Abstract
Primary bladder large cell neuroendocrine carcinoma (LCNEC) is a rare, aggressive neoplasm with high recurrence rates and poor prognosis. Traditional management has heavily relied on radical cystectomy, which, despite its aggressiveness, often results in unsatisfactory outcomes. Emerging evidence suggests the potential for less invasive, bladder-sparing approaches, yet detailed reports and long-term outcomes remain scarce. We report a groundbreaking case of a 59-year-old male diagnosed with primary bladder LCNEC, managed through a pioneering bladder-sparing multimodal treatment. This novel strategy included transurethral resection followed by a tailored chemoradiation protocol, resulting in exceptional disease control and preservation of bladder function over a 20-month follow-up period, without evidence of recurrence. This case underscores the viability of bladder conservation strategies as a legitimate alternative to radical cystectomy for managing LCNEC, presenting a beacon of hope for patients wishing to preserve bladder functionality. It prompts a reevaluation of traditional treatment paradigms and advocates for further research into multimodal, organ-sparing approaches for this challenging malignancy.
Background
Bladder cancer is a heterogeneous disease, with neuroendocrine carcinoma (NEC) representing a rare and particularly aggressive subset. Among these, large cell neuroendocrine carcinoma (LCNEC) of the bladder is exceedingly rare, accounting for less than 1% of all bladder malignancies.Citation1 The standard treatment for LCNEC has traditionally been radical cystectomy, often complemented by adjuvant chemotherapy.Citation2 However, the prognosis for patients undergoing these treatments remains poor, with high rates of recurrence and metastasis. This grim outlook necessitates exploration into alternative treatment strategies that not only aim to manage the disease effectively but also improve the quality of life for patients by preserving bladder function. Recent advancements in multimodal therapy have shown promise in the management of various aggressive cancers, suggesting a potential paradigm shift in the treatment of bladder LCNEC.Citation3,Citation4 Other therapeutic approaches encompass molecular alterations and targeted treatments for diverse neuroendocrine tumors of the genitourinary tract.Citation5 Bladder preservation, as part of a multimodal approach, offers a compelling alternative, potentially enabling effective cancer control while maintaining organ functionality. The rationale for exploring such strategies in LCNEC treatment stems from the limited success and substantial morbidity associated with traditional approaches, coupled with an increasing understanding of the disease’s biology and responses to different therapeutic modalities.Citation6
By detailing the management of a patient with this rare condition through a unique combination of transurethral resection, chemoradiation, and careful follow-up, we seek to highlight the feasibility and effectiveness of this strategy. Furthermore, this report aims to contribute to the scant literature on bladder LCNEC and stimulate further research into alternative treatment modalities that can offer patients both effective management and quality of life.
Case Presentation
In May 2022, a 59-year-old male with a notable medical history of acoustic neuroma, hypertension, hyperglycemia, and a previous episode of gastric duodenal ulcer presented to our department with a three-week history of intermittent gross hematuria. The initial diagnostic assessments, including cystoscopy () and imaging studies (CT urography and pelvic MRI), revealed a solid tumor measuring 2.1×2.0 x 2.1 cm on the left posterior wall of the bladder. There was no evidence of tumor extension beyond the bladder or significant lymph node enlargement (). Comprehensive CT scans of multiple areas, including the chest, revealed no evidence of tumor metastasis.
Figure 1 Cystoscopic visualizations of the patient. (A) Initial cystoscopy showing large cell neuroendocrine carcinoma within the bladder. (B) Posttreatment visualization indicating local scar tissue formation following comprehensive treatment, including TURBT, secondary resection, chemotherapy, and radiotherapy. (C and D) Comparative cystoscopy images captured before and after electrocauterization of the bladder scar tissue one year after TURBT.
