?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Treatment for opioid use disorder is important because of the negative health, societal and economic consequences of illicit opioid use, but treatment adherence can be a challenge. This study assessed the association between buprenorphine medication-assisted treatment (MAT) adherence and relapse, health care utilization and costs.
Patients and methods
Patients with opioid use disorder who were newly initiating a buprenorphine MAT regimen were identified in the 2008–2014 MarketScan® Commercial and Medicaid Databases and followed for 12 months after their earliest outpatient pharmacy claim for buprenorphine. Adherence was categorized using proportion of days covered (PDC) with buprenorphine, and patients with PDC≥0.80 were classified as adherent. Descriptive and adjusted analyses compared relapse prevalence, utilization and costs, all measured in the 12 months following buprenorphine MAT initiation, of adherent patients to patients in non-adherent PDC categories (PDC<0.20, 0.20≤PDC<0.40, 0.40≤PDC<0.60, 0.60≤PDC<0.80).
Results
Adherent patients were 37.1% of the Commercial sample (N=16,085) and 41.3% of the Medicaid sample (N=5,688). In both samples, non-adherent patients were significantly more likely than adherent patients to relapse and to have hospitalizations and emergency department visits. As a result, as buprenorphine MAT adherence increased, pharmacy costs increased, but medical costs decreased. Total costs (pharmacy plus medical costs) in the 12 months following buprenorphine MAT initiation decreased with adherence in Commercial patients ($28,525 for PDC<0.20 to $17,844 for PDC≥0.80). A slight decrease in total costs in the 12 months following buprenorphine MAT initiation was also observed in Medicaid patients ($21,292 for PDC<0.20 to $18,621 for PDC≥0.80). After adjustment, total costs of adherent patients in the Commercial sample ($17,519) were significantly lower compared with those of non-adherent patients (range $20,294–$24,431). In the Medicaid sample, adjusted total costs were not significantly different between adherence groups.
Conclusion
Buprenorphine MAT adherence in the 12 months following treatment was associated with reduced odds of relapse and reduced unadjusted medical costs. For Commercial patients who were adherent to treatment, the adjusted total costs were predicted to be 30% lower than those for patients with PDC<0.20.
Introduction
The misuse of opioids, including prescription pain relievers, illegally produced opioids and heroin, is a growing public health concern. In 2015, an estimated 2.0 million people aged 12 years or older in the USA met the criteria for an opioid use disorder (OUD) involving prescription pain relievers in the past 12 months, and 0.6 million individuals had an OUD involving heroin in the past 12 months.Citation1 (The term “opioid use disorder” was introduced in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition.Citation2 Previously, the terms “opioid dependence” and “opioid abuse” were used to diagnose corresponding conditions). Furthermore, the USA is amid a national opioid overdose epidemic with the rate of opioid-related overdose deaths increasing by more than 200% in the past 15 years.Citation3 In addition to death from overdose, OUD has been associated with blood-borne infections such as hepatitis and HIV, criminal involvement, productivity losses and increased health care utilization and costs.Citation4–Citation10 The resulting economic burden is significant with recent estimates of the societal costs of OUD and opioid overdose exceeding $78 billion.Citation6
Treatment for OUD is important because of the negative consequences of illicit opioid use; however, treating OUD patients is often challenging as OUD is a chronic disease characterized by episodes of relapse and remission.Citation11 Current treatment options include psychosocial therapy and medication-assisted treatment (MAT) with methadone, buprenorphine (alone or in combination with naloxone) or naltrexone.Citation11 Buprenorphine MAT, a commonly used therapy and the focus of this study, is an effective treatment option that is available in outpatient office-based settings through waivered clinicians.Citation12,Citation13 From 2000 to 2016, only physicians were eligible to apply for and receive waivers. In 2016, US federal regulations extended waivering to nurse practitioners (NPs) and physician assistants (PAs). In some states, buprenorphine prescribing may be limited to waivered physicians, or NPs and PAs working under a waivered physician, due to state-level scope-of-practice laws.
A previous study found that buprenorphine MAT was associated with a reduced incidence of relapse among patients with OUD.Citation14 In addition, it has been shown that opioid-dependent patients adherent to buprenorphine MAT in the year following treatment initiation had reduced utilization of expensive health care servicesCitation15–Citation17 and total health care costs.Citation15,Citation16 However, these reductions were among patients in a single Commercial health planCitation15,Citation16 or in one state Medicaid plan,Citation17 and to the authors’ knowledge no study has explored the effect of adherence on outcomes in large real-world samples of patients from different health plans throughout the USA.
The primary objective of this study was to understand the relationship between buprenorphine MAT adherence and odds of relapse, health care resource utilization and costs among both commercially insured and publicly insured (ie, Medicaid) patients with OUD who were newly initiating buprenorphine MAT. The secondary objective was to identify factors associated with buprenorphine MAT adherence. This study expands on the insights gained from prior studies by examining the impact of adherence among a large, national sample of individuals with OUD.
Patients and methods
Study design and data source
This retrospective, observational cohort study used administrative claims data from the MarketScan® Commercial Claims and Encounters (Commercial)Citation18 and Medicaid Multi-State (Medicaid)Citation19 Research Databases. The Commercial Database includes fully adjudicated medical and pharmacy claims for more than 100 million employees and their dependents from across the USA including more than 38 million lives in 2013 alone. Major data contributors include employers and health plans that cover employees and their dependents through different insurance plan structures including fee-for-service, fully capitated and partially capitated health plans. The Medicaid Database includes similar information for Medicaid beneficiaries in several geographically dispersed states. Both databases provide detailed cost, utilization and outcome data for health care services performed in both inpatient and outpatient settings, including retail and mail order outpatient pharmacies. All study data were de-identified and fully compliant with the Health Insurance Portability and Accountability Act (HIPAA) of 1996. The study entailed analyses of existing databases in which subjects could not be identified. Therefore, the study was not considered human subject research and did not require institutional review board (IRB) approval.
