Abstract
Pluripotent stem cells have been derived from various embryonic, fetal and adult sources. Embryonic stem cells (ESCs) and parthenogenic ESCs (pESCs) are derived from the embryo proper while embryonic germ cells (EGCs), embryonal carcinoma cells (ECCs), and germ-line stem cells (GSC) are produced from germ cells. ECCs were the first pluripotent stem cell lines established from adult testicular tumors while EGCs are generated in vitro from primordial germ cells (PGCs) isolated in late embryonic development. More recently, studies have also demonstrated the ability to produce GSCs from adult germ cells, known as spermatogonial stem cells. Unlike ECCs, the source of GSCs are normal, non-cancerous adult tissue. The study of these unique cell lines has provided information that has led to the ability to reprogram somatic cells into an ESC-like state. These cells, called induced pluripotent stem cells (iPSCs), have been derived from a number of human fetal and adult origins. With the promises pluripotent stem cells bring to cell-based therapies there remain several considerations that need to be carefully studied prior to their clinical use. Many of these issues involve understanding key factors regulating their generation, including those which define pluripotency. In this regard, the following article discusses critical aspects of pluripotent stem cell derivation and current issues about their therapeutic potential.
Keywords:
Introduction
Pluripotent stem cells have been derived from a multitude of embryonic, fetal and adult sources including somatic and germ cells (). Embryonal carcinoma cells (ECCs) were the first to be identified in the 1960s, from the mouseCitation1 and subsequently in human tissues.Citation2 ECCs are pluripotent cells derived from adult testicular teratocarcinomas (or mixed germ cell tumors) from which genetic, immunological and morphological evidence suggest a primordial germ cell (PGC) origin.Citation3 Building from these studies, pluripotent stem cells have been derived from blastocysts (embryonic stem cells, ESCs); PGCs in vitro (embryonic germ cells, EGCs) and more recently from adult germ cells (germ-line stem cells, GSCs) and unfertilized eggs (parthenogenetic pESCs).Citation4 Of significance are gene discoveries in these stem cells that have led to the ability to produce pluripotent stem cells from differentiated adult cells. These cells, known as induced pluripotent stem cells (iPSCs), have been accomplished by genetic and biochemical engineering of adult and progenitor cells with pluripotent regulators. This review will summarize current issues regarding the derivation and potential clinical applications of pluripotent cells, with a focus on human-derived stem cells ().
Figure 1 Human pluripotent stem cells include embryonic stem cells cultured from cells of the inner cell mass of normal or parthenogenetic blastocysts, embryonic germ cells generated from primordial germ cells in late embryonic development, embryonal carcinoma cells isolated from adult teratocarcinomas, germline stem cells derived from spermatogonia, and induced pluripotent stem cells generated by reprogramming differentiated adult cells. Pluripotent stem cells exhibit the potential to produce all cell types of the body. Thus, directed differentiation of these cells holds promise for treating a wide variety of diseases and injuries.
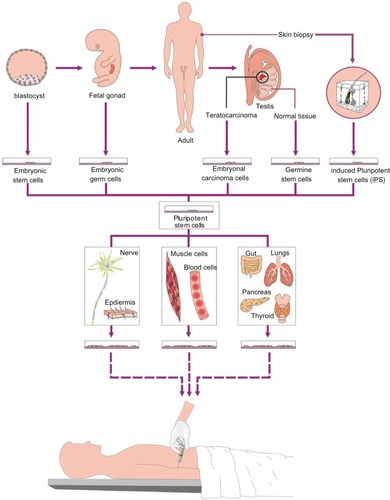
Table 1 Types of pluripotent stem cell lines
These unique cell lines share the general properties of pluripotent stem cells including unlimited self-renewal and the ability to give rise to derivatives of all three embryonic germ layers. Pluripotency of these cell types is demonstrated experimentally by producing cell types representing all three germ layers either spontaneously during embryoid body formation, using directed differentiation protocols in culture or in teratomas after injection into adult mice. ESCs, EGCs and iPSCs have also demonstrated the ability to produce representatives of the germ cell lineage.Citation5–Citation20 The most stringent test for pluripotency involves the ability of these cells to contribute to the development of the embryo proper either partially, in chimeric mice or solely by tetraploid complementation. While most pluripotent cell types have demonstrated their contributions in chimeric mice, ESCs and now more recently, iPSCs have also proven the ability to produce viable offspring through tetraploid complementation.Citation21,Citation22
Methodology
Embryonic stem cells
The derivation and maintenance of sustainable human ESCs were first performed in 1998 by James Thomson when his team cultured the inner cell mass of developing blastocysts (embryo proper) from donated embryos received from in vitro fertilization (IVF) programs.Citation23 During this same time John Gearhart derived EGCs from cultured PGCs of the genital ridge.Citation24 Both research teams developed techniques learned from several decades of prior animal studies deriving pluripotent cell lines from mouse blastocysts.Citation25,Citation26 Since this time, rapid progress has been achieved in improving culture conditions as more lines are developed. These improvements have primarily addressed two important issues with ESC derivation, the ability to acquire viable starting material and for clinical purposes to derive them under xeno-free conditions. This section will focus on recent updates and novel approaches for deriving and maintaining ESCs. Importantly, advances in ESC derivation will be critical given the shift in the political climate toward expanding stem cell research.
ESCs are isolated from the inner cell mass of 5- to 7-day-old blastocysts and cultured with mitotically-inactivated fibroblast cells. An in depth review of embryo-derived stem cells has been undertaken by Smith,Citation27 and comprehensive reviews with methodologies can be found in several books.Citation28–Citation30 Successful derivation of ESCs is limited by the quality and quantity of the inner cell mass obtained.Citation31 As most lines have been developed from blastocysts for IVF purposes that would have otherwise been discarded, their quality is less than optimal for implantation. Recently, Daley and colleagues have reported an improved method for deriving ESCs from discarded poor-quality embryos from infertility centers.Citation32 This study utilized hypoxic conditions based on previous studies which show hypoxia was beneficial for preimplantation development, ESC cloning and maintaining pluripotency in culture.Citation33–Citation35 However, the researchers were careful to state that their experiments were not designed to conclude whether hypoxia was beneficial for derivation. More recently, Yamanaka and colleagues have also reported 40-fold higher efficiency rates in iPSC production under hypoxic conditions compared to controls.Citation36 Another issue raised by Lerou and colleaguesCitation37 was eliminating the standard immunosurgery and other manipulations normally used to remove the inner cell mass from the blastocysts. As these structures are usually disrupted in poor quality embryos this step would alleviate the stress these procedures apply to the cells. Using this combined strategy their results demonstrated 4% to 6% derivation efficiencies similar to lines derived by frozen embryos.Citation38
Optimizing derivation of human ESC lines under animal free conditions is continually evolving as it remains a fundamental concern for their use in cell based therapies. Several reports have demonstrated the ability to derive human ESCs under serum-free and feeder-free conditions but the stability of these cells over long term culture is uncertain.Citation39,Citation40 Importantly, it will remain to be seen if the approaches that are now employed to enhance iPSC derivation can in fact also enhance the efficiency of human ESC derivation under these conditions as well. For instance, a recent study demonstrated that epigenetic modifying reagents like 5-aza-2’-deoxycytidine (AZA) and trichostatin A (TSA) significantly improve efficiency rates in mouse ESC derivation (40% when both AZA and TSA are added compared to 5% in controls).Citation41 In the mouse, epigenetic modifying reagents have been shown to dedifferentiate ESCs after embryoid formation and prevent ESC differentiation.Citation42,Citation43 These studies exemplify how defining pluripotency and the factors that regulate self-renewal will be critical for improving pluripotent stem cell derivation as well as for the development of new stem cell types.