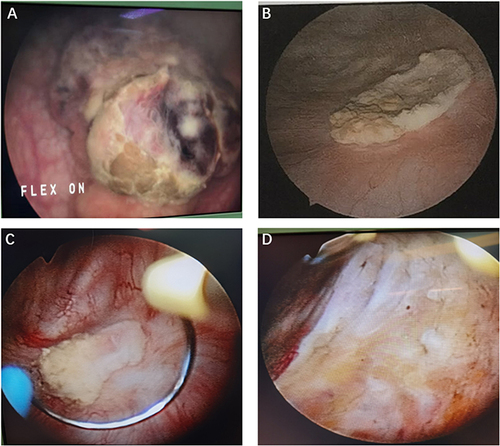
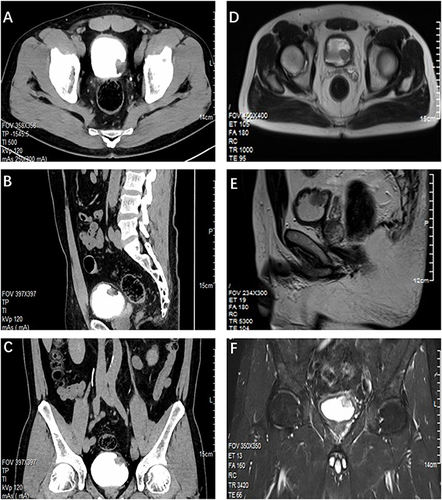
Figure 2 Radiological Imaging of the Patient. (A) Axial CTU view. (B) Sagittal CTU projection. (C) Coronal CTU image. CT urography revealed a nodular soft tissue mass measuring approximately 2.1 cm × 2.0 cm × 2.1 cm on the bladder’s left posterior wall, with surface calcification and mild to moderate enhancement following contrast agent administration. (D) Axial MR image. (E) Sagittal MR image. (F) Coronal MR image. MRI scans display heterogeneous signal intensities within the bladder lesion, with isointense T1 and slightly hyperintense T2 signals.
The patient underwent a transurethral resection of the bladder tumor (TURBT), during which a high-grade neuroendocrine carcinoma infiltrating the muscular layer was identified. The pathological evaluation revealed a high-grade LCNEC with adjacent noninvasive high-grade urothelial carcinoma. Immunohistochemical staining showed positivity for CD56, Chromogranin A, Synaptophysin, and GATA3, with a Ki-67 labeling index of 80%, confirming the diagnosis of LCNEC ().
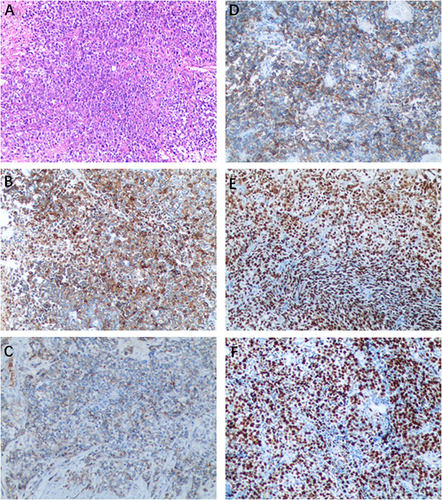
Figure 3 Histopathological and immunohistochemical staining of the tumor. (A) LCNEC of the bladder showed large cells with large nuclear size, prominent nucleoli, and abundant cytoplasm. (H&E staining, ×100). (B) The neoplasm displayed positive expression of Synaptophysin. (C) The neoplasm displayed positive expression of CgA. (D) Positive for CD56. (E) Positive for GATA3. (F) High proliferative index with Ki67 at 80%.
Given the aggressive nature of LCNEC and the patient’s desire to preserve bladder function, a bladder-sparing multimodal treatment approach was adopted. This included a second TURBT to ensure complete removal of the visible tumor and adjacent tissues, followed by a chemotherapeutic regimen comprising cisplatin and etoposide (EP regimen), and targeted radiotherapy delivering 64 Gy in 32 fractions. This comprehensive treatment strategy was designed to maximize tumor control while minimizing the risk of recurrence and preserving the bladder.
The patient demonstrated an exceptional response to the treatment, with no evidence of tumor recurrence on follow-up cystoscopy 20 months post-diagnosis. The bladder-sparing approach not only achieved effective local control of the neoplasm but also allowed the patient to maintain normal bladder function, significantly enhancing his quality of life.
Discussion
Primary bladder large-cell neuroendocrine carcinoma (LCNEC) is a rare and formidable malignancy that presents significant diagnostic and therapeutic potential.Citation7,Citation8 While LCNEC predominantly occurs in the respiratory and digestive tracts, its manifestation in the urinary system is notably infrequent.Citation9 This cancer predominantly affects older males, typified by a median onset age of 65 years and a male-to-female ratio of 4:1.Citation10 Its pathological spectrum includes small-cell neuroendocrine carcinoma (SCNEC), paraganglioma, carcinoid, and LCNEC. Clinically, LCNEC most commonly manifests as painless gross hematuria, although some patients may exhibit no symptoms.Citation10 The challenge in diagnosing bladder LCNEC arises from its nonspecific clinical and imaging presentation, making pathological examination crucial for a definitive diagnosis.Citation11
Bladder LCNEC is characterized by distinct pathological features, including enlarged cells with low nuclear-to-cytoplasmic ratio, prominent nucleoli, and unique cellular arrangements resembling chrysanthemums or nested structures. High mitotic activity, often accompanied by tumor necrosis, further contributes to its aggressive nature. Immunohistochemical analysis typically reveals the presence of neuroendocrine markers like synaptophysin, chromogranin A, and CD56, alongside a significant Ki67 proliferation index. Understanding these traits is crucial for accurate diagnosis and management of this aggressive cancer.Citation12 Our case demonstrated neuroendocrine differentiation through the expression of CD56, chromogranin A (CgA), and synaptophysin (Syn), underscored by a high Ki-67 labeling index, with coexpression of urothelial markers such as P63, suggesting a possible urothelial tumor origin, although the precise histogenesis remains elusive because of the rarity and complexity of LCNEC.