Study population
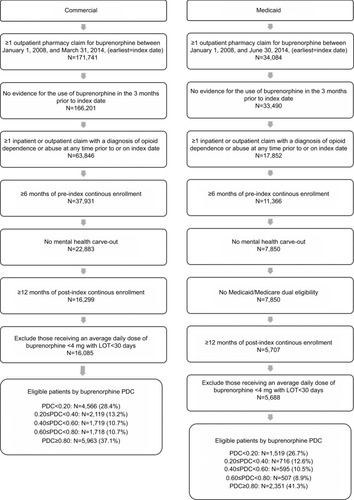
Patients of any age with at least one outpatient pharmacy claim for buprenorphine from January 1, 2008, to March 31, 2014, in the Commercial Database or June 30, 2014, in the Medicaid Database were selected for analysis. The date of the earliest buprenorphine claim was set as the index date. Patients were required to have at least one inpatient or outpatient service claim including a diagnosis of opioid dependence or abuse (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] 304.0×, 304.7× or 305.5×) prior to or on the index date. Patients with index dates in 2008 may have been identified in the middle of a treatment episode; thus, patients with outpatient pharmacy claims for buprenorphine in the 3 months prior to the index date were excluded to of the index date. Continuous enrollment with medical and pharmacy benefits for 6 months prior to and 12 months following the index date was required for study inclusion. Patients from database contributors that may have mental health and substance abuse carve-outs that do not share data with the plan were excluded because claims in the database for these patients may not represent all covered health care utilization. Patients treated with an average daily dose of buprenorphine of less than 4 mg/day and who remained on treatment less than 30 days were excluded, as these patients were likely to be detoxification patients rather than patients using buprenorphine MAT as maintenance therapy. Medicaid patients were excluded if they had dual Medicaid/Medicare eligibility because services covered in full by Medicare may not be included in the MarketScan Medicaid Multi-State Database. Patients were followed up over 12 months, commencing on the index date (ie, at buprenorphine initiation).
Adherence
Buprenorphine MAT adherence was measured using the proportion of days covered (PDC) by buprenorphine in the 12 months following treatment initiation. The total days of possession of buprenorphine were calculated from the days of supply information on outpatient pharmacy claims, regardless of gaps in therapy. For patients who refilled early, overlapping days were appended to the total days of supply. PDC was then calculated as:
Patients were grouped by PDC into the following categories: PDC<0.20, 0.20≤PDC<0.40, 0.40≤PDC<0.60, 0.60≤PDC<0.80 and PDC≥0.80. Patients with PDC≥0.80 were classified as adherent, consistent with previous studies in this disease areaCitation15,Citation16 and other chronic conditions.Citation20
Outcome measures
Relapse prevalence
Relapse prevalence was measured during the follow-up period of the study. Relapses are not directly captured in claims data, so a proxy measure based on services that may be indicators of relapse was employed. The proxy measure, which was a modification of a measure used in previous claims-based studies,Citation21,Citation22 defined relapse as the presence of claims for any of the following binary (yes/no) relapse indicators:
Diagnosis code of opioid dependence, continuous or episodic (ICD-9-CM 304.01, 304.02, 304.71, 304.72), following an opioid dependence in remission code (ICD-9-CM 304.03, 304.73)
Inpatient admission with a primary diagnosis related to opioid use (opioid dependence, opioid abuse or opioid overdose [ICD-9-CM 965.09])
Detoxification with any diagnosis related to opioid use
Emergency department (ED) visit with any diagnosis related to opioid use
Health care utilization and costs
All-cause health care utilization and costs were evaluated during the 12-month post-index period. Specific utilization measures included inpatient admissions, ED visits, physician office visits and outpatient pharmacy services. Corresponding costs were measured from the paid amounts on relevant claims, including both the patient responsibility (eg, deductible, copay or coinsurance) and the health plan payment (including coordination of benefit amount). Cost categories were created by summing costs across all relevant claims. Inpatient costs were defined as all costs related to an admission. Outpatient costs included costs from ED visits, physician office visits and other non-pharmacy outpatient services. Medical costs comprised inpatient plus outpatient costs, and total costs comprised medical costs plus outpatient pharmacy costs. If the sum of a patient’s costs across all claims in a category was less than zero, which occasionally happens in claims data if claims are erroneously reconciled, the patient’s costs for that category were set to zero. No trimming of high-end cost outliers was conducted. Similar health care utilization and cost measures were calculated from claims in the 6-month pre-index period to compare against the post-index measures. All cost estimates were inflation adjusted to 2014 US dollars, using the medical component of the Consumer Price Index.
Other variables
Patient demographics including age in years, sex, race (Medicaid Database only), geographic region of residence (Commercial Database only), urban/rural residence, insurance plan type and relationship to the policyholder (Commercial Database only) were measured on the index date. Sex is a binary variable (male, female) in the databases used for this analysis. Race, in the Medicaid Database was categorized as follows: White (not of Hispanic ethnicity); Black (not of Hispanic ethnicity); Hispanic (regardless of race) and others (American Indian/Alaskan Native, Asian or native Hawaiian/other Pacific Islander). Geographic region in the Commercial Database is based on US census regions, which include Northeast, Northcentral (Midwest), South and West. The urban/rural residence designation in the databases is based on whether place of residence is located within a US metropolitan statistical area (urban) or not (rural). Insurance plan type (eg, preferred provider organization [PPO] and health maintenance organization [HMO]) and the relationship to the policyholder (Commercial Database only, ie, employee, spouse or child/others) were categorized as recorded on the index buprenorphine claim.
The Deyo-Charlson Comorbidity Index (DCI)Citation23 was calculated from claims during the 6-month pre-index period. The DCI is an aggregate measure of comorbidity, expressed as a numeric score, based on the presence of diagnoses for selected chronic conditions (ie, cerebrovascular disease, congestive heart failure, chronic pulmonary disease, dementia, diabetes, hemiplegia or paraplegia, HIV/AIDS, liver disease, malignancy [any], metastatic solid tumor, myocardial infarction, peptic ulcer disease, peripheral vascular disease, renal disease and rheumatologic disease), which are assigned weights ranging from 1 to 6 points. Weights for all conditions recorded in the patient’s claims are summed to produce the DCI score. The range of possible scores is 0–33 with higher scores reflective of greater comorbid burden.