Induced pluripotent stem cells
Since the development of the first iPSCs almost 3 years ago, multiple laboratories have reported on the ability to derive iPSCs in mouse, human, rat and monkey cells by genetic and/or chemical manipulation using a small set of transcription factors and in some cases, chemical modifiers.Citation44 Unlike traditional methods which utilize viral integration to introduce gene expression, current iPSC strategies focus on reprogramming cells more suitable for clinical applications.Citation45 These methods include transduction with proteins alone (protein transduction), utilizing small molecules that are able to facilitate expression of reprogramming factors, and by employing nonintegrating vectors that transiently express reprogramming factors. Most studies involve the exogenous expression of known genes regulating pluripotency, such as Oct4, Nanog, and Sox2 (Lin28 and Fbx12) in addition to oncogenic factors such as c-Myc and Klf4.Citation46–Citation48 In every case, the combination of these factors expressed in more differentiated cell types successfully produced ESC-like colonies. These studies demonstrate that Oct4 and Sox2 are critical for reprogramming cells, while c-Myc and Klf4 expression, though not critical for transformation, does significantly increase reprogramming efficiency. In three cases, iPSCs were generated without the use of an oncogenic factor. In the first study by Thompson and colleagues, they successfully reprogrammed human fetal and neonatal fibroblasts using Lin28 and Nanog in place of Myc and Klf4. The ability to eliminate oncogenic factors like Myc and Klf4 may be attributable to differences in plasticity of reprogramming less and more differentiated cells.Citation49,Citation50 In the remaining two studies, Oct4 and Sox2 were utilized along with either SV40 large T antigen to reprogram human fibroblasts or with a histone deacetylase inhibitor to reprogram mouse fibroblasts.Citation49,Citation50
Genetic integration using either a lenti- or retrovirus has been the most common method for iPSC production. One problem with generating iPSCs using lentiviral integration occurs when the inserted pluripotent genes are not silenced over time, thereby preventing differentiation of the cells for clinical application.Citation50 For this reason, retroviruses have been employed using a similar strategy. With retroviruses, gene silencing usually occurs a few weeks after host cell integration. However, compared to lentiviruses, retroviruses exhibit lower transduction efficiencies since these viruses specifically target replicating cells and in some cases, silencing does not occur.Citation51
One main issue in iPSC induction is avoiding factors which promote tumorigenesis. To circumvent the issue of oncogenic transgene integration into the host genome, several methods are used including adenoviral transduction, transient transfection, piggyBac transposon gene-delivery system, and various chemical reagents including the direct delivery of the reprogramming proteins themselves.Citation51–Citation55 The minimal set of transcription factors required to induce reprogramming is constantly being refined, as well as the application of chemical inhibitors and signaling molecules. Chemical inhibitors involved in DNA methylation, histone methylation, and acetylation not only improve reprogramming efficiencies and kinetics, but also prevent the use of additional reprogramming factors. For instance, chemical inhibitors such as AZA, valproic acid (VPA), and BIX-01294 (BIX), involved in epigenetic processes have been demonstrated to improve reprogramming when combined with conventional reprogramming factors, such as Oct4 and Nanog.Citation49,Citation56–Citation60 Other molecules such as Wnt3a, 2i, and A-83-01, have also been employed to target specific pathways which appear to enhance the transition to fully programmed iPSCs.Citation58–Citation60 Nonetheless, it still remains a challenge to reprogram somatic cells by chemical treatment alone. Another critical issue in iPSC derivation is the safety of small molecules used to generate therapeutically relevant iPSCs. For example, some of these chemicals not only exert known localized changes in cells, but they also promote global modifications which will most likely result in genetic aberrations and/or dysregulation of genes. A specific example of this concept is AZA, which is known to induce DNA damage.Citation61
Independent of the iPSC derivation method used, iPSC-like colonies appear to form 1 to 4 weeks after transfection. This time depends, in part, on the differentiated state of the host cells with less differentiated cells requiring a shorter time to form colonies. These colonies are then selected for clonal propagation based on morphology. Pluripotent expression patterns normally take an additional 3 to 4 weeks to develop depending on the cells and methods involved. At this point, colonies should exhibit greater than 70% to 90% OCT4+ cells. Many colonies will never completely transform into pluripotent stem cells so it is very important to select and purify cells based on pluripotent cell surface markers.Citation62 It is the authors’ experience that TRA-1-60 or TRA-1-81 appear to be more effective than SSEA4 for identifying pluripotent human cells.
Another issue in iPSC induction is the low rate of transformation of the transfected host cells. With the use of transgene expression alone 0.001% to 0.1% efficiencies have been reported, but with the addition of other “enhancing” molecules or hypoxic cell culture conditions,Citation36 this rate is reported to be at most 3% in human cells and 10% in mouse cells.Citation46,Citation49 It also appears that higher efficiency rates are correlated with cells from earlier developmental tissue. For instance, work from our laboratory has shown efficiencies of ~2% with only the addition of two genes when applying iPSC technology to human PGCs (). Pera and colleagues also demonstrated that a subpopulation of human adult fibroblasts expressing the pluripotency marker stage specific embryonic antigen 3 (SSEA3) were the source of iPSC colonies after transduction with Oct4, Sox2, Klf4 and cMyc.Citation63 In this report, the efficiency of iPSC derivation was increased by 8-fold while no colonies were generated from SSEA3 negative cells. This evidence lends support to one of two models recently proposed by Yamanaka to explain the low efficiency and partial nature of iPSC generations. Here, the possibility that only SSEA3+ cells generate colonies supports that only an “elite” subset of cells are competent for reprogramming.Citation64 Alternatively, Yamanaka also proposes a stochastic model in which most, if not all, differentiated cells have the potential to become iPSCs. His laboratory and others provide evidence for this model by demonstrating that iPSC formation is impaired by general mechanisms involved in regulating senescence including the p53 and p21 pathways.Citation65,Citation66 Thus, while studies generating iPSCs provide hope for reprogramming adult cells for therapeutic uses, iPSC research also emphasizes the need to continue finding mechanisms that regulate pluripotency and cellular reprogramming. For this purpose, future studies identifying the factors that regulate cellular reprogramming of lineage-restricted cells will be critical.
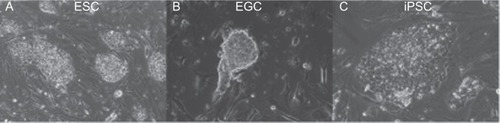
Figure 2 Pluripotent stem cell derivation produce colonies similar in morphology and culturing conditions. A) ESC colonies. B) EGC and C) PGC-derived iPSC colony.
Lineage reprogramming has been shown to occur naturally in lower vertebrates by several different strategies including de differentiationCitation62 and transdifferentiation.Citation67 In fact, transdifferentiation in mammalian cells has been recently highlighted by two studies. Graf and colleagues have shown that lineage switching is possible in the hematopoietic system whereby the overexpression of C/EBPα and β is sufficient to reprogram B lymphocytes into macrophages. Melton and colleagues have also demonstrated lineage switching by directly converting mature pancreatic exocrine cells to endocrine β cells in adult mice.Citation68,Citation69 In this study a genome-wide expression analysis of > 1100 transcription factors from the developing pancreas was employed which revealed specific expression in distinct progenitor cells. Using knockout studies to pinpoint genes required for β cell fate specification, they were able to identify just three factors that together reprogrammed adult exocrine cells into β cells. These studies illustrate the significance of elucidating the molecular machinery that underlies reprogramming which can in turn be utilized to develop strategies to reprogram cell fate that do not require the pluripotent state.