Due to the scarcity of LCNEC, the literature on bladder LCNEC is limited, underscoring the absence of a standardized treatment framework. The prognosis of LCNEC patients with LCNEC is poor, and LCNEC is characterized by high recurrence and metastasis rates.Citation13 In practice, treatment modalities for lung LCNEC are often adapted for bladder LCNEC, with therapeutic options generally borrowed from strategies for other neuroendocrine or urothelial carcinomas of the bladder.Citation14 A multimodal treatment approach, typically integrating surgery and chemotherapy, serves as the cornerstone of management. Ensuring complete tumor resection through histopathological examination of the tumor’s basal tissue, coupled with subsequent transurethral resections and regular cystoscopies, is essential for posttreatment monitoring.Citation15 Currently, the efficacy of TURBT in treating early-stage localized lesions remains underexplored. Radical cystectomy is the preferred surgical choice for bladder LCNEC given its tendency for distant metastasis, with the liver, lungs, brain, and bones being common metastatic sites.Citation16
Therefore, the role of neoadjuvant or adjuvant chemotherapy in the management of bladder LCNEC cannot be overstated. Currently, cisplatin combined with an etoposide chemotherapy regimen, which is primarily used to treat lung LCNEC, serves as a valuable reference for bladder cancer treatment.Citation17 Statistical analyses of follow-up data from reported bladder LCNEC patients revealed decreasing survival rates: 51.5% at 1 year, 21.2% at 2 years, and 9.1% at 3 years.Citation18 Our case report details successful bladder-preserving treatment for primary bladder LCNEC. This involved transurethral resection, followed by cisplatin-based chemotherapy with etoposide and local radiation therapy. Decision-making factored in patient age, comorbidities, and the desire to maintain bladder function. Complete resection of the tumor with negative margins, achieved through transurethral resection of bladder tumor (TURBT) and secondary transurethral resection, is a crucial step in minimizing the risk of local recurrence. By completely removing the visible tumor and adjacent tissue, we were able to reduce the likelihood of residual disease and improve patient prognosis.
Administering adjuvant chemotherapy with cisplatin and etoposide aims to eliminate micrometastatic disease and enhance survival prospects.Citation19 The synergy of cisplatin, a broad-spectrum chemotherapeutic agent, with etoposide, a topoisomerase inhibitor effective against neuroendocrine tumors, notably improves antitumor efficacy and minimizes relapse risks. Integrating local radiation therapy bolstered local control and further mitigated recurrence risks, underscoring the role of radiation in managing various malignancies, including bladder cancer. The 20-month tumor-free survival outcome achieved herein favorably compares the outcomes of radical cystectomy and chemotherapy for LCNEC, underscoring the efficacy of our approach while maintaining the patient’s quality of life.
While our study yields promising results, further investigation is necessary to validate this treatment approach. Larger patient cohorts and longer-term follow-up assessments are crucial for establishing its efficacy and safety profile. This research is essential for confirming bladder-sparing therapy as a standard-of-care option for primary bladder LCNEC. Additionally, future research endeavors can drive improvements in clinical practices and patient outcomes. By examining long-term efficacy, recurrence rates, and quality of life outcomes, we can refine therapeutic protocols and enhance patient selection criteria. Ultimately, this evidence-based approach holds the potential to improve survival rates and quality of life for individuals with this rare and aggressive malignancy.
Conclusions
Our study highlights the effectiveness and benefits of a multimodal bladder-sparing treatment for primary bladder LCNEC. This approach offers a promising option for those unsuitable for or preferring to avoid radical cystectomy. However, further research with larger patient groups and longer-term follow-ups is crucial to confirm its status as a standard therapy for this rare and aggressive malignancy, potentially leading to improved clinical practices and survival rates.
Abbreviations
LCNEC, Large cell neuroendocrine carcinoma; NEC, Neuroendocrine carcinoma; EP: Etoposide and cisplatin; TURBT, Transurethral resection of bladder tumor.
Ethics Approval and Consent to Participate
The study was approved by the ethics committee of Second People’s Hospital of Yichang. Written informed consent was obtained from the patient for publication of the details of the medical case and any accompanying images.
Consent for Publication
Informed consent has been obtained from the patient included in this study.
Disclosure
The authors declare no competing interests in this work.