Specific pre-index comorbid conditions were measured based on the presence of one or more non-diagnostic claim in the 6-month pre-index period carrying a diagnosis code indicative of the condition. Only non-diagnostic claims – that is, claims other than for laboratory and radiology services – were used to create the comorbid condition variables, because diagnostic claims may list rule-out conditions rather than actual comorbidities. Comorbidity variables included non-opioid drug use disorder, alcohol use disorder, depressive disorder, bipolar disorder, generalized anxiety disorder, schizophrenia, chronic pain condition (eg, migraine, headache syndromes, spondylosis, disc disorders, cervicalgia, torticollis, neuropathies, osteoarthritis, rheumatoid arthritis, endometriosis, chronic pancreatitis, chronic postoperative or trauma pain), HIV/AIDS, hepatitis B and hepatitis C. The comorbid non-opioid drug use disorder variable measured disorders involving substances other than opioids, alcohol and tobacco, including sedatives, hypnotics, anxiolytics, cocaine, cannabis, amphetamines, non-amphetamine psychostimulants, hallucinogens, antidepressants and other unspecified substances. Opioids were not included in this variable because all patients in the study had evidence of OUD on or prior to the index date, and the intent was to measure comorbid drug use disorders related to non-opioid substances. Alcohol was not included when measuring non-opioid drug use disorder because pre-index comorbid alcohol use disorder was measured with a separate variable.
Concomitant medication use was measured based on one or more outpatient pharmacy claim in the 6-month pre-index period for the following medication classes: opioid analgesics excluding buprenorphine and methadone (eg, codeine, fentanyl, hydrocodone, hydromorphone, meperidine, morphine, oxycodone, oxymorphone, pentazocine, propoxyphene and tapentadol), benzodiazepines, non-benzodiazepine sedative/hypnotics, antidepressants and antipsychotics. An outpatient services claim for an injectable antipsychotic also was considered evidence of concomitant antipsychotic use. MAT other than buprenorphine MAT (ie, methadone MAT, oral naltrexone MAT and extended-release injectable naltrexone MAT) was measured based on one or more relevant claim in the 6-month pre-index period. Psychosocial treatment was measured in the 12-month post-index period.
Buprenorphine dosing variables were created to include as covariates in modeling because previous studies suggested that buprenorphine dose may impact subsequent treatment retention and adherence.Citation24,Citation25 The average daily dose of buprenorphine was measured over the first 6 months post-index to assess the association between dosing in the initial months of treatment and medication adherence over the 12-month follow-up period. Average daily dose was calculated from information on pharmacy claims by determining the milligrams of product dispensed at each fill (ie, tablet strength × number of tablets) and performing the following calculation:
Access restrictions (eg, prior authorization) imposed by state Medicaid agencies may affect buprenorphine adherence. Thus, for the Medicaid analysis, a variable was created to indicate the presence of any of the following restrictions on access to buprenorphine: daily dose limit of 16 mg or less, lifetime treatment length limit of 1 year or less or prior authorization frequency of ≤6 months. This variable could not be reported descriptively due to confidentiality agreements with database contributors, but was included as a covariate when modeling buprenorphine adherence.
Statistical analyses
The Commercial and Medicaid samples were analyzed separately in parallel analyses. The patient group with PDC≥0.80 (ie, patients defined as adherent) was the reference category for all statistical comparisons. Chi-squared tests and Student’s t-tests were used to evaluate the statistical significance of differences in patient characteristics between PDC categories for categorical variables and continuous variables, respectively. For categorical variables, when more than 20% of cells in a comparison had expected observations of 5 or fewer, Fisher’s exact tests were used. An a priori p-value of <0.05 was set as the threshold for statistical significance.
Adjusted analyses were conducted to examine the impact of PDC on relapse and total health care costs in the year following buprenorphine MAT initiation and to assess the factors of adherence to buprenorphine MAT. Relapse in the 12 months following treatment initiation, a binary (yes/no) variable, was modeled via standard logistic regression. In addition to the PDC category, covariates in the relapse models included the following: age, sex, race (Medicaid only), relationship to policyholder (Commercial only), insurance plan type, pre-index alcohol use disorder diagnosis, pre-index non-opioid drug use disorder diagnosis, pre-index severe mental illness diagnosis (schizophrenia and/or bipolar disorder), pre-index other mental illness diagnosis (depressive disorder and/or generalized anxiety disorder) and pre-index chronic pain condition diagnosis. Examination of Schoenfeld residualsCitation26 and variation inflation factorsCitation27 for each covariate confirmed the appropriateness of the model structure and absence of high correlation between covariates, respectively.
Generalized linear models (GLMs) with a log link and underlying gamma distribution were used to model the cost data. The same set of covariates used in the relapse models was included in the cost models. Model diagnostics suggested that the models may overestimate costs for some covariates among very expensive patients. Costs were, therefore, adjusted based on the GLM coefficients for the key explanatory variable of PDC category as well as for other variables that the analysis suggested may be cost drivers (Commercial: relationship to policyholder, pre-index chronic pain condition diagnosis; Medicaid: insurance plan type, pre-index severe mental illness diagnosis) in case model trends were dissimilar across subgroups.
Factors associated with adherence were identified in a logistic regression model that estimated the impact of patient characteristics on being in the adherent cohort (ie, having PDC≥0.80 vs. PDC<0.80). Covariates were similar to those used in the relapse and cost models, except that PDC categories were not included as covariates since PDC was used to define the dependent variable of adherence. In addition, the following covariates were added to assess their association with adherence: geographic region (Commercial only); urban/rural residence; Medicaid access restrictions on buprenorphine (Medicaid only); pre-index MAT other than buprenorphine MAT; buprenorphine average dose in the first 6 months post-index and post-index psychosocial treatment. The review of variation inflation factorsCitation27 confirmed that there was no high multicollinearity between model covariates.