Embryonic germ cells
Primordial germ cells are the progenitor cells of the germ cell lineage, which are the sole source of gametes in the adult. During human development, 50 to 100 PGCs are first distinguishable at ~22 days in the endoderm of the dorsal wall of the yolk sac, near the allantois and in the mesenchyme of the stalk. From there, they proceed to migrate through the hindgut during the fourth week and dorsal mesentery in the fifth week to reach the genital ridge.Citation70–Citation72 By the end of the fifth week or early in the sixth week, ~1000 PGCs begin to actively migrate from the dorsal mesentery to the genital ridge.Citation73–Citation75 At this time, in the female, premeiotic PGCs begin extensive mitotic expansion until they arrest in prophase of meiosis I beginning around week 10 in gestation.Citation70 In contrast, at 8 weeks male PGCs begin extensive mitotic expansion and then arrest around 10 weeks gestation.Citation73,Citation74,Citation76–Citation78 To derive human EGCs, PGCs are isolated from the fetal gonad between 5 and 10 weeks’ gestation. This time period coincides with peak PGC proliferation and encompasses the period in which the gonad undergoes sexual dimorphism into either an ovary or testis starting in the 7th week.Citation79 PGCs are unipotent in that they are lineage-restricted to become germ cells, do not exhibit self renewal and do not survive past one week under standard tissue culture conditions.Citation80 The derivation of EGCs from human tissue has been performed by adapting methods based in part from the original EGC derivation in the mouse. Unlike other pluripotent stem cells which are primarily derived on primary mouse embryonic feeder cells (MEF), EGCs have been mainly derived using the transformed mouse embryonic fibroblast line, Sandoz Thioguanine- and Ouabain-resistant mouse fibroblasts (STO). A few reports deriving mouse and recently one involving human PGCs has successfully utilized MEFs for EGC derivation.Citation81–Citation84
In most studies, EGC growth media has consisted of serum as first reported for human ESC derivation.Citation23 However, a recent report suggests that MEFs with serum replacement are more efficient for human EGC derivation.Citation81 These results highlight that more research in this area is needed to improve EGC derivation. For instance, deriving EGCs is a unique process when compared to deriving other ESCs by the addition of several different factors. Forskolin is one such pharmacological agent which raises intracellular cAMP levels and has been shown to stimulate mitosis in PGC cultures.Citation80 Forskolin has been uniquely employed in EGC derivation to increase derivation efficiency, however, it is not required. Derivation of human EGCs also relies on leukemia inhibitor factor (LIF). LIF was originally known for its inhibitory role in liver cell differentiationCitation85 and later employed for the derivation of mouse embryonic stem cells (mESC) where it signals via the LIF receptor (LIFR), gp130 and intracellular stat3b to maintain mESC pluripotency.Citation86 However, activation of this pathway does not maintain self-renewal of human ESCs, but is required for human and mouse EGC culture. Another growth factor which may be critical for EGC derivation is stem cell factor (SCF), also known as c-kit ligand. Used for mouse EGC derivation, this factor is well known for its role in mPGC proliferation and survival and has been attributable to the reduced ability of MEFs when compared to STO for culturing mPGCs.Citation85 Although our laboratory has not seen an effect of adding SCF in our human EGC cultures, we have shown a positive correlation in PGC proliferation and survival in cultures with subcloned STO feeder cells expressing increased concentrations of transmembrane SCF.Citation87 Finally, EGC derivation like all other pluripotent stem cells relies on fibroblast growth factor (FGF2) for proliferation and survival. FGF2 functions as a potent mitogen in many cell types, and was also the determining factor which led to the first EGC derivation from mice.Citation88–Citation90
In the first week, most human PGC cultures do not produce visible EGC colonies. Staining for tissue non-specific alkaline phosphatase (TnAP) activity demonstrates the presence of solitary PGCs with either stationary or migratory morphology. After 2 to 3 weeks, large and recognizable EGC colonies develop at approximately 10% to 20% efficiency rates. Compared to other pluripotent stem cells, EGCs are challenging to maintain due to the difficulty in disaggregating colonies. This issue together with problems in obtaining PGCs significantly hampers research in this area.
Germ-line stem cells
Recent studies have shown that pluripotent stem cells can also be produced from germ cells isolated further along in male development.Citation91–Citation93 These cells, known as germ-line stem cells (GSCs), were first generated from mouse spermatogonia, and last year, the first study was reported deriving GSCs from testicular biopsies of men.Citation91–Citation93 Spermatogonia stem cells (SSCs) of the male germ line are present at birth. They develop from PGCs in the fetal gonad and consist of multiple subpopulations that either self renew or continue the differentiation process leading to sperm development. Although GSCs derived from the neonate mouse produced teratomas, cells initially reported from the adult testis did not, suggesting that they were multipotent.Citation94,Citation95 However, Scholer and colleagues using a different method derived GSCs from adult mice which demonstrated teratoma and chimera formation, including germ cell contribution and transmission.Citation96 Similar experiments have also been performed on human adult testicular biopsies which in one study produced GSCs capable of teratoma formation.Citation94,Citation97 As with any human germ cell line, these cells cannot be utilized for chimera testing.
In both the mouse and human studies, the ability to generate fully reprogrammed GSCs that can form teratomas and contribute to chimeras appears to be dependent on the method of cell selection and possibly the growth factors employed. For instance, these studies utilized different cell-surface markers including CD49f, CD90, GDNFRα1, and CD133 in combination with various cell culture matrices like collagen and laminin, to select the “appropriate” spermatogonial subpopulation.
The success of deriving GSCs is attributable to a long history of studying SSCs in culture.Citation98–Citation100 Interesting comparisons can be made between GSC culture conditions and culture conditions used to produce other pluripotent stem cells. For instance, like EGCs, several lines of evidence suggest that GSCs may require LIF for complete reprogramming. This is supported by Conrad and colleagues who demonstrated by testing various conditions with and without LIF, that LIF alone was sufficient to produce viable GSC lines capable of forming teratomas. His study also noted that the addition of fibroblast growth factor 2 (FGF2) or glial-derived neurotrophic factor (GDNF) did not increase the efficiency rate.Citation94 In contrast, Kossack and colleagues attempted to generated human GSCs without the use of LIF and were unable to demonstrate teratoma formation.Citation97 In addition, culturing cells over a longer period of time in the presence of LIF under ESC culture was attributed to the generation of mouse GSCs which not only formed teratomas, but also produced chimeras with germ-line transmission.Citation96 Interestingly though, unlike EGCs, human GSC generation does not appear to require FGF2 or GDNF which have been shown previously to support SSC survival in culture.
One benefit of GSCs it that they provide an adult source for pluripotent stem cells without the complications of reprogramming. Ye t further analysis and functional validation in animal studies are required to evaluate their potential for clinical applications. Two primary concerns about the use of GSCs for clinical applications include their uniparental epigenetic imprints and the potential availability they may have for only male patients.
Parthenogenetic stem cells
Parthenogenesis is the development of a diploid embryo from a female gamete without contributions from a male. This process occurs naturally in some invertebrate and vertebrate species (such as reptiles, fish), but is very rare in mammals. Parthenogenetic activation can also be induced experimentally using chemicals to mimic sperminduced Ca2+ oscillations (such as alcohol, ionomycin, or cycloheximide) or by physical stimulation including mechanical stimulation, cold temperatures and electrical shock.Citation101–Citation103 After activation, exposure to cytoskeletal inhibitors (such as 6-dimethylaminopurine) prevent the extrusion of the second polar body creating a diploid parthenote. In mammals, parthenogenetic embryos are unable to thrive beyond the early postimplantation stage, largely because of the lack of paternally expressed imprinted genes required for the normal development of extra embryonic tissues.Citation104,Citation105 In fact, Kono and colleagues have demonstrated the ability to produce the birth of live parthenogenetic mice which were able to produce offspring when the appropriate imprinting of key genes were expressed.Citation106,Citation107 In humans, this concept is also demonstrated in a single case report of spontaneous parthenogenetic chimerism in which the patient survived with a mixed makeup of normal and parthenogenetic cells.Citation108
The first report of the intentional creation of human patient specific pESC lines was published by Pryzhkova and colleagues.Citation109 These lines possessed all of the typical characteristics of human ESC lines generated from IVF embryos. This includes pluripotent marker expression (summarized in ), the ability to differentiate into cellular derivatives of all three germ layers in vitro and the ability to form teratomas in immuno-deficient mice (reviewed in).Citation110 Although chimeric studies cannot be applied to human pESCs, mouse pESCs have contributed to adult tissue in chimeras including germ-line transmission.Citation111–Citation113 Human pESCs lines have also been generated by other laboratories.Citation114,Citation115 In fact, the erroneous report by Hwang and colleagues declaring the first successful derivation of a human somatic cell nuclear transfer (SCNT) ESC-line was later identified by Daley and colleagues to be pESCs which contained genetic material solely from the oocyte donor.Citation116,Citation117 Together these studies have shown that pESCs can be derived successfully at relatively high efficiency rates, ~10% to 16% when compared with other stem cells.Citation102,Citation114,Citation115,Citation117,Citation118
Table 2 Pluripotent stem cell markers
From work in mice, it was originally thought that human pESCs would also be for the most part genetically homozygous.Citation119 This is critical from a clinical standpoint in terms of minimizing the risk of immunological rejection in patients. However, two landmark reports on this issue demonstrated several human pESC lines that were heterozygous at several loci which resulted from genetic recombination events during oocyte maturation. Importantly, loci included the major histocompatibility complex (MHC) which plays a defining role in autoimmunity.Citation109,Citation114 This issue was resolved by two groups who demonstrated methods to generate HLA-homozygous pESC lines by pre-selecting an HLA-homozygous egg donorCitation120 or by generating haploid parthenogenetic embryos.Citation115,Citation120 These cells are produced by eliminating the cytoskeletal inhibitor step which permits the extrusion of the second polar body after oocyte activation.Citation120
In general, pESC derivation mimics those of ESCs in terms of blastocyst isolation and cell culture. Oocytes are collected from donors after hormonal stimulation for in vitro fertilization (IVF) purposes and then subjected to electric stimulation or as Pryzhkova and colleagues have shown, chemical induction alone with ionophore, for egg activation followed by kinase inhibitor 6-dimethylaminopurine (6-DMAP) to prevent the extrusion of the second polar body (this step eliminated for homozygous lines).Citation102,Citation109 After activation, embryos are cultured and the inner cell mass (ICM) is isolated from days 5–6 blastocysts. Like ESCs, derivation requires either human or mouse fibroblasts as a feeder layer. Growth media and serum requirements for pESC derivation are also similar to those used for ESC derivation which include knockout serum replacement (Invitrogen) or human serum. While all studies utilize FGF2 most, but not all, used LIF demonstrating LIF is not critical for deriving pESCs (pers comm, Dr Marina Pryzhkova).Citation115
Another possible source for human pESCs has been demonstrated in reproductively incompetent oocytes, including those that are immature, failed or abnormally fertilized from IVF. These cells are normally discarded during IVF and so alleviate some of the ethical concerns that surround using normal embryos. The process of IVF or intracytoplasmic sperm injection (ICSI) can sometimes produce aneuploid embryos with none or multiple pronuclei.Citation121 In 2004, Suss-Toby and colleagues reported the generation of a human ESC line from a mononuclear zygote following ICSI, which demonstrated normal diploid female karyotype (46,XX) and corresponding ESC characteristics.Citation121 These authors suggest, that mononuclear zygotes can develop into normal blastocysts after sperm penetration as a result of asynchronous formation of pronuclei. Although, this study did not determine the parthenogenetic origin of the ESC line, others have now shown that mononuclear zygotes discarded from IVF can be an additional source for creating human pESCs. Of importance, these parthenogenetic lines expressed all of the properties of a normal euploid hESC line.Citation115,Citation118,Citation122
One important factor in the therapeutic application of pESCs is that currently human pESCs have only been derived from fresh oocytes making this stem cell limited to women who are able to donate eggs. However, if human pESCs could be derived from cryopreserved oocytes this would provide the opportunity to treat women with decreased ovarian reserve and women needing chemotherapy. Two recent studies show great promise in this area. For example, mouse pESC lines that have been derived from cryopreserved ovaries, express ESC-specific markers and differentiate into embryoid bodies in vitro and teratomas in vivo.Citation123 Another study has also demonstrated the ability to produce parthenogenetic human blastocysts from cryopreserved oocytes at high efficiency rates.Citation124 Together these studies provide promise for cryopreserved eggs in the future as a potential source for pESCs.