Acknowledgments
Our thanks are extended to the patient who graciously consented to the publication of this case.
References
- Pósfai B, Kuthi L, Varga L, et al. The colorful palette of neuroendocrine neoplasms in the genitourinary tract. Anticancer Res. 2018;38:3243–3254.
- Pompas-Veganzones N, Gonzalez-Peramato P, Sanchez-Carbayo M. The neuroendocrine component in bladder tumors. Curr Med Chem. 2014;21:1117–1128. doi:10.2174/0929867321666131201141346
- Sroussi M, Elaidi R, Fléchon A, et al. Neuroendocrine carcinoma of the urinary bladder: a large, retrospective study from the French genito-urinary tumor group. Clin Genitourin Cancer. 2020;18:295–303.e293. doi:10.1016/j.clgc.2019.11.014
- Wang G, Yuan R, Zhou C, et al. Urinary large cell neuroendocrine carcinoma: a clinicopathologic analysis of 22 cases. Am J Surg Pathol. 2021;45:1399–1408. doi:10.1097/PAS.0000000000001740
- Shehabeldin AN, Ro JY. Neuroendocrine tumors of genitourinary tract: recent advances. Ann Diagn Pathol. 2019;42:48–58. doi:10.1016/j.anndiagpath.2019.06.009
- Bote H, Alaoui A, Oussama Z, et al. Neuroendocrine carcinoma of the bladder: about 5 cases. Pan Afr Med J. 2017;26(92):1.
- He B, Chen Y, Hui Z. Primary pure bladder large cell neuroendocrine carcinoma: a case report. Asian J Surg. 2023;46:5454–5455. doi:10.1016/j.asjsur.2023.08.037
- Mahmoudnejad N, Mohammadi Torbati P, Lashay A, et al. Achieving a one-year-tumor-free survival in a female with primary large cell neuroendocrine carcinoma of the urinary bladder and liver metastasis; a case report. Urology Case Rep. 2023;47(102347):102347. doi:10.1016/j.eucr.2023.102347
- Akamatsu S, Kanamaru S, Ishihara M, et al. Primary large cell neuroendocrine carcinoma of the urinary bladder. Internat J Urol. 2008;15:1080–1083. doi:10.1111/j.1442-2042.2008.02168.x
- Radović N, Turner R, Bacalja J. Primary ”pure” large cell neuroendocrine carcinoma of the urinary bladder: a case report and review of the literature. Clin Genit Can. 2015;13:e375–377. doi:10.1016/j.clgc.2015.03.005
- Treglia G, Paone G, Flores B, et al. A rare case of large cell neuroendocrine carcinoma of the urinary bladder evaluated by ¹⁸F-FDG-PET/CT. Revista Esp de Med Nuclear e Imagen Mol. 2014;33:312–313. doi:10.1016/j.remn.2013.10.007
- Hailemariam S, Gaspert A, Komminoth P, et al. Primary, pure, large-cell neuroendocrine carcinoma of the urinary bladder. Modern Pathol. 1998;11:1016–1020.
- Mollica V, Massari F, Andrini E, et al. Prognostic factors of survival for high-grade neuroendocrine neoplasia of the bladder: a SEER database analysis. Current Oncol. 2022;29:5846–5854. doi:10.3390/curroncol29080461
- Xia K, Zhong W, Chen J, et al. Clinical characteristics, treatment strategy, and outcomes of primary large cell neuroendocrine carcinoma of the bladder: a case report and systematic review of the literature. Front Oncol. 2020;10(1291). doi:10.3389/fonc.2020.01291
- Xiao P, Liu J, Sun W, et al. Large cell neuroendocrine carcinoma of the urinary bladder: a case report and literature review. Asian J Surg. 2023;46:6049–6050. doi:10.1016/j.asjsur.2023.09.041
- Zhou H, Liu L, Yu G, et al. Analysis of clinicopathological features and prognostic factors in 39 cases of bladder neuroendocrine carcinoma. Anticancer Res. 2017;37:4529–4537. doi:10.21873/anticanres.11850
- Chen K, Dai P, Ni J, et al. The prognosis analysis of organ metastatic patterns in lung large cell neuroendocrine carcinoma: a population-based study. Front Oncol. 2022;12:1050800.
- Coelho HM, Pereira BA, Caetano PA. Large cell neuroendocrine carcinoma of the urinary bladder: case report and review. Curr Urol. 2013;7:155–159. doi:10.1159/000356270
- Filosso P, Guerrera F, Evangelista A, et al. Adjuvant chemotherapy for large-cell neuroendocrine lung carcinoma: results from the European society for thoracic surgeons lung neuroendocrine tumours retrospective database. Europ J Cardio Surg. 2017;52:339–345. doi:10.1093/ejcts/ezx101