All data management, descriptive analyses, bivariate analyses and adjusted analyses were conducted using SAS 9.4 (SAS Institute Inc, Cary, NC, USA).
Results
Patient selection and PDC groups
A total of 16,085 commercially insured patients and 5,688 Medicaid patients were qualified for the analysis (). In the Commercial sample, 37.1% of patients were classified as adherent based on PDC≥0.80. Among the non-adherent patients, 28.4% had PDC<0.20, 13.2% had 0.20≤PDC<0.40, 10.7% had 0.40≤PDC<0.60 and 10.7% had 0.60≤PDC<0.80. In the Medicaid sample, 41.3% of patients were adherent. Among the non-adherent patients, 26.7% had PDC<0.20, 12.6% had 0.20≤PDC<0.40, 10.5% had 0.40≤PDC<0.60 and 8.9% had 0.60≤PDC<0.80.
Patient characteristics
Baseline patient demographics and clinical characteristics are presented in (Commercial) and (Medicaid). The majority of patients in the Commercial sample were male (62.7%), while the majority of Medicaid patients (72.6%) were female. The high proportion of female patients in the Medicaid sample is consistent with the high female-to-male ratio among Medicaid beneficiaries in the underlying database, which is likely related to Medicaid eligibility requirements. The overall study sample in both the Commercial and Medicaid samples had an average age of 32 years. Commercial patients with PDC≥0.80 were slightly older and more likely to be the insured employee (as opposed to a dependent) than those with lower adherence. Among Medicaid patients, the proportion of Black patients was higher among patients with PDC<0.20 (12.1%), 0.20≤PDC<0.40 (6.3%) and 0.40≤PDC<0.60 (6.4%) as compared to patients with PDC≥0.80 (4.3%).
Table 1 Baseline demographic and clinical characteristics among adult patients with OUD who were newly initiating buprenorphine MAT: Commercial sample
Table 2 Baseline demographic and clinical characteristics among adult patients with OUD who were newly initiating buprenorphine MAT: Medicaid sample
In both the Commercial and Medicaid samples, significantly higher (p<0.001) rates of baseline alcohol use disorder, non-opioid drug use disorder, depressive disorder and bipolar disorder were observed in those with PDC<0.20 than among adherent patients (PDC≥0.80). A higher rate of chronic pain conditions was observed among Medicaid patients with the lowest level of adherence (PDC<0.20) compared to adherent patients (p<0.001). Medicaid patients with PDC<0.20 had higher rates of concomitant medication use including opioid analgesics, benzodiazepines, non-benzodiazepine sedative/hypnotics, antidepressants and antipsychotics than adherent patients (opioid analgesics, p=0.007; all other medication classes, p<0.001).
Relapse prevalence
In the Commercial sample, 21.2% of patients had at least one indicator of relapse during the 12-month post-index period with the lowest prevalence of relapse observed in patients with PDC≥0.80 (11.4%). The proportion of patients with at least one indicator of relapse was significantly higher in each of the lower adherence groups compared to the adherent group (). The same trend was observed when each relapse indicator was considered individually, except for the indicator measuring a diagnosis code of opioid dependence, continuous or episodic, following an opioid dependence in remission code, which was significantly different between the lowest adherence group (PDC<0.20) and the adherent group (PDC≥80%), but similar across other PDC groups.
Table 3 Unadjusted relapse prevalence, health care resource utilization and costs in the 12-month post-index period among adult patients with OUD who were newly initiating buprenorphine MAT
In the Medicaid sample, 15.0% of patients had at least one indicator of relapse during the 12-month post-index period with the lowest relapse prevalence observed in adherent patients (10.0%). Similar to the trend observed in the Commercial sample, the proportion of Medicaid patients with an indicator of relapse was significantly higher in each of the lower adherence groups compared to the adherent group (). The same trend was observed when each relapse indicator was considered individually, except for the indicator measuring a diagnosis code of opioid dependence, continuous or episodic, following an opioid dependence in remission code, which was significantly different between the two lowest adherence groups (PDC<0.20, 0.20≤PDC<0.40) and the adherent group (PDC≥80%), but similar across other PDC groups. In contrast to relapse prevalence in the Commercial sample, an ED visit with any diagnosis related to opioid use was the most common individual relapse indicator among Medicaid patients.
After adjustment to control for differences between cohorts, Commercial patients in all other PDC groups had significantly higher odds of experiencing at least one indicator of relapse in the 12 months post buprenorphine MAT initiation compared to the reference group of adherent patients with PDC≥80% (PDC<0.20: adjusted odds ratio [AOR]=2.02, 95% CI=1.77–2.32; 0.20≤PDC<0.40: AOR=2.33, 95% CI=1.99–2.74; 0.40≤PDC<0.60: AOR=2.27, 95% CI=1.92–2.70; 0.60≤PDC<0.80: AOR=1.65, 95% CI=1.37–1.98; all p<0.001). Similar results were observed among Medicaid patients in the 12-month post-index period (PDC<0.20: AOR=1.76, 95% CI=1.40–2.20; 0.20≤PDC<0.40: AOR=1.90, 95% CI=1.45–2.50; 0.40≤PDC<0.60: AOR=1.65, 95% CI=1.22–2.23; 0.60≤PDC<0.80: AOR=1.48, 95% CI=1.06–2.06; all p<0.01; ).