Unlike work done in ESCs and EGCs, few studies have explored the clinical application of pESCs using transplant models.Citation125 For instance, one study has shown the stable and functional hematopoietic engraftment of mouse pESCs derived from uniparental genomes.Citation126 Another study demonstrated the ability of rabbit pESCs to differentiate into myogenic, osteogenic, adipogenic, and endothelial lineages. These cells were injected into a chemically induced injured tibialis muscle of nude mice, where they were able to integrate and form muscle- and bone-like tissues.Citation127 In addition to these studies, pESCs from nonhuman primates have also been derived that could differentiate into dopamine neurons that restore function in a rat model of Parkinson’s disease.Citation128
Clinical considerations
Given their properties of unlimited self renewal and differentiating potential, pluripotent stem cells hold the promise of providing sufficient numbers of differentiated cells that could potentially be used to treat a wide variety of human conditions, including heart disease, diabetes, and many neurological disorders. However, it is unknown which source of pluripotent stem cells will provide the best therapy for any given disease or affliction. In fact, it seems more reasonable given the uniqueness of different pluripotent stem cells that there will not be only one given stem cell line or approach that provides the single resolution for the diverse needs across all cell-based therapies. Most importantly, the ideal candidate must be easily and reproducibly cultured and manipulated so that the stem cells possess the necessary characteristics for successful differentiation, transplantation and engraftment. This includes taking steps to prevent unregulated proliferation, unwanted cell migration from the lesion site, incorrect differentiation, and poor functioning of transplanted cells.
Ethical and scientific hurdles remain when using pluripotent stem cells in cell-based therapy. For instance, the ethical issues surrounding the use of embryonic and fetal sources for many of these lines present a challenge. In this respect, iPSCs from adult tissues are less controversial and provide an avenue for producing patient-specific cell lines which would eliminate complications involving allograft rejection.Citation46,Citation129–Citation131 However, one of the main obstacles for utilizing these cells is the use of oncogenic factors or vectors which may cause tumorigenesis. In fact, all of the iPSCs reported to date require an oncogenic factor to produce lines from adult tissue at a notable efficiency.Citation4 Several studies have reported on karyotypic abnormalities which develop in iPSC lines.Citation50,Citation132 For instance, Cheng and colleagues found that utilizing the SV40 large T antigen (Simian Vacuolating Virus 40 TAg) to increase transduction efficiency, led to the majority of iPSC lines with abnormal karyotypes. This is not surprising given that the large T antigen is an oncogene associated with the transformation in a variety of cell types.Citation133 Another problem facing iPSC technology is poor efficiency with reported induction rates of only 0.001% to 10% from transfected cells. These rates strongly suggest the involvement of other factors that are critical to regulate reprogramming.
Several reports of the therapeutic use of human pluripotent stem cell-derived cells have been reported across animal models representing a variety of treatable diseases and injuries. First clinical reports were shown in neural-derived cells from human ECCs and EGCs.Citation130 These studies included the use of EGC-derived neural stem cells in animal models of stroke and motor neuron injury. In a rat model of spinal cord injury, transplanted cells appeared to promote partial recovery of the spine by protecting motor neuron death.Citation134 However, in a mouse excitotoxic brain damage model, EGC-derived cells migrated away from the lesion sight and toward damaged areas within the striatum, hippocampus, thalamus, and white matter tracts.Citation135 Models other than neuronal differentiation have also been employed using EGCs-derived cells. For example, EGC-derived cells have also been shown to successfully replace certain bladder defects induced in rats.Citation136
Reports using animal models have also shown the therapeutic use of human ESC-derived cells. These have included a gamut of cell types including insulin secreting islets, retinal cells, liver, chondrocytes, cardiomyocytes, and cells of the neural lineage.Citation129,Citation137–Citation139 Studies have shown that cells derived from human ESCs led to improvements in animal models of osteochondral defects,Citation140,Citation141 diabetes,Citation142 heart ischemia,Citation143–Citation146 Parkinson’s,Citation147,Citation148 spinal cord injury,Citation134,Citation149–Citation152 stroke,Citation153–Citation156 liver disease,Citation157 macular degenerationCitation158 and multiple sclerosis.Citation159,Citation160 In fact, work led by Keirstead and colleagues involving ESC-derived glial cells in rat spinal cord injury models almost led to the first FDA-approved human clinical trials involving ESC-derived progenitors in the summer of 2009.Citation161 This trial sponsored by Geron Corp (Menlo Park, CA, USA), involved injecting human ESC-derived oligodendrocyte progenitors into patients with severe spinal cord injury, but was halted when benign appearing cysts began developing in some of the animal trials. Other clinical trials have also been reported in the near future using ESC-derived retinal pigmented epithelium for macular degeneration and ESC-derived β islet cells for the treatment of diabetes.Citation158,Citation162
Despite there being no current reports using human iPSC-derived cells, studies have begun to show the utility of mouse iPSCs in animal transplant models. Several of these models involve neural and cardiac afflictions. For instance, one study has shown that mouse iPSC-derived neurons have the ability to not only integrate themselves into fetal brains, but also improve symptoms of rats with Parkinson’s disease.Citation163 Another study implicated iPSC-derived progenitors in the treatment of acute myocardial infarction.Citation164 With the exciting promise of iPSC-based therapies studies have begun demonstrating the ability to derive iPSCs from patients with a specific disease. The first report of patient-specific lines were those developed by Cheng and colleagues from patients with sickle cell anemia.Citation50 Since then, human iPSCs have also been generated from skin biopsies of patients with spinal muscular atrophy, amyotrophic lateral sclerosis and familial dysautonomia which demonstrated the ability to differentiate into motor neurons.Citation165–Citation167 Likewise, iPSCs from patients with type 1 diabetes have been derived that could differentiate into insulin-producing cells.Citation168 Proof of iPSC potential for patient-specific treatments comes from a landmark paper describing the treatment of a humanized sickle cell anemia mouse model with iPSCs generated from autologous skin.Citation169 In this study, Hanna and colleagues derived an iPSC line from a transgenic mouse expressing a human sickle cell gene, corrected the mutant α-globin gene producing this disease and then showed that the differentiated cells from the corrected iPSC line were able to treat the disease when injected back into the knock-in mice. More recently, iPSCs have also been generated from β-thalassemia patients.Citation170
Two primary concerns for the use of pluripotent stem cell-derived tissue are host-graft rejection and tumor formation. Graft-versus-host rejection is a critical factor in nonpatient-derived pluripotent stem cells. Therefore, it is critical to have stem cell-derived transplants that are a similar match to the histocompatibility complex of the patient in order to prevent complications associated with long-term immune suppression.Citation171 This is especially pertinent to stem cell-based therapies where cells are integrated into host tissue and as such cannot be surgically removed. However, one solution is to generate a registry of HLA-typed pluripotent stem cell lines from various ethnic groups. The possibility to create a bank of HLA-homozygous stem cell lines, which could be MHC, matched for the majority of human population, is dependent on determining a realistic number that would satisfy a sizable population. However, the number of actual ESC lines that would be needed for a perfect tissue match is still under considerable debate, with estimates by different groups ranging from the hundreds to the thousands.Citation171,Citation172 The considerable range in these studies can be in part contributed to the genetic diversity of the population as well as the criteria set forth for HLA mismatch. In contrast, a few papers have also estimated smaller numbers for pESC lines. These studies estimate that 10 to 70 lines of homozygous human pESC lines would be needed to cover the majority of the Japanese, UK and US populations.Citation172–Citation174
Several issues about pluripotent stem cells have raised concerns on their potential for tumor formation in clinical applications. First, all pluripotent stem cell lines have the propensity to become chromosomally abnormal over long-term culture, a characteristic feature of carcinogenesis. In fact, ESCs and iPSCs share similarities with the pluripotent cancer stem cells, ECCs, including abnormalities in chromosomes 12, 17, and X all of which are implemented in generating teratocarcinomas.Citation47,Citation175–Citation178 Using array-based comparative genomic hybridization (aCGH), a recent report comparing 17 human ESC lines also identified amplification at 20q11.21 and a derivative of chromosome 18.Citation179 It will remain to be seen if the application of this relatively new technology for stem cell purposes will identify more abnormalities missed by chromosomal banding techniques. Secondly, there is always the possibility that some stem cells remain pluripotent even after long-term culturing conditions which promote differentiation. Thus, efficient differentiation protocols along with rigid cell selection must be available to provide pure populations prior to transplantation.
