal variation in blood pressure in manNature196118923513743262
- BrennanPJGreenbergGMiallWEThompsonSGSeasonal variation in arterial blood pressureBr Med J (Clin Res Ed)19822856346919923
- YoshimuraKTeraiAFluctuation of night time frequency in patients with symptomatic nocturiaInt J Urol200512546947315948746
- MaSMorilakDAChronic intermittent cold stress sensitises the hypothalamic-pituitary-adrenal response to a novel acute stress by enhancing noradrenergic influence in the rat paraventricular nucleusJ Neuroendocrinol2005171176176916219005
- YamoriYIkedaKKulakowskiECMcCartyRLovenbergWEnhanced sympathetic-adrenal medullary response to cold exposure in spontaneously hypertensive ratsJ Hypertens19853163663998461
- AnderssonKETreatment of the overactive bladder: possible central nervous system drug targetsUrology200259182412007518
- AnderssonKEWeinAJPharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinencePharmacol Rev200456458163115602011
- ImamuraTIshizukaOAizawaNCold environmental stress induces detrusor overactivity via resiniferatoxin-sensitive nerves in conscious ratsNeurourol Urodyn200827434835217701982
- ChenZIshizukaOImamuraTRole of alpha1-adrenergic receptors in detrusor overactivity induced by cold stress in conscious ratsNeurourol Urodyn200928325125618837433
- YoshimuraKKamotoTTsukamotoTOshiroKKinukawaNOgawaOSeasonal alterations in nocturia and other storage symptoms in three Japanese communitiesUrology200769586487017482923
- HommaYArakiIIgawaYJapanese Society of Neurogenic BladderClinical guideline for male lower urinary tract symptomsInt J Urol2009161077579019811547
- MadersbacherSAlivizatosGNordlingJSanzCREmbertonMde la RosetteJJEAU 2004 guidelines on assessment, therapy and follow-up of men with lower urinary tract symptoms suggestive of benign prostatic obstruction (BPH guidelines)Eur Urol200446554755415474261
- McVaryKTRoehrbornCGAvinsALUpdate on AUA guideline on the management of benign prostatic hyperplasiaJ Urol201118551793180321420124