Table 4 Adjusted odds of at least one indicator of relapse in the 12-month post-index period among adult patients with OUD who were newly initiating buprenorphine MATTable Footnotea
Health care utilization and costs
presents the unadjusted health care resource utilization and costs by the PDC group in the 12 months following buprenorphine MAT initiation. In both the Commercial and Medicaid samples, the proportion of patients with an inpatient admission in the 12 months following the index date was significantly higher in the non-adherent groups compared to the adherent cohort. The proportion of patients hospitalized in the lowest non-adherent group (PDC<0.20) was about twice that of patients with PDC≥80% (Commercial: 44.3% vs. 17.9%, p<0.001; Medicaid: 39.0% vs. 19.9%, p<0.001). A similar pattern was observed for ED utilization (Commercial: 55.0% vs. 34.5%, p<0.001; Medicaid: 79.6% vs. 65.0%, p<0.001). Adherent patients had a significantly higher number of physician office visits post buprenorphine MAT initiation than non-adherent patients. In both the Com mercial and Medicaid samples, patients with PDC≥80% incurred about 13 physician office visits, on average, over the 12-month post-index period, compared to about eight visits for patients with PDC<0.20 (p<0.01). The difference may be explained, in part, by office visits required for buprenorphine MAT initiation and monitoring. As expected given the association between medication adherence and prescription fills, adherent patients also had a higher number of pharmacy claims than non-adherent patients.
The average unadjusted total health care costs in the 12-month post-index period were $23,006 and $19,888 for the patients in the Commercial and Medicaid samples, respectively. In the Commercial sample, the mean unadjusted total health care costs decreased as adherence levels increased from $28,525 in patients with PDC<0.20 to $17,844 in patients with PDC≥0.80. The average health care costs in the Medicaid sample decreased slightly from $21,292 in patients with PDC<0.20 to $18,621 in patients with PDC≥0.80. Mean unadjusted outpatient pharmacy costs increased with adherence in both Commercial and Medicaid patients while medical costs decreased with adherence levels. The large standard deviations as given in are typical of health care cost data, which are often highly skewed. This study’s inclusion criteria required all patients to have buprenorphine MAT utilization at index so there was not a cluster of patients with zero total costs; however, there were some patients with very high costs that resulted in large SDs around the mean costs.
presents the adjusted mean total health care costs in the 12 months post buprenorphine MAT initiation for the Commercial and Medicaid samples. After adjusting for differences between PDC groups using GLMs, mean total costs in the Commercial sample decreased as adherence increased, with adherent patients (PDC≥0.80) having significantly lower adjusted mean total costs ($17,519) as compared to patients in all non-adherent groups (PDC<0.80; range from $20,294 to $24,431). Other factors that were found to significantly affect the health care costs in the Commercial sample included relationship to policyholder and having a comorbid chronic pain condition. Adjusted mean total costs were significantly lower for insured employees ($18,570) than for covered dependents (spouses, $20,325; children/others, $27,073). Contrarily, the adjusted mean total costs of Commercial patients with a pre-index chronic pain condition were significantly higher ($24,995) than similar patients with no pre-index claims indicative of a chronic pain condition ($18,840).
Table 5 Adjusted total costs in the 12-month post-index period based on GLMs among adult patients with OUD who were newly initiating buprenorphine MATTable Footnotea
In the Medicaid sample, adjusted mean total costs were not significantly different between adherent (PDC≥0.80) and non-adherent groups (PDC<0.80). Factors found to significantly affect the health care costs in the Medicaid sample included managed care plan type and severe mental illness comorbidity status. Adjusted mean total costs for patients in Medicaid managed care plans were higher ($22,777) than for Medicaid patients in non-managed care plans ($19,371). Patients with a pre-index severe mental illness had significantly higher adjusted mean total costs in the 12-month post-index period ($24,146) than patients with no claims evidence of such a condition ($18,273).
Factors associated with adherence
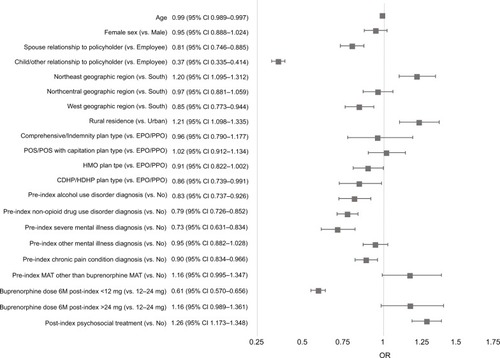
Variables in the logistic regression models that were significantly associated with increased odds of adherence (PDC≥0.80) in Commercial patients () included receipt of psychosocial therapy post-index (AOR=1.26, 95% CI=1.173–1.348), rural residence vs. urban (AOR=1.21, 95% CI=1.098–1.335), and Northeast geographic region vs. South (AOR=1.20, 95% CI=1.095–1.312). Factors significantly associated with decreased odds of adherence were being a child or other dependent of the policyholder vs. being the employee (AOR=0.37, 95% CI=0.335–0.414), being a spouse of the policyholder vs. being the employee (AOR=0.81, 95% CI=0.746–0.885), average daily dose of buprenorphine MAT <12 mg vs. 12–24 mg (AOR=0.61, 95% CI=0.570–0.656), pre-index severe mental illness diagnosis (AOR=0.73, 95% CI=0.631–0.834), pre-index non-opioid drug use disorder diagnosis (AOR=0.79, 95% CI=0.726–0.852), pre-index alcohol use disorder diagnosis (AOR=0.83, 95% CI=0.737–0.926), pre-index chronic pain diagnosis (AOR=0.90, 95% CI 0.834–0.966) and West geographic region vs. South (AOR=0.85, 95% CI=0.773–0.944).
Figure 2 Predictors of adherence (PDC≥0.80) among adult patients with OUD who were newly initiating buprenorphine MAT: Commercial sample.
Abbreviations: CDHP, consumer-driven health plan; HDHP, high deductible health plan; HMO, health maintenance organization; M, months; MAT, medication-assisted treatment; OUD, opioid use disorder; PDC, proportion of days covered; POS, point of service plan; PPO, preferred provider organization.