Nevertheless, there is a risk that less differentiated progenitors derived from pluripotent cells may also generate tumor formation. This can be caused by either the innate properties of the cells themselves or by the host environment. For example, it has been shown that leukemias develop more frequently when hematopoietic stem cells are derived from umbilical cord blood as compared to bone marrow or blood suggesting that immature cells may carry higher risks for malignancy.Citation180 There are also numerous examples of bone marrow transplantation where donor-derived human bone marrow cells contributed to solid organ cancers.Citation181–Citation183 Whether these bone-marrow-derived cells are responsible for tumor formation or contributed to a microenvironment that supports tumor growth is not clear.Citation47,Citation184,Citation185
Studies have suggested that the frequent presence of fetal-derived cells in the stroma of malignant breast cancer tumors associated with pregnancy and in some cervical cancers may play a role in their cancer progression.Citation186,Citation187 This issue was further highlighted by the first report of tumor formation from nonmarrow-derived stem/progenitors involving the therapeutic use of human neural stem cells to treat a young patient with inherited ataxia telangiectasia.Citation188 In this case, the quality of stem cells was not reported, raising concerns of proper quality controls as well as raising an important issue about the age of the patient at the time of therapy. In fact, the authors of that report, who did not perform the therapy, caution the use of any progenitors or stem cells in a young environment which alone may drive oncogenesis in otherwise stable cells. This is also consistent with the last decade of human fetal neural stem cell therapy in older adults for Parkinson’s disease, where tumor formation was not demonstrated. Or in the only reported study involving cells derived from a human pluripotent stem cell source. In this clinical study (performed by Layton Bioscience, Inc, Sunnyvale, CA), human ECCs were used to produce postmitotic neurons to treat stroke patients. After almost a decade of follow-up with multiple older patients there are still no reports of tumor formation.Citation189–Citation192
Future directions to eliminate possible stem cell-derived tumor formation have been proposed.Citation47 These include genetic controls and cell targeting to selectively eliminate tumor forming stem cells after transplantation. Although experimental animal models and clinical trials in cancer gene therapy provide support for the utility of these strategies the need in the future will be to test these strategies in pluripotent stem cell derived transplants.
Theoretical considerations
Not only does the study of pluripotent stem cell derivation provide a potential source for patient-derived stem cell sources, but they also provide an excellent experimental model for regenerative medicine. Specifically, processes involved in cellular programming can be elucidated that can then be applied to other adult tissues. For instance, studying the process of derivation of iPSCs and EGCs from their differentiated predecessors can provide critical information regarding pathways involved in dedifferentiation. While iPSCs provide hope for reprogramming adult cells for therapeutic uses, they also stress the necessity in finding mechanisms regulating pluripotency and avoiding those associated with oncogenesis. For this purpose, the factors regulating cellular reprogramming of lineage-restricted cells like that seen in PGCs and adult germ cells during their derivation into pluripotent stem cells may be helpful.
Acknowledgments
We like to thank Fei Fei Liu and Marc Hiller for their assistance in cell culture and photography.
Disclosures
The authors have no conflicts of interest that are directly relevant to the content of this review.
References
- StevensLCDevelopment of resistance to teratocarcinogenesis by primordial germ cells in miceJ Natl Cancer Inst19663768598676005945
- AndrewsPWTeratocarcinomas and human embryology: pluripotent human EC cell lines. Review articleApmis199810611581679524574
- StevensLCOrigin of testicular teratomas from primordial germ cells in miceJ Natl Cancer Inst19673845495526025005
- YuJThomsonJAPluripotent stem cell linesGenes Dev200822151987199718676805
- EguizabalCShovlinTCDurcova-HillsGSuraniAMcLarenAGeneration of primordial germ cells from pluripotent stem cellsDifferentiation2009782–311612319683852
- YuZJiPCaoJDazl promotes germ cell differentiation from embryonic stem cellsJ Mol Cell Biol2009129310319783541
- HastonKMTungJYReijo PeraRADazl functions in maintenance of pluripotency and genetic and epigenetic programs of differentiation in mouse primordial germ cells in vivo and in vitroPLoS One200945e565419468308
- GeijsenNHoroschakMKimKGribnauJEgganKDaleyGQDerivation of embryonic germ cells and male gametes from embryonic stem cellsNature2004427697014815414668819
- HubnerKFuhrmannGChristensonLKDerivation of oocytes from mouse embryonic stem cellsScience200330056231251125612730498
- NayerniaKNolteJMichelmannHWIn vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring miceDev Cell200611112513216824959
- ToyookaYTsunekawaNAkasuRNoceTEmbryonic stem cells can form germ cells in vitroProc Natl Acad Sci U S A200310020114571146214504407
- Lacham-KaplanOChyHTrounsonATesticular cell conditioned medium supports differentiation of embryonic stem cells into ovarian structures containing oocytesStem Cells200624226627316109761
- KerkisAFonsecaSASerafimRCIn vitro differentiation of male mouse embryonic stem cells into both presumptive sperm cells and oocytesCloning Stem Cells20079453554818154514
- ClarkATBodnarMSFoxMSpontaneous differentiation of germ cells from human embryonic stem cells In vitroHum Mol Genet200413772773914962983
- AflatoonianBMooreHHuman primordial germ cells and embryonic germ cells, and their use in cell therapyCurr Opin Biotechnol200516553053516154336
- KeeKGonsalvesJMClarkATPeraRABone morphogenetic proteins induce germ cell differentiation from human embryonic stem cellsStem Cells Dev200615683183717253946
- TilgnerKAtkinsonSPGolebiewskaAStojkovicMLakoMArmstrongLIsolation of primordial germ cells from differentiating human embryonic stem cellsStem Cells200826123075308518802037
- ParkTSGalicZConwayAEDerivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cellsStem Cells200927478379519350678
- WestFDMachacekDWBoydNLPandiyanKRobbinsKRSticeSLEnrichment and differentiation of human germ-like cells mediated by feeder cells and basic fibroblast growth factor signalingStem Cells200826112768277618719225
- BucayNYebraMCirulliVA novel approach for the derivation of putative primordial germ cells and sertoli cells from human embryonic stem cellsStem Cells2009271687718845765
- ZhaoXYLiWLvZiPS cells produce viable mice through tetraploid complementationNature20094617260869019672241
- NagyAGoczaEDiazEMEmbryonic stem cells alone are able to support fetal development in the mouseDevelopment199011038158212088722
- ThomsonJAEmbryonic stem cell lines derived from human blastocysts [comment][erratum appears in Science 1998 4;282(5395):1827]Science19982825391114511479804556
- ShamblottMJAxelmanJWangSDerivation of pluripotent stem cells from cultured human primordial germ cellsProc Natl Acad Sci U S A1998952313726137319811868
- EvansMJKaufmanMHEstablishment in culture of pluripotential cells from mouse embryosNature198129258191541567242681
- MartinGRIsolation of a pluripotent cell line from early mouse embryos cultured in media conditioned by teratocarcinoma stem cellsProc Natl Acad Sci U S A198178763476386950406
- SmithAGEmbryo-derived stem cells: of mice and menAnnu Rev Cell Dev Biol20011743546211687496
- AmitMItskovitz-EldorJDerivation and maintenance of human embryonic stem cellsTurksenTMethods in Molecular Biology, Volume 331: Human Embryonic Stem Cell ProtocolsNew YorkHumana Press20064353