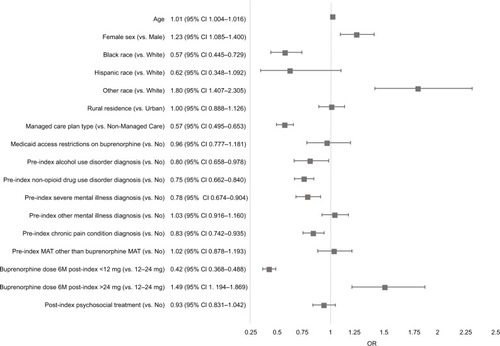
In the Medicaid sample, variables significantly associated with increased odds of adherence based on logistic regression models () included female (AOR=1.23, 95% CI=1.085–1.400) and average daily dose of buprenorphine MAT >24 mg vs. 12–24 mg (AOR=1.49, 95% CI=1.194–1.869). Factors significantly associated with decreased odds of PDC≥0.80 were average daily dose of buprenorphine MAT <12 mg vs. 12–24 mg (AOR=0.42, 95% CI=0.368–0.488), managed care plan type vs. non-managed care plan type (AOR=0.57, 95% CI=0.495–0.653), pre-index non-opioid drug use disorder diagnosis (AOR=0.75, 95% CI=0.662–0.840), pre-index severe mental illness diagnosis (AOR=0.78, 95% CI=0.674–0.904), pre-index chronic pain condition diagnosis (AOR=0.83, 95% CI=0.742–0.935) and pre-index alcohol use disorder diagnosis (AOR=0.80, 95% CI 0.658–0.978).
Figure 3 Predictors of adherence (PDC≥0.80) among adult patients with OUD who were newly initiating buprenorphine MAT: Medicaid sample.
Abbreviations: M, months; MAT, medication-assisted treatment; OUD, opioid use disorder; PDC, proportion of days covered.
Discussion
This study assessed adherence in the 12 months following buprenorphine MAT initiation among OUD patients and found that 37% of Commercial patients and 41% of Medicaid patients were classified as adherent, based on PDC of 0.80 or above. The Commercial adherence rate is similar to the findings of two previous retrospective claims database studies utilizing a single US commercial health plan population. One studyCitation15 found that 32% of patients were adherent to buprenorphine MAT in the year following treatment initiation, while the otherCitation16 determined that 36%–43% were adherent, depending on the adherence definition used. The Medicaid adherence rate in this study based on a multistate sample appeared higher than results from a previous analysis of data from one state Medicaid program.Citation17 Different adherence measures between the two studies make direct comparison difficult, but the previous analysis found that only 21% of patients persistently refilled buprenorphine over 12 months following treatment initiation.
The association between adherence and reduced odds of relapse was consistent with expectations and confirmed previous studies in other patient samples.Citation14,Citation16,Citation22 Patients with lower levels of buprenorphine MAT adherence had significantly increased odds of relapse compared to adherent patients, lending further evidence that buprenorphine MAT adherence is associated with improved treatment outcomes.
Among Commercial patients, after controlling for demographic characteristics and comorbidities, higher levels of adherence were associated with lower total costs, and the effect was larger as the adherence level increased. Adherent patients’ (PDC≥0.80) adjusted total health care costs were nearly 30% less than non-adherent patients’, despite the higher pharmacy costs of buprenorphine MAT for adherent patients. This analysis did not separately measure buprenorphine pharmacy costs, but the $350 to $420 estimated monthly cost of buprenorphineCitation28 might account for part of the outpatient pharmacy cost difference between adherent and non-adherent patients. For Medicaid patients, descriptive analysis found that adherence was associated with higher outpatient pharmacy costs and lower medical costs, resulting in lower total costs. The models controlling for demographic characteristics and comorbidities found no association between buprenorphine MAT adherence and total costs, which indicates that the reduction in total costs among adherent patients in the descriptive analyses of the Medicaid population is likely explained by other clinical or demographic factors that are prevalent in the adherent population.
The total cost trends observed in the Commercial sample after adjustment are consistent with two recent analyses of buprenorphine-treated patients.Citation15,Citation16 Tkacz et alCitation15 analyzed health care charges (as opposed to paid amounts) and found adherent patients to have significantly lower adjusted total charges over the 12-month post-index period, as compared to non-adherent patients ($28,458 vs. $49,051, p=0.001). Ruetsch et alCitation16 assessed costs (paid amounts) and similarly found adjusted total costs over the 12-month post-index period to be lower among adherent than non-adherent patients ($7,581 vs. $10,638, p<0.01). Both these studies analyzed a single health plan population, and findings from analyses of a single plan may reflect a benefit design or member or clinician population that is unique to that plan. However, the similar trends in the current study, which included patients from several commercial fee-for-service and managed care plans across the USA, suggest that buprenorphine MAT adherence may be a key factor for reducing costs across commercial plans.
The current study’s Medicaid cost trends appear to conflict with the intuitive association between buprenorphine adherence and better health outcomes leading to reduced health care costs, but several factors may have impacted this association. First, when comparing the distribution of total costs among Medicaid patients to that of Commercial patients, the cost variation within group was notably higher among the Medicaid patients. This may mean Medicaid patients are a more heterogeneous group and include patients with multiple preexisting comorbidities who require personalized approaches to OUD management. Furthermore, Medicaid patients often struggle with limited and restricted provider networks that result in limited access to addiction specialists, long waiting times for appointments and transportation issues. In addition, Medicaid patients may have also experienced access challenges in other treatment areas. Health care systems for substance use disorder treatment are often fragmented and poorly funded.Citation29 As found in our study, comorbidities such as alcohol use disorder, schizophrenia, depressive disorder and bipolar disorder were more prevalent among patients who were not adherent to buprenorphine MAT. These comorbidities may complicate navigating the health care system efficiently to receive treatments to address the consequences of non-adherence to the medication, especially when being forced to rely on Medicaid providers and funding. Certain patients may be adherent due to close monitoring by physicians because of the seriousness of the patient’s situation (eg, patients with multiple comorbidities or legal system interaction), thus resulting in improved medication adherence and also health care costs for close monitoring and treatment. Unmeasurable characteristics or interactions between risk factors and adherence that could not be controlled for in modeling also may have impacted the cost model results.
There is no optimal duration of maintenance therapy with buprenorphine. Treatment guidelinesCitation11,Citation30 suggest that short- or long-term treatment may be appropriate depending on several factors, most of which cannot be measured in claims data (eg, patient preference for continued treatment, psychosocial support, stable home situation and absence of legal problems). Thus, while patients with PDC of 0.80 or above (about 9 or 10 months of therapy) were the reference group in the current study, there could be treatment successes across all PDC groups. Other studiesCitation16 found patients with PDC between 0.60 and 0.79 to have costs similar to those of patients with PDC≥0.80, suggesting that some patients may have adequate cost outcomes at slightly lower adherence levels.
A number of patient characteristics were found to be predictive of non-adherence, suggesting several opportunities for patients, clinicians and payers to improve buprenorphine MAT adherence. Comorbid conditions such as alcohol use disorder, non-opioid drug use disorder, severe mental illness (schizophrenia and/or bipolar disorder), chronic pain conditions and lower average daily dose of buprenorphine MAT (<12 mg) were significantly associated with buprenorphine MAT non-adherence. In commercially insured patients, the use of psychosocial therapy in addition to buprenorphine MAT was associated with increased odds of adherence while being a spouse, child or other dependent of the primary policyholder was associated with decreased odds of adherence. The adequate identification and management of mental health conditions and other substance use disorders among opioid-dependent patients receiving buprenorphine MAT should be a key treatment focus. Careful selection and monitoring of buprenorphine MAT doses may also improve patient adherence. In addition, children or spouses of commercial insurance policyholders should be given extra support to remain on therapy.
As with any retrospective claims analysis, this study had several limitations. Administrative claims data are subject to data coding limitations and data entry error. Substance use disorders tend to be under-recorded in claims data, due to various factors including access to care (including screening and treatment) issues,Citation29 stigma,Citation31 privacy concernsCitation32 and billing practices.Citation33 Sensitivity of substance use disorder coding in claims data is generally high but specificity may be low,Citation34 such that there is potential for misclassification of outcomes that depend on the presence of OUD diagnoses on claims. This study was limited to individuals with commercial coverage through an employer and Medicaid beneficiaries from select states and results may not be generalizable to patients with other types of coverage or with no health insurance coverage. The PDC cohorts were created based on information present on claims and may be subject to measurement error. PDC was based on days’ supply of medication dispensed. It was not possible to ascertain from claims whether patients actually took all days of therapy that was dispensed. Similarly, potential drug diversion could not be ascertained from claims data. It is possible that patients who appeared to be adherent to buprenorphine MAT actually were not due to diversion. Relapse was identified using service-based proxies that, while informed by expert opinion and prior study, have not been validated and, thus, may have misclassified some patients. It is likely the relapse proxy underestimates actual relapse prevalence since relapse does not necessarily result in health care utilization within a defined time frame. That said, false positives also are possible, as reasons other than relapse may result in some of the relapse indicator services. For example, the presence of a diagnosis code of opioid dependence, continuous or episodic, following an opioid dependence in remission code could represent patients undergoing evaluation when switching buprenorphine MAT clinicians. Finally, the adjusted analysis was limited to controlling for factors that could be measured from claims data. Differences between cohorts could have remained after adjustment and may have impacted findings. For example, it was not possible to measure and control for disease severity, psychosocial supports, legal system involvement, ease of access to heroin/opioids or other related factors that may have impacted outcomes.
Conclusion
Adherence to buprenorphine MAT in the 12 months following treatment initiation was low, with only 37% of Commercial and 41% of Medicaid patients having PDC≥0.80. In contrast, treatment adherence was associated with reduced odds of relapse and medical costs in both Commercial and Medicaid patients. Specifically, for Commercial patients who were treatment adherent, the reduction in their medical costs exceeded the increased pharmacy costs to the level where the total costs for adherent patients were predicted to be 30% lower than those among the PDC<0.20 group. In comparison, the reduction in medical costs among Medicaid patients was just enough to offset the increased pharmacy costs, which may be because Medicaid patients are a more heterogeneous group who require personalized approaches to OUD management. Additional studies, especially prospective studies that follow patients over multiple years, are needed to better understand the relationship between treatment, adherence and long-term health outcomes in patients with OUD to optimize treatment for these patients.
Acknowledgments
The authors thank the following Truven Health Analytics® (Cambridge, MA, USA) employees for their contributions: Ashley Cole who was responsible for SAS programming and data management and Kavya Thelakkat who contributed to the manuscript preparation.
Disclosure
This study was funded by Indivior Inc, (Richmond, VA). BAW, MD, received funding from Indivior Inc. for his role as a project consultant and previously served on Indivior-sponsored advisory boards. VRN and NAR are employees of Indivior Inc. LBM is an employee of Truven Health Analytics®, part of the IBM Watson Health™ business, which received funding from Indivior Inc. to conduct this analysis. TMW was an employee of Truven Health Analytics when this study was conducted and is currently at the Department of Internal Medicine, Division of Epidemiology, University of Utah, Salt Lake City, UT, USA. The authors report no other conflicts of interest in this work. Portions of this study were presented at the Academy of Managed Care Pharmacy (AMCP) Nexus Conference, National Harbor, MD, USA, October 3–6, 2016 and the US Psychiatric and Mental Health Congress, San Antonio, TX, USA, October 21–24, 2016.