- SullivanSCowanCAEgganKHuman Embryonic Stem Cells: The Practical HandbookWest SussexJohn Wiley and Sons, Ltd2007
- KlimanskayaILanzaRMethods in Enzymology, Volume 418: Embryonic Stem CellsSan DiegoAcademic Press2006
- LerouPHYabuuchiAHuoHDerivation, growth and applications of human embryonic stem cells from poor-quality in vitro fertilization embryosReproduction2004128325926715333777
- LerouPHYabuuchiAHuoHDerivation and maintenance of human embryonic stem cells from poor-quality in vitro fertilization embryosReprod Biomed Online200835923933
- BavisterBOxygen concentration and preimplantation developmentReprod Biomed Online20049548448615588462
- EzashiTDasPRobertsRMLow O2 tensions and the prevention of differentiation of hES cellsProc Natl Acad Sci U S A2005102134783478815772165
- ForsythNRMusioAVezzoniPSimpsonAHNobleBSMcWhirJPhysiologic oxygen enhances human embryonic stem cell clonal recovery and reduces chromosomal abnormalitiesCloning Stem Cells200681162316571074
- YoshidaYTakahashiKOkitaKIchisakaTYamanakaSHypoxia enhances the generation of induced pluripotent stem cellsCell Stem Cell20095323724119716359
- LerouPHYabuuchiAHuoHDerivation and maintenance of human embryonic stem cells from poor-quality in vitro fertilization embryosNat Protoc20083592393318451800
- CowanCAVSvetlakovAVPolstianoyAMDerivation of a novel human embryonic stem-cell line under serum-free and feeder-free conditionsDokl Biol Sci20043501313531356
- LudwigTEBergendahlVLevensteinMEYuJProbascoMDThomsonJAFeeder-independent culture of human embryonic stem cellsNat Methods20063863764616862139
- LudwigTELevensteinMEJonesJMDerivation of human embryonic stem cells in defined conditionsNat Biotechnol200624218518716388305
- KimCAmanoTParkJCarterMGTianXYangXImprovement of embryonic stem cell line derivation efficiency with novel medium, glucose concentration, and epigenetic modificationsCloning Stem Cells20091118910019226216
- Tsuji-TakayamaKInoueTIjiriYDemethylating agent, 5-azacytidine, reverses differentiation of embryonic stem cellsBiochem Biophys Res Commun20043231869015351705
- LeeJHHartSRSkalnikDGHistone deacetylase activity is required for embryonic stem cell differentiationGenesis2004381323814755802
- FengBNgJHHengJCNgHHMolecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cellsCell Stem Cell20094430131219341620
- LeeHParkJForgetBGGainesPInduced pluripotent stem cells in regenerative medicine: an argument for continued research on human embryonic stem cellsRegen Med20094575976919761400
- AmabileGMeissnerAInduced pluripotent stem cells: current progress and potential for regenerative medicineTrends Mol Med2009152596819162546
- KiuruMBoyerJLO’ConnorTPCrystalRGGenetic control of wayward pluripotent stem cells and their progeny after transplantationCell Stem Cell20094428930019341619
- FengBJiangJKrausPReprogramming of fibroblasts into induced pluripotent stem cells with orphan nuclear receptor EsrrbNat Cell Biol200911219720319136965
- HuangfuDOsafuneKMaehrRInduction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2Nat Biotechnol200826111269127518849973
- MaliPYeZHommondHHImproved efficiency and pace of generating induced pluripotent stem cells from human adult and fetal fibroblastsStem Cells20082681998200518511599
- StadtfeldMNagayaMUtikalJWeirGHochedlingerKInduced pluripotent stem cells generated without viral integrationScience2008322590394594918818365
- OkitaKNakagawaMHyenjongHIchisakaTYamanakaSGeneration of mouse induced pluripotent stem cells without viral vectorsScience2008322590394995318845712
- KajiKNorrbyKPacaAMileikovskyMMohseniPWoltjenKVirus-free induction of pluripotency and subsequent excision of reprogramming factorsNature2009458723977177519252477
- WoltjenKMichaelIPMohseniPpiggyBac transposition reprograms fibroblasts to induced pluripotent stem cellsNature2009458723976677019252478
- KimDKimCHMoonJIGeneration of human induced pluripotent stem cells by direct delivery of reprogramming proteinsCell Stem Cell20094647247619481515
- MikkelsenTSHannaJZhangXDissecting direct reprogramming through integrative genomic analysisNature20084547200495518509334
- ShiYDoJTDespontsCHahmHSScholerHRDingSA combined chemical and genetic approach for the generation of induced pluripotent stem cellsCell Stem Cell20082652552818522845
- MarsonAForemanRChevalierBWnt signaling promotes reprogramming of somatic cells to pluripotencyCell Stem Cell20083213213518682236
- SilvaJBarrandonONicholsJKawaguchiJTheunissenTWSmithAPromotion of reprogramming to ground state pluripotency by signal inhibitionPLoS Biol2008610e25318942890
- LiWWeiWZhuSGeneration of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitorsCell Stem Cell200941161919097958
- PaliiSSVan EmburghBOSankpalUTBrownKDRobertsonKDDNA methylation inhibitor 5-Aza-2’-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3BMol Cell Biol200828275277117991895
- BrockesJPKumarAPlasticity and reprogramming of differentiated cells in amphibian regenerationNat Rev Mol Cell Biol20023856657412154368
- ByrneJANguyenHNReijo PeraRAEnhanced generation of induced pluripotent stem cells from a subpopulation of human fibroblastsPLoS One200949e711819774082
- YamanakaSElite and stochastic models for induced pluripotent stem cell generationNature20094607251495219571877
- HongHTakahashiKIchisakaTSuppression of induced pluripotent stem cell generation by the p53-p21 pathwayNature200946072591132113519668191
- BanitoARashidSTAcostaJCSenescence impairs successful reprogramming to pluripotent stem cellsGenes Dev200923182134213919696146
- EguchiGOkadaTSDifferentiation of lens tissue from the progeny of chick retinal pigment cells cultured in vitro: a demonstration of a switch of cell types in clonal cell cultureProc Natl Acad Sci U S A1973705149514994576021
- XieHYeMFengRGrafTStepwise reprogramming of B cells into macrophagesCell2004117566367615163413
- ZhouQBrownJKanarekARajagopalJMeltonDAIn vivo reprogramming of adult pancreatic exocrine cells to beta-cellsNature2008455721362763218754011
- BakerTGFranchiLLThe fine structure of oogonia and oocytes in human ovariesJ Cell Sci1967222132244933750
- SunELGondosBSquash preparation studies of germ cells in human fetal testesJ Androl19849553343386542097
- MakabeSNaguroTMottaPMA new approach to the study of ovarian follicles by scanning electron microscopy and ODO macerationArch Histol Cytol199255Suppl1831901283951
- HeynRMakabeSMottaPMUltrastructural dynamics of human testicular cords from 6 to 16 weeks of embryonic development. Study by transmission and high resolution scanning electron microscopyItal J Anat Embryol19981034 Suppl 1172911315948
- BendsenEByskovAGLaursenSBLarsenHPAndersenCYWestergaardLGNumber of germ cells and somatic cells in human fetal testes during the first weeks after sex differentiationHum Reprod2003181131812525434
- FrancavillaSCordeschiGProperziGConcordiaNCappaFPozziVUltrastructure of fetal human gonad before sexual differentiation and during early testicular and ovarian developmentJ Submicrosc Cytol Pathol19902233894002390761
- WitschiEEmbryology of the OvaryGradyHGSmithDEThe OvaryBaltimoreWilliams and Wilkins1963110
- WartenbergHDevelopment of the early human ovary and role of the mesonephros in the differentiation of the cortexAnat Embryol (Berl)198216522532807158813
- GondosBHobelCJUltrastructure of germ cell development in the human fetal testisZ Zellforsch Mikrosk Anat197111911204327535
- LaboskyPAHoganBLMouse primordial germ cells: isolation and in vitro cultureMethods Mol Biol200846118719919030797
- DolciSPesceMDe FeliciMCombined action of stem cell factor, leukemia inhibitory factor, and cAMP on in vitro proliferation of mouse primordial germ cellsMol Reprod Dev19933521341397686377
- HuaJYuHLiuSDerivation and characterization of human embryonic germ cells: serum-free culture and differentiation potentialReprod Biomed Online200919223824919712561
- GodinIWylieCCTGF beta 1 inhibits proliferation and has a chemotropic effect on mouse primordial germ cells in cultureDevelopment19911134145114571811953