References
- Substance Abuse and Mental Health Services Administration (SAM-HSA), Center for Behavioral Health Statistics and QualityKey Substance Use and Mental Health Indicators in the United States: Results from the 2015 National Survey on Drug Use and HealthRockville, MDSubstance Abuse and Mental Health Services Administration2016HHS Publication No. SMA 16-4984, NSDUH Series H-51
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders-DSM-55th edWashington, DCAmerican Psychiatric Publishing2013
- RuddRAAleshireNZibbellJEGladdenRMIncreases in drug and opioid overdose deaths – United States, 2000–2014MMWR Morb Mortal Wkly Rep20166450–511378138226720857
- BirnbaumHGWhiteAGSchillerMWaldmanTClevelandJMRolandCLSocietal costs of prescription opioid abuse, dependence, and misuse in the United StatesPain Med201112465766721392250
- HansenRNOsterGEdelsbergJWoodyGESullivanSDEconomic costs of nonmedical use of prescription opioidsClin J Pain201127319420221178601
- FlorenceCSZhouCLuoFXuLThe economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013Med Care2016541090190627623005
- WhiteAGBirnbaumHGMarevaMNDirect costs of opioid abuse in an insured population in the United StatesJ Manag Care Pharm200511646947915998164
- StrasselsSAEconomic burden of prescription opioid misuse and abuseJ Manag Care Pharm200915755656219739878
- McAdam-MarxCRolandCLClevelandJOderdaGMCosts of opioid abuse and misuse determined from a Medicaid databaseJ Pain Palliat Care Pharmacother201024151820345194
- OderdaGMLakeJRudellKRolandCLMastersETEconomic burden of prescription opioid misuse and abuse: a systematic reviewJ Pain Palliat Care Pharmacother201529438840026654413
- KampmanKJarvisMAmerican Society of Addiction Medicine (ASAM) National Practice Guideline for the use of medications in the treatment of addiction involving opioid useJ Addict Med20159535836726406300
- McCance-KatzEFOffice-based buprenorphine treatment for opioid-dependent patientsHarv Rev Psychiatry200412632133815764468
- ParranTVAdelmanCAMerkinBLong-term outcomes of office-based buprenorphine/naloxone maintenance therapyDrug Alcohol Depend20101061566019717249
- TkaczJSevertJCacciolaJRuetschCCompliance with buprenorphine medication-assisted treatment and relapse to opioid useAm J Addict2012211556222211347
- TkaczJVolpicelliJUnHRuetschCRelationship between buprenorphine adherence and health service utilization and costs among opioid dependent patientsJ Subst Abuse Treat201446445646224332511
- RuetschCTkaczJNadipelliVRHeterogeneity of nonadherent buprenorphine patients: subgroup characteristics and outcomesAm J Manag Care2017236e172e17928817294
- Lo-CiganicWHGelladWFGordonAJAssociation between trajectories of buprenorphine treatment and emergency department and in-patient utilizationAddiction2016111589290226662858
- IBM Watson Health [webpage on the Internet]Truven Health Analytics®, part of the IBM Watson Health™ businessMarketScan Commercial Claims and Encounters Research Database, 2007–2015 [Data file]. Available from: http://truvenhealth.com/markets/life-sciences/products/data-tools/marketscan-databasesAccessed July 10, 2018
- IBM Watson Health [webpage on the Internet]Truven Health Analytics®, part of the IBM Watson Health™ businessMarketScan Medicaid Multi-State Research Database, 2007–2015 [Data file]. Available from: http://truvenhealth.com/markets/life-sciences/products/data-tools/marketscan-databasesAccessed July 10, 2018
- Nau DP [webpage on the Internet]Proportion of Days Covered (PDC) as a Preferred Method of Measuring Medication AdherenceAlexandria, VirginiaPharmacy Quality Alliance2017 Available from: http://pqaalliance.org/resources/adherence.aspAccessed August 30, 2017
- ClarkRESamnalievMBaxterJDYoungGYThe evidence doesn’t justify steps by state Medicaid programs to restrict opioid addiction treatment with buprenorphineHealth Aff201130814251433
- ClarkREBaxterJDAwehGO’ConnellEFisherWHBartonBARisk factors for relapse and higher costs among Medicaid members with opioid dependence or abuse: opioid agonists, comorbidities, and treatment historyJ Subst Abuse Treat201557758025997674
- DeyoRACherkinDCCiolMAAdapting a clinical comorbidity index for use with ICD-9 CM administrative databasesJ Clin Epidemiol19924566136191607900
- FareedAVayalapalliSCasarellaJDrexlerKEffect of buprenorphine dose on treatment outcomesJ Addict Dis201231181822356665
- KhemiriAKharitonovaEZahVRubyJToumiMAnalysis of buprenorphine/naloxone dosing impact on treatment duration, resource use and costs in the treatment of opioid-dependet adults: a retrospective study of US public and private health care claimsPostgrad Med2014126511312025295655
- HessKRGraphical methods for assessing violations of the proportional hazards assumption in Cox regressionStat Med19951415170717237481205
- O’BrienRMA caution regarding rules of thumb for variance inflation factorsQual Quant200741673680
- SchackmanBRLeffJAPolskyDCost-effectiveness of long-term outpatient buprenorphine-naloxone treatment for opioid dependence in primary careJ Gen Intern Med201227666967622215271
- Medicaid and CHIP Payment Access Commission (MACPAC)Medicaid and the Opioid Epidemic2017 Available from: https://www.macpac.gov/publication/medicaid-and-the-opioid-epidemic/Accessed August 30, 2017
- Center for Substance Abuse Treatment (CSAT) [webpage on the Internet]Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid AddictionTreatment Improvement Protocol (TIP) Series 40Rockville, MDSubstance Abuse and Mental Health Services Administration2004DHS Publication No (SMA) 04-3939
- KuleszaMLarimerMERaoDSubstance use related stigma: what we now and the way forwardJ Addict Behav Ther Rehabil20132278225401117
- RoughKBatemanBTPatornoESuppression of substance abuse claims in Medicaid data and rates of diagnoses for non-substance abuse conditionsJAMA2016315111164116626978213
- O’MalleyKJCookKFPriceMDWildesKRHurdleJFAshtonCMMeasuring diagnoses: ICD Code accuracyHealth Serv Res2005405 pt 21620163916178999
- KimHMSmithEGStanoCMValidation of key behaviorally based mental health diagnoses in administrative data: suicide attempt, alcohol abuse, illicit drug abuse and tobacco useBMC Health Serv Res2012121822270080