- MatsuiYToksozDNishikawaSEffect of Steel factor and leukaemia inhibitory factor on murine primordial germ cells in cultureNature199135363467507521719421
- DolciSWilliamsDEErnstMKRequirement for mast cell growth factor for primordial germ cell survival in cultureNature199135263388098111715518
- LaboskyPABarlowDPHoganBLEmbryonic germ cell lines and their derivation from mouse primordial germ cellsCiba Found Symp19941821571687835148
- NiwaHBurdonTChambersISmithASelf-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3Genes Dev19981213204820609649508
- ShamblottMJKerrCLAxelmanJDerivation and Differentiation of Human Embryonic Germ CellsLanzaRHoganBMeltonDPedersenRThomsonJWestMHandbook of Stem Cells1New YorkElsevier Academic Press2004459469
- De FeliciMDolciSPesceMProliferation of mouse primordial germ cells in vitro: a key role for cAMPDev Biol199315712772808387035
- ResnickJLBixlerLSChengLDonovanPJLong-term proliferation of mouse primordial germ cells in cultureNature199235963955505511383830
- MatsuiYZseboKHoganBLDerivation of pluripotential embryonic stem cells from murine primordial germ cells in cultureCell19927058418471381289
- GuanKNayerniaKMaierLSPluripotency of spermatogonial stem cells from adult mouse testisNature200644070881199120316565704
- IzadyarFPauFMarhJGeneration of multipotent cell lines from a distinct population of male germ line stem cellsReproduction2008135677178418502893
- Kanatsu-ShinoharaMInoueKLeeJGeneration of pluripotent stem cells from neonatal mouse testisCell200411971001101215620358
- ConradSRenningerMHennenlotterJGeneration of pluripotent stem cells from adult human testisNature2008456722034434918849962
- KossackNMenesesJShefiSIsolation and characterization of pluripotent human spermatogonial stem cell-derived cellsStem Cells200927113814918927477
- KoKTapiaNWuGInduction of pluripotency in adult unipotent germline stem cellsCell Stem Cell200951879619570517
- KossackNMenesesJShefiSIsolation and characterization of pluripotent human spermatogonial stem cell-derived cellsStem Cells200927113814918927477
- DymMHeZJiangJPantDKokkinakiMSpermatogonial stem cells: unlimited potentialReprod Fertil Dev2009211152119152741
- OatleyJMBrinsterRLSpermatogonial stem cellsMethods Enzymol200641925928217141059
- Kanatsu-ShinoharaMTakehashiMShinoharaTBrief history, pitfalls, and prospects of mammalian spermatogonial stem cell researchCold Spring Harb Symp Quant Biol200873l 723
- RougierNWerbZMinireview: Parthenogenesis in mammalsMol Reprod Dev200159446847411468784
- RevazovaESTurovetsNAKochetkovaODPatient-specific stem cell lines derived from human parthenogenetic blastocystsCloning Stem Cells20079343244917594198
- CibelliJBCunniffKVranaKEEmbryonic stem cells from parthe-notesMethods Enzymol200641811713517141033
- SuraniMABartonSCNorrisMLDevelopment of reconstituted mouse eggs suggests imprinting of the genome during gametogenesisNature198430859595485506709062
- McGrathJSolterDInability of mouse blastomere nuclei transferred to enucleated zygotes to support development in vitroScience19842264680131713196542249
- KonoTObataYWuQBirth of parthenogenetic mice that can develop to adulthoodNature2004428698586086415103378
- WuQKumagaiTKawaharaMRegulated expression of two sets of paternally imprinted genes is necessary for mouse parthenogenetic development to termReproduction2006131348148816514191
- StrainLWarnerJPJohnstonTBonthronDTA human parthenogenetic chimaeraNat Genet19951121641697550344
- RevazovaESTurovetsNAKochetkovaODHLA Homozygous Stem Cell Lines Derived from Human Parthenogenetic BlastocystsCloning Stem Cells2008101112418092905
- HaoJZhuWShengCYuYZhouQHuman parthenogenetic embryonic stem cells: one potential resource for cell therapySci China C Life Sci200952759960219641863
- AllenNDBartonSCHiltonKNorrisMLSuraniMAA functional analysis of imprinting in parthenogenetic embryonic stem cellsDevelopment19941206147314828050357
- KimKLerouPYabuuchiAHistocompatible embryonic stem cells by parthenogenesisScience2007315581148248617170255
- ThomsonJASolterDThe developmental fate of androgenetic, parthenogenetic, and gynogenetic cells in chimeric gastrulating mouse embryosGenes Dev1988210134413513203909
- MaiQYuYLiTDerivation of human embryonic stem cell lines from parthenogenetic blastocystsCell Res200717121008101918071366
- LinGOuYangQZhouXA highly homozygous and parthenogenetic human embryonic stem cell line derived from a onepronuclear oocyte following in vitro fertilization procedureCell Res20071712999100718040289
- HwangWSRohSILeeBCPatient-specific embryonic stem cells derived from human SCNT blastocystsScience200530857291777178315905366
- KimKNgKRugg-GunnPJRecombination signatures distinguish embryonic stem cells derived by parthenogenesis and somatic cell nuclear transferCell Stem Cell20071334635218371368
- De SousaPAGardnerJSneddonSClinically failed eggs as a source of normal human embryo stem cellsStem Cell Res20092318819719393594
- LinHLeiJWiningerDMultilineage potential of homozygous stem cells derived from metaphase II oocytesStem Cells200321215216112634411
- RevazovaESTurovetsNAKochetkovaODHLA homozygous stem cell lines derived from human parthenogenetic blastocystsCloning Stem Cells2008101112418092905
- Suss-TobyEGerecht-NirSAmitMManorDItskovitz-EldorJDerivation of a diploid human embryonic stem cell line from a mononuclear zygoteHum Reprod200419367067514998969
- McElroySLKeeKTranNMensesJGiudiceLCReijo PeraRADevelopmental competence of immature and failed/abnormally fertilized human oocytes in nuclear transferReprod Biomed Online200816568469318492373
- XingFFangZQinBLiYHouJChenXParthenogenetic embryonic stem cells derived from cryopreserved newborn mouse ovaries: a new approach to autologous stem cell therapyFertil Steril20099141238124418353322
- de FriedEPRossPZangGHuman parthenogenetic blastocysts derived from noninseminated cryopreserved human oocytesFertil Steril200889494394717706204
- MullerRLengerkeCPatient-specific pluripotent stem cells: promises and challengesNat Rev Endocrinol20095419520319352317
- EckardtSLeuNABradleyHLKatoHBuntingKDMcLaughlinKJHematopoietic reconstitution with androgenetic and gynogenetic stem cellsGenes Dev200721440941917322401
- KohCJDeloDMLeeJWParthenogenesis-derived multipotent stem cells adapted for tissue engineering applicationsMethods2009472909718799133
- Sanchez-PernauteRLeeHPattersonMParthenogenetic dopamine neurons from primate embryonic stem cells restore function in experimental Parkinson’s diseaseBrain2008131Pt 82127213918669499
- BongsoAFongCYGauthamanKTaking stem cells to the clinic: Major challengesJ Cell Biochem200810561352136018980213
- LoBGaranNHistorical perspective and evolving concerns for human researchCancer Treat Res200713211017305014
- ChaerkadyRKerrCLMarimuthuATemporal analysis of neural differentiation using quantitative proteomics (dagger)J Proteome Res2009831315132619173612
- AasenTRayaABarreroMJEfficient and rapid generation of induced pluripotent stem cells from human keratinocytesNat Biotechnol200826111276128418931654
- AliSHDeCaprioJACellular transformation by SV40 large T antigen: interaction with host proteinsSemin Cancer Biol2001111152311243895
- KerrDALladoJShamblottMJHuman embryonic germ cell derivatives facilitate motor recovery of rats with diffuse motor neuron injuryJ Neurosci200323125131514012832537
- MuellerDShamblottMJFoxHEGearhartJDMartinLJTransplanted human embryonic germ cell-derived neural stem cells replace neurons and oligodendrocytes in the forebrain of neonatal mice with excitotoxic brain damageJ Neurosci Res200582559260816247803
- FrimbergerDMoralesNShamblottMGearhartJDGearhartJPLakshmananYHuman embryoid body-derived stem cells in bladder regeneration using rodent modelUrology200565482783215833555
- FurthMEAtalaAStem cell sources to treat diabetesJ Cell Biochem2009106450751119130494
- LindvallOKokaiaZProspects of stem cell therapy for replacing dopamine neurons in Parkinson’s diseaseTrends Pharmacol Sci200930526026719362379
- KarussisDKassisIThe potential use of stem cells in multiple sclerosis: an overview of the preclinical experienceClin Neurol Neurosurg2008110988989618375051
- HwangNSVargheseSLeeHJIn vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cellsProc Natl Acad Sci U S A200810552206412064619095799
- HwangNSElisseeffJApplication of stem cells for articular cartilage regenerationJ Knee Surg2009221607119216354
- KroonEMartinsonLAKadoyaKPancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivoNat Biotechnol200826444345218288110
- KehatIKhimovichLCaspiOElectromechanical integration of cardiomyocytes derived from human embryonic stem cellsNat Biotechnol200422101282128915448703
- LafammeMAGoldJXuCFormation of human myocardium in the rat heart from human embryonic stem cellsAm J Pathol2005167366367116127147
- XueTChoHCAkarFGFunctional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakersCirculation20051111112015611367
- LeorJGerechtSCohenSHuman embryonic stem cell transplantation to repair the infarcted myocardiumHeart200793101278128417566061
- Ben-HurTIdelsonMKhanerHTransplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian ratsStem Cells20042271246125515579643
- RoyNSClerenCSinghSKYangLBealMFGoldmanSAFunctional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytesNat Med200612111259126817057709
- LiuSQuYStewartTJEmbryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantationProc Natl Acad Sci U S A200097116126613110823956
- KeirsteadHSNistorGBernalGHuman embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injuryJ Neurosci200525194694470515888645
- FaulknerJKeirsteadHSHuman embryonic stem cell-derived oligodendrocyte progenitors for the treatment of spinal cord injuryTranspl Immunol200515213114216412957
- CloutierFSiegenthalerMMNistorGKeirsteadHSTransplantation of human embryonic stem cell-derived oligodendrocyte progenitors into rat spinal cord injuries does not cause harmRegen Med20061446947917465839
- DaadiMMMaagALSteinbergGKAdherent self-renewable human embryonic stem cell-derived neural stem cell line: functional engraftment in experimental stroke modelPLoS One200832e164418286199
- DaadiMMLeeSHAracAFunctional Engraftment of the Medial Ganglionic Eminence Cells in Experimental Stroke ModelCell Transplant200918781582619500468
- HicksAULappalainenRSNarkilahtiSTransplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: cell survival and functional recoveryEur J Neurosci200929356257419175403
- OyamadaNItohHSoneMTransplantation of vascular cells derived from human embryonic stem cells contributes to vascular regeneration after stroke in miceJ Transl Med200865418823569
- Soto-GutierrezAKobayashiNRivas-CarrilloJDReversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytesNat Biotechnol200624111412141917086173
- VuglerACarrAJLawrenceJElucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantationExp Neurol2008214234736118926821
- NistorGITotoiuMOHaqueNCarpenterMKKeirsteadHSHuman embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantationGlia200549338539615538751
- AharonowizMEinsteinOFainsteinNNeuroprotective effect of transplanted human embryonic stem cell-derived neural precursors in an animal model of multiple sclerosisPLoS One200839e314518773082
- CouzinJBiotechnology. Celebration and concern over US trial of embryonic stem cellsScience2009323591456819179496
- TrounsonANew perspectives in human stem cell therapeutic researchBMC Med200972919519878
- WernigMZhaoJPPruszakJNeurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s diseaseProc Natl Acad Sci U S A2008105155856586118391196
- NelsonTJMartinez-FernandezAYamadaSPerez-TerzicCIkedaYTerzicARepair of acute myocardial infarction by human stemness factors induced pluripotent stem cellsCirculation2009120540841619620500
- EbertADYuJRoseFFJrInduced pluripotent stem cells from a spinal muscular atrophy patientNature2009457722727728019098894
- DimosJTRodolfaKTNiakanKKInduced pluripotent stem cells generated from patients with ALS can be differentiated into motor neuronsScience200832158931218122118669821
- LeeGPapapetrouEPKimHModelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCsNature2009461726240240619693009
- MaehrRChenSSnitowMGeneration of pluripotent stem cells from patients with type 1 diabetesProc Natl Acad Sci U S A2009106155231552419805208
- HannaJWernigMMarkoulakiSTreatment of sickle cell anemia mouse model with iPS cells generated from autologous skinScience200731858581920192318063756
- WangYJiangYLiuSSunXGaoSGeneration of induced pluripotent stem cells from human beta-thalassemia fibroblast cellsCell Res20091991120112319690515
- CondicMLRaoMRegulatory issues for personalized pluripotent cellsStem Cells200826112753275818669906
- FadenRRDawsonLBateman-HouseASPublic stem cell banks: considerations of justice in stem cell research and therapyHastings Cent Rep2003336132714983554
- NakajimaFTokunagaKNakatsujiNHuman leukocyte antigen matching estimations in a hypothetical bank of human embryonic stem cell lines in the Japanese population for use in cell transplantation therapyStem Cells200725498398517185611
- TaylorCJBoltonEMPocockSSharplesLDPedersenRABradleyJABanking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matchingLancet200536695022019202516338451
- StruloviciYLeopoldPLO’ConnorTPPergolizziRGCrystalRGHuman embryonic stem cells and gene therapyMol Ther200715585086617356540
- HarrisonNJBakerDAndrewsPWCulture adaptation of embryonic stem cells echoes germ cell malignancyInt J Androl200730427528117488340
- DraperJSSmithKGokhalePRecurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cellsNat Biotechnol2004221535414661028
- BlumBBenvenistyNThe tumorigenicity of human embryonic stem cellsAdv Cancer Res200810013315818620095
- SpitsCMateizelIGeensMRecurrent chromosomal abnormalities in human embryonic stem cellsNat Biotechnol200826121361136319029912
- GreavesMFCord blood donor cell leukemia in recipientsLeukemia20062091633163416791267
- BarozziPLuppiMFacchettiFPost-transplant Kaposi sarcoma originates from the seeding of donor-derived progenitorsNat Med20039555456112692543
- AvitalIMoreiraALKlimstraDSDonor-derived human bone marrow cells contribute to solid organ cancers developing after bone marrow transplantationStem Cells200725112903290917690178
- GolfinopoulosVPentheroudakisGKamakariSMetaxa-MariatouVPavlidisNDonor-derived breast cancer in a bone marrow transplantation recipientBreast Cancer Res Treat2009113221121318264757
- DubernardGAractingiSOsterMBreast cancer stroma frequently recruits fetal derived cells during pregnancyBreast Cancer Res2008101R1418271969
- RoordaBDter ElstAKampsWAde BontESBone marrow-derived cells and tumor growth: contribution of bone marrow-derived cells to tumor micro-environments with special focus on mesenchymal stem cellsCrit Rev Oncol Hematol200969318719818675551
- JohnsonKLBianchiDWFetal cells in maternal tissue following pregnancy: what are the consequences?Hum Reprod Update200410649750215319378
- ChaDKhosrotehraniKKimYStrohHBianchiDWJohnsonKLCervical cancer and microchimerismObstet Gynecol2003102477478114551008
- AmariglioNHirshbergAScheithauerBWDonor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patientPLoS Med200962e100002919226183
- StilleyCSRyanCMKondziolkaDBenderADeCesareSWechslerLChanges in cognitive function after neuronal cell transplantation for basal ganglia strokeNeurology20046371320132215477565
- WatsonDJLonghiLLeeEBGenetically modified NT2N human neuronal cells mediate long-term gene expression as CNS grafts in vivo and improve functional cognitive outcome following experimental traumatic brain injuryJ Neuropathol Exp Neurol200362436838012722829
- KondziolkaDWechslerLGoldsteinSTransplantation of cultured human neuronal cells for patients with strokeNeurology200055456556910953194
- WechslerLRClinical trials of stroke therapy: which cells, which patients?Stroke2009403 SupplS149S15119